Abstract
A total of 45 cyanobacterial strains isolated from rice fields near Loktak Lake in Manipur, India were tested for their rice root colonization capacity under light and under darkness. Twenty-one of these strains showed significant colonization of rice roots. The average colonization values were 637 and 381 μg chl a g−1 root dry wt in N2 medium and 792 and 451 μg chl a g−1 root dry wt in NO −3 medium under light and darkness, respectively. Thus, while the colonization was higher under light and in NO −3 medium, there was significant level of colonization under darkness in N2 medium (381 μg chl a g−1 root dry wt). A 16S rRNA gene fragment-based denaturing gradient gel electrophoresis analysis revealed difference in the competence of individual strains to colonize rice roots exposed to individual or mixed population. The colonization pattern of seven strains used in competition experiments was found to be biphasic. A 16S rRNA gene-based phylogenetic analysis revealed high level of molecular similarity among strains of Nostoc and Anabaena.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Demand for industrially produced chemical fertilizer as sources of nitrogen (N) and phosphorus (P) has increased considerably for improving crop production. Concomitantly, this has led to increased level of N in leaching water. A significant portion of applied N fertilizer is also lost through denitrification, ammonia volatilization, and runoff. Moreover, industrial production of N fertilizer is a highly energy intensive process costing about US$ 45 billion each year. Development of sustainable and environmentally friendly alternatives to chemical fertilizers is necessary for enhancing crop production. Diazotrophic cyanobacterial strains growing under water-logging rice fields are known to meet the N requirement of crop plants. Past studies have emphasized the biofertilizer potentials of N2-fixing photosynthetic bacteria and cyanobacteria together contributing 10–80 kg N ha−1 averaging about 30 kg N ha−1 crop−1 (Roger and Watanabe 1986). The ability of cyanobacteria to grow in symbiosis with various plants has an obvious implication for their use in agricultural biotechnology. Biological nitrogen fixation by free-living and plant-associated diazotrophs (Ladha et al. 1993; Watanabe and Roger 1984) and mineralization of soil N (Bouldin 1986; Kundu and Ladha 1995) contribute significantly to the tropical rice production.
Various free-living cyanobacterial strains can associate with a wide range of plants (Gantar et al. 1991a; Korzhenevskaya et al. 1999) and they can be used as biofertilizers for agronomically important crop plants (Nilsson et al. 2002). The colonization ability of different cyanobacterial strains varies. A study involving mixed population of symbiotic cyanobacterial strains in mixed population revealed the association of only one strain with rice roots (Nilsson et al. 2005). In addition, non-heterocystous cyanobacterial isolates from rice field have been shown to associate with rice plants with their filaments penetrating the root cells (Mehboob et al. 2010). To our knowledge, there has been no study on the screening of rice field heterocystous cyanobacterial strains exhibiting ability to associate with rice plants. In the present study, 45 rice field cyanobacterial isolates belonging to the genera Nostoc, Anabaena, Calothrix, Cylindrospermum, and Mastigocladus were checked for their potential to colonize rice roots. Rice roots colonization of 21 selected cyanobacterial strains were quantified in terms of chlorophyll a in the presence and absence of combined N under light as well as under darkness. Further, study of Anabaena and Nostoc strains 16S rRNA gene-based denaturing gradient gel electrophoresis (DGGE) revealed variation in colonization of individual strains when rice roots were exposed to individually or mixed populations.
Materials and methods
Organisms and growth conditions
Heterocystous cyanobacterial strains of Nostoc, Anabaena, Calothrix, Cylindrospermum, and Mastigocladus were isolated from rice fields near Loktak Lake, Manipur as described in Rippka et al. (1979). Morphological identification of individual isolates was performed according to Desikachary (1959) (Table 1). The isolates were grown and maintained in combined N- free BG-110 medium at 25°C and a photon fluence rate of 50 μmol m−2 s−1.
Growth study
Cyanobacterial growth was determined by measuring increase in concentrations of chlorophyll a (Mackinney 1941) and phycobiliprotein contents (Bennett and Bogorad 1973), 6 days after inoculation in N2 medium, NO −3 medium, or NH +4 medium. N2 medium corresponds to BG-110 medium without combined N source, whereas NO −3 medium and NH +4 medium included 5 mM NaNO3 or 2 mM NH4Cl in combined N-free medium.
Generation of DNA fingerprint profile
DNA was amplified by using STRR1A primer (Rasmussen and Svenning 1998). Six-day-old axenic culture of cyanobacterial strains were washed twice and suspended in appropriate volume of sterile milli-Q water to obtain few filaments in every 2-μl suspension. This suspension was heated at 100°C for 10 min and the released DNA was amplified by PCR (Chingkheihunba and Singh 2011). The PCR thermal cycles were carried out in a total volume of 50 μl containing 100 pmol STRR1A primer, 1.25 mM deoxynucleoside triphosphate (dNTP), 2 μl template DNA, 2 U of DNA polymerase (Bioline), and the buffer supplied with the enzyme. We used the Applied Biosystems 2720 thermal cycler. Six microliters of PCR product was electrophoresed at 80 V in 1.5% agarose gels in 1x TAE buffer (0.04 M Tris–acetate, 0.001 M EDTA, pH 8.0) for 1.5 h. The gels were stained for 30 min in the same buffer containing ethidium bromide (5 μg ml−1) and photographed under UV transilluminator using a CCD camera (Olympus Camedia C4000).
Germination of rice seed
Rice (Oryza sativa) variety RCM-10 obtained from the Indian Council of Agricultural Research, Imphal, India, was surface sterilized by washing in sterile distilled water followed by washing in 1% (v/v) sodium hypochlorite solution for 5 min. The seeds were then finally rinsed in sterile distilled water. The germination of seeds was carried out by sowing on autoclaved perlite surface floating on top of tenfold diluted BG-110 medium supplemented with 0.5 mM NaNO3 and buffered with equimolar concentration of HEPES–NaOH, in a plastic bucket. The perlite was irrigated from time to time with similarly diluted sterile 0.5 mM NaNO3 supplemented BG11 medium. Germination was carried out in growth cabinet at 25°C at saturating relative humidity and a 12-h light–dark cycle at photon fluence rate of 50 μmol m−2 s−1.
Co-cultivation of cyanobacteria with rice plant
Rice seedlings grown for 15 days were uprooted from the perlite and washed in distilled water and then suspended in 20 ml of tenfold diluted BG-110 medium with and without 0.5 mM NaNO3. To this, cyanobacterial filaments were added to a final concentration of 2 μg chl a ml−1. Co-cultivation of cyanobacteria and rice plants was carried out at 25°C under light or under darkness. Five days after co-culturing, the rice seedlings were uprooted and washed to remove loosely attached cyanobacterial filaments. The excised roots were then used for measuring colonization by cyanobacteria in terms of chl a per gram root dry weight. All the experiments were performed twice with three replicates each.
Competition experiments
Competition among randomly selected strains of either Anabaena or Nostoc to associate with rice roots was carried out by co-culturing 15-day-old rice seedlings with a mixture comprising either two strains or three strains or four strains, at a final concentration of 2 μg chl a ml−1 containing an equal amount of chlorophyll a from each cyanobacterial constituent. After 7 days, rice roots were sonicated at 40 kHz for 30 s in Powersonic LUC405 to remove loosely attached cyanobacterial filaments. The roots were then excised and crushed separately using sterile pestle and mortar in an appropriate volume of sterile milli-Q water. The DNA was then extracted using GeNeiUltrapure Bacterial genomic DNA purification kit and used as template in subsequent PCR for generating DNA amplicons for DGGE. Amplification of 16S rRNA gene by PCR was carried out using CYA106FGC (5′-CGCCCGCCGCGCCCCGCGCCGGTCCCGCCGCCCCCGCCCGCGGACGGGTGAGTAACGCGTGA-3′) and CYA781R (CYA781Ra 5′-GACTACTGGGGTATCTAATCCCATT-3′ and CYA781Rb 5′-GACTACAGGGGT ATCTAATCCCTTT-3′) sequences as forward and reverse primers as already described (Nübel et al. 1997). We used Applied Biosystems 2720 thermal cycler. Fifty microliters of PCR mixture comprised 20 pmol of each primer, 2 U of DNA polymerase (Bioline), 10 μM dNTP, 1 μl template DNA, and the buffer supplied with the enzyme. DGGE was performed using Bio-Rad Dcode universal mutation detection system. PCR product was applied directly onto 1-mm thick 8% (w/v) polyacrylamide (37.5:1 acrylamide–bisacrylamide) gel with a linear denaturing gradient of 20% to 60% [100% denaturant is defined as 7 M urea and 40% (v/v) formamide]. The gel was electrophoresed in 1x TAE buffer at 60°C and 150 V for 5.5 h. Gels were stained in 1x TAE buffer containing 5 μg ml−1 ethidium bromide and photographed under UV light by using a CCD camera (Olympus Camedia 4000). A mixture of 3 μl 16S rDNA amplicons of each strain was used as a marker for the identification of DNA band corresponding to individual strains on DGGE gel as indicated in the figures.
Association kinetics and inhibition experiments
The kinetics of association and effects of co-cyanobacterial strains on the growth of other strains used in competition experiment were done as described by Nilsson et al. (2002, 2005).
16S rDNA sequencing and phylogenetic analysis
16S rDNA fragment of cyanobacterial strains amplified partially (approximately 700 bp) with primers CYA106F and CYA781R (a + b) (Nübel et al. 1997) was gel purified by using Geneipure gel extraction kit in accordance with the manufacturer’s instructions. The purified amplicons were sequenced with the cycle sequencing method using BigDye terminator v3.1 cycle sequencing kit supplied by Applied Biosystems using ABI 3130 genetic analyzer (Applied Biosystems).
The sequences thus obtained were manually corrected by comparing forward and reverse sequences. The length of the corrected sequences varied between 449 and 597 bp. For phylogenetic analysis, each sequence was subjected to a BLAST analysis. The sequences with the highest similarities indicated by BLAST were selected. The phylogenetic tree of the isolates and their nearest neighbors were derived from UPGMA analysis using MEGA 4 software (Tamura et al. 2007).
Results
Growth
The growth of cyanobacterial isolates was determined as increase in chlorophyll a content (Table 2) and phycobilisome contents 6 days after inoculation. Wide variations in the chlorophyll a concentration of cyanobacterial isolates was observed. The increase in chlorophyll a content ranged from 0.39 ± 0.06 to 3.47 ± 0.08 μg ml−1, 0.99 ± 0.04 to 4.34 ± 0.28 μg ml−1, and 0.19 ± 0.07 to 1.22 ± 0.03 μg ml−1 in N2 medium, NO −3 medium, and NH +4 medium, respectively. Strain RN7 showed highest growth in N2 medium and NH +4 medium, whereas strain RCY1 showed maximum growth in NO −3 medium. Strain RL3 showed least growth in N2 medium and NH +4 medium, whereas strain RC3 grew least in NO −3 medium. Similar pattern was observed with regards to changes in phycobiliprotein contents (data not shown).
DNA fingerprint profile
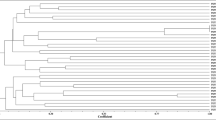
In order to determine the genetic diversity of the cyanobacterial isolates and to establish their molecular identity, a STRR1A-based fingerprint profile of all 21 strains was generated (Fig. 1). Each strain produced a unique fingerprint except strain numbers RN1 and RN17. The latter two strains produced identical fingerprints although they were isolated from two different rice field sites. Maximum size of the DNA amplicon was found to be approximately 3,500 bp for Cylindrospermum strains RCY1.
Co-cultivation of cyanobacterial strains and rice plants
Twenty-one strains were selected for colonization study as they form visible blue-green colonies on the root surface. The quantification of colonization of rice roots by the selected cyanobacterial strains was performed in the presence and absence of combined N under light as well as under darkness. The results of colonization are shown in Table 3. The degree of colonization by each strain varied. The average colonization capacity of Nostoc strains (556 μg chl a g−1 root dry wt) was lower than the average colonization capacity of Anabaena strains (761 μg chl a g−1 root dry wt) in N2 medium under light. Similarly, the average values under darkness in N2 medium and NO −3 medium were found to be 311 and 451 μg chl a g−1 root dry wt, respectively, for Nostoc strains and 533 and 548 μg chl a g−1 root dry wt, respectively, for Anabaena strains. However, in NO −3 -supplemented media, the average colonization ability was higher for Nostoc strains (946 μg chl a g−1 root dry wt) compared to Anabaena strains (765 μg chl a g−1 root dry wt) under light. The overall intensity of colonization ranged from 224 ± 30 to 1,642 ± 102 μg chl a g−1 root dry wt and 261 ± 84 to 1,595 ± 202 μg chl a g−1 root dry wt under light in N2 medium and NO −3 medium, respectively. Corresponding values under darkness ranged from 139 ± 70 to 1,234 ± 110 μg chl a g−1 root dry wt and 187 ± 43 to 1,424 ± 166 μg chl a g−1 root dry wt in N2 medium and NO −3 medium, respectively.
Although the degree of colonization exhibited wide variation, colonization was higher under light and under NO −3 -supplemented medium. Fourteen of the 21 strains exhibited higher level of colonization in the NO −3 medium under light, whereas the rest of the strains showed higher level of colonization in N2 medium under light. Maximum association was observed in the case of strain RA32 that showed consistently high levels of root colonization under all conditions.
Competition
The competitive colonization ability of randomly selected individual cyanobacterial strains in mixed population was investigated using 16S rDNA-based DGGE fingerprint profile. A mixture prepared by mixing PCR amplicons from individual strains was used as a marker for establishing identity of individual strains in DGGE gel. During competition assay involving three Nostoc strains (RN1, RN7, and RN16), strain RN16 was the most competent in its ability to colonize rice roots (Fig. 2). As seen in Fig. 2, DNA bands specific only for RN16 were detected in the DGGE profile of rice root-associated cyanobacteria, although strains RN1 and RN7 were included along with the RN16 in the colonization test. When Nostoc strain RN16 was excluded from the cyanobacterial mixture, the second most successful strain was Nostoc strain RN1. Among Anabaena strains (Fig. 3), strain RA36 was on root surface in all the combinations used. On the other hand, association of strain RA42 was always suppressed by the presence of other Anabaena strains in the mixed population. Strain RA25 inhibited the association of strain RA32. However, strains RA25 and RA32 both colonized rice root successfully when strain RA42 was included in the mixed inocula. The results obtained show the capacity of more than one strain to colonize rice roots simultaneously. The competitive ability of four Anabaena strains was in the order of RA36>RA25>RA32>RA42.
DGGE-based profiling of Nostoc genotypes using 16S rDNA amplicons generated with PCR primer pair CYA106FGC (5′-CGCCCGCCGCGCCCCGCGCCGGTCCCGCCGCCCCCGCCCGCGGACGGGTGAGTAACG CGTGA-3′) and CYA781R (CYA781Ra 5′-GACTACTGGGGTATCTAATCCCATT-3′ and CYA781Rb 5′-GACT ACAGGGGTATCTAATCCCTTT-3′) over a denaturing gradient of 20% to 60%. a 16S rDNA profile of individual Nostoc strains RN7, RN16, and RN1; b 16S rDNA profile-based identification of Nostoc genotypes exposed to rice roots. Captions on top of lanes represent cyanobacterial mix used in competition experiments. Lane “M” represents mixture of 16S rDNA amplicons
DGGE-based profiling of Anabaena genotypes using 16S rDNA amplicons generated with PCR primer CYA106FGC (5′-CGCCCGCCGCGCCCCGCGCCGGTCCCGCCGCCCCCGCCCGCGGACGGGTGAGTAACG CGTGA-3′) and CYA781R (CYA781Ra 5′-GACTACTGGGGTATCTAATCCCATT-3′ and CYA781Rb 5′-GACT ACAGGGGTATCTAATCCCTTT-3′) over a denaturing gradient of 20% to 60%. a 16S rDNA profile of individual Anabaena strains RA25, RA36, RA42, and RA32; b 16S rDNA profile-based identification of Anabaena genotypes exposed to rice roots. Captions on top of lanes represent cyanobacterial mix used in competition experiments. Lane “M” represents mixture of 16S rDNA amplicons
Kinetics of cyanobacterial association
The association kinetics of randomly selected strains used in competition experiment was investigated (Table 4). The result revealed a biphasic nature of association with a fast first phase extending for a period of about 60 to 120 min and a slower second phase. Strain RA25 exhibited maximum association capacity among all the seven strains and RN16 showed maximum association capacity among Nostoc strains. Success of cyanobacterial association with host rice plant was confirmed by planting rice seeding in perlite. The persistence of cyanobacterial strains on root surfaces after 3 weeks confirmed the success of this inoculation method.
Inhibition
The influence of each of the Anabaena strains RA25, RA32, RA36, and RA42 on the growth of other three Anabaena strains and similar experiments using Nostoc strains RN1, RN7, and RN16 revealed no growth inhibition of cyanobacterial strains by another strain (data not shown).
Molecular affiliation of 16S rRNA genes
Evolutionary relationship of Nostoc and Anabaena strains characterized for their rice root colonization competence was deduced by generating UPGMA-based phylogenetic tree (Fig. 4). The strains were distributed in four clusters. The sequence of strain RN16 grouped with Anabaena strains in cluster I showed 99% similarity with Nostoc PCC 7120 (sequence accession number AY768390.1). Similarly, Anabaena strains RA32 in cluster IV showed 98% similarity with Anabaena flos-aquae UTEX LB2391 (sequence accession number DQ234824.1). The 16S rRNA gene sequence from strains RA36 and RA25 showed 99% and 97% similarity with A. flos-aquae UTCC 64 (sequence accession number FJ830582.1), respectively. Strain RN1 showed 96% similarity with Nostoc muscorum (sequence accession number GQ451425.1).
Evolutionary relationships of Nostoc and Anabaena strains used in the rice root colonization experiment inferred by using UPGMA method conducted in MEGA4. The optimal tree with the sum of branch length = 0.25293396 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated from the dataset (complete deletion option). There were a total of 447 positions in the final dataset
Discussion
Traditional use of cyanobacteria as a biofertilizer involves the inoculation of N2-fixing cyanobacteria in rice fields where N released after death/decay of cyanobacterial biomass is taken up by rice plants. A more efficient biotrophic transfer of N from N2-fixing cyanobionts occurs in natural symbioses (Rai 1990; Rai et al. 2000). Several recent studies therefore have focused on selection and use of N2-fixing cyanobacteria that can associate and colonize roots of crop plants (Gantar et al. 1991a, b; Nilsson et al. 2002, 2005; Whitton and Potts 2000).
In an effort to increase the contribution of biologically fixed N as an economical and environmentally friendly N source for rice plant, the artificial association capacity of free-living rice field cyanobacterial isolates to associate individually or in mixed inoculation with rice plant was checked. The symbiotic association of cyanobacterial species with various host plants is well documented and involves mainly species of Nostoc and Anabaena (Rai 1990). A number of symbiotic cyanobacteria isolated from different geographic locations have been characterized in terms of their rice root association kinetics and association capacity (Nilsson et al. 2002). Strategies for selection of promising cyanobacterial biofertilizer strains must involve identification of native isolates that can successfully colonize and associate tightly with crop plants in addition to showing multiple herbicide resistance and salinity tolerance. Our study have characterized rice root colonization ability of rice field cyanobacterial isolates (Table 1) by monitoring their colonization of rice roots, when exposed to mixed strain population (Figs. 2 and 3). Attempts towards developing cyanobacterial biofertilizer technology have shown moderate success rates. One of reasons for this has been the loss of the ability of laboratory-constructed biofertilizer strains to compete with native flora. The problem can be overcome by using native rice field cyanobacterial isolates. In the present investigation, out of 45 strains tested, 21 strains showed appreciable and tight association with rice plants (Table 3) which underscores the necessity for screening cyanobacterial strains with capacity to colonize rice plants. STRR1A-based PCR fingerprinting revealed a high level of diversity among the cyanobacterial isolates (Fig. 1). Nitrogen fertilizers are used routinely for enhancing crop productivity that can influence the pattern of cyanobacterial growth. Therefore, the growth of rice root colonizing cyanobacterial strains was checked and found to be least in NH +4 medium (Table 2). This could be due to high intracellular NH +4 concentration as a result of inhibition of glutamine synthetase activity (García-Domínguez et al. 2000). The association ability was higher under light and in NO −3 -supplemented media, and this could be a result of decreased growth rate in N2 medium and under darkness. However, strain RA25 showed high level of colonization under darkness. Appreciable colonization exhibited by cyanobacterial isolates in darkness reflects their potential to associate with rice roots in field condition. The ability of associated cyanobacteria to fix N2 in the presence of NO −3 –N highlights their biofertilizer potential (Nilsson et al. 2002). In addition to meeting N requirement (Ghosh and Saha 1997; Rai et al. 2000; Whitton and Potts 2000), cyanobacteria can also facilitate the availability of immobilized forms of nutrients to crop plants after mineralization (Pilar et al. 2007). The competition among strains exhibiting different degree of rice root colonization was carried out in order to determine the influence of individual strains on the association ability of other strains in mixed population. The capacity of the individual cyanobacterial strain to associate with rice plants in a one to one co-culture seems to depend on the growth attributes with faster growing strains showing better association than the slower growing strain. However, colonization behavior of individual strains changed in mixed cyanobacterial populations as shown by the association experiment performed using three Nostoc strains or four Anabaena strains (Figs. 2 and 3). It may be a case of metabolic superiority of the strains making them more efficient to associate with the host rice plants and creating unfavorable condition for the other strains. A short-term exposure of rice seedling to cyanobacterial suspension appears to be a promising method of introducing competent cyanobacterial strains on rice roots as shown by the association kinetics experiment. All the seven strains analyzed for their rice root colonization potential grouped into four distinct phylogenetic clusters. Interestingly, strains RN16 and RA36 falling in the same cluster were the most competent strains to colonize rice root in mixed inocula (Fig. 4).
The cyanobacterial strains belonging to the genera Nostoc and Anabaena can comprise 80% of the rhizosphere isolates (Prasanna et al. 2009). The predominance of genera Nostoc and Anabaena irrespective of chemical fertilizer treatments and stages of crop growth in rice field indicates that strains of these two genera have adapted well to rice field conditions either as floating assemblages or in the rhizosphere. Nostoc is known to be one of the most versatile diazotrophic cyanobacterial genera observed in free-living state and in symbiotic association with fungi, bryophytes, gymnosperms, and angiosperms (Prasanna et al. 2009; Rai 1990). Soil-dwelling cyanobacterial isolates belonging to the genera Nostoc, Anabaena, and Cylindrospermum have been reported to associate with wheat seedlings (Gantar et al. 1991a, b). In such associations, cyanobacterial strains were found to fix nitrogen in the presence of NO −3 that supports the growth of wheat seedling (Gantar et al. 1991b). Competent Nostoc strains are known to form both loose and tight association as compared to Anabaena, which appears to form only loose association. Tightly associated Nostoc strains can be present on the root surface as well as in the intracellular spaces and in the cells of cortical parenchyma (Gantar et al. 1991b). Non-heterocystous, non-symbiotic cyanobacteria have also been described to colonize the rhizosphere, infect epidermal cells, and multiply intracellularly (Mehboob et al. 2010). The nitrogen fixation activity in rhizosphere of rice roots has been found to be higher than that of the activity observed in surface soil or floodwater (Watanabe 1978). Cyanobacteria growing in such associations show increased level of nitrogenase activity and transfer of fixed nitrogen to the host plants (Ghosh and Saha 1997; Rai et al. 2000; Whitton and Potts 2000). Thus, creation of effective association between nitrogen-fixing cyanobacteria and agronomically important crop plant would enhance the biofertilizer N transfer efficiency to the host plants. The N transfer activity has been observed to be ranging from 5% to 12% depending on the growth stages of the rice plants (Pilar et al. 2007). Cyanobacterial populations, in addition to meeting N requirements of crop plants, also contribute to the soil quality through improved nutrient transformation and plant growth-promoting effects (Rao et al. 1998). Apart from their desired biofertilizer role, cyanobacteria can enhance oxygen availability to submerged rhizosphere, ameliorate salinity, and contribute to pH homeostasis and phosphate solubilization (Kaushik 2004; Mandal et al. 1998).
Our results reflect that rice root association capacity of different cyanobacterial strains differs and get influenced by strains in the mixed inocula. This is evident from the fact that among three Nostoc strains in mixed population, strain RN16 with intermediate colonization ability (Table 3 and Fig. 2) was the most competent rice root-colonizing strain. Similarly, among four Anabaena strains, RA36 with intermediate growth rate and colonization ability exhibited maximum rice root colonization ability (Tables 2 and 3 and Fig. 3). Our results suggest that individual strains with better colonization and growth ability in the laboratory may not necessarily be the most effective and competent strains for rice root colonization in natural environmental conditions.
References
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in filamentous blue green algae. J Cell Biol 58:419–435
Bouldin DR (1986) The chemistry and biology of flooded soils in relation to the nitrogen economy in rice fields. In: De Datta SK, Patrick WH Jr (eds) Nitrogen economy of flooded rice soils. Martinus Nijh off, The Netherlands, pp 1–14
Chingkheihunba A, Singh AK (2011) Molecular typing and distribution of filamentous cyanobacteria isolated from two distinctly located regions of North-Eastern India. World J Microbiol Biotechnol 27:2187–2194
Desikachary TV (1959) Cyanophyta. Indian Council of Agricultural Research, New Delhi
Gantar M, Kerby NW, Rowell P, Obreht Z (1991a) Colonization of wheat (Triticum vulgare L.) by N2-fixing cyanobacteria: I. A survey of soil cyanobacterial isolates forming associations with roots. New Phytol 118:477–483
Gantar M, Kerby NW, Rowell P (1991b) Colonization of wheat (Triticum vulgare L.) by N2-fixing cyanobacteria: II. An ultrastructural study. New Phytol 118:485–492
García-Domínguez M, Reyes JC, Florencio FJ (2000) NtcA represses transcription of gifA and gifB, genes that encode inhibitors of glutamine synthetase type I from Synechocystis sp. PCC 6803. Mol Microbiol 35:1192–1201
Ghosh TK, Saha KC (1997) Effects of inoculation with N2-fixing cyanobacteria on the nitrogenase activity in soils and rhizosphere of wetland rice (Oryza sativa L.). Biol Fertil Soils 16:16–20
Kaushik BD (2004) Use of blue-green algae and Azolla biofertilizers in rice cultivation and their influence on soil properties. In: Jain PC (ed) Microbiology and biotechnology for sustainable development. CBS, New Delhi, pp 166–184
Korzhenevskaya TG, Lobakova ES, Dol’nikova GA, Gusev MV (1999) Topography of microsymbionts in apogeotropic roots of the cycads Cycas revolute Thunb. and Encephalartoshorridus (Jacq.) Lehm. Microbiology 68:437–442
Kundu DK, Ladha JK (1995) Efficient management of soil and biologically fixed nitrogen in intensively cultivated rice fields. Soil Biol Biochem 27:431–439
Ladha JK, Tirol-Padre A, Reddy CK, Ventura W (1993) Prospects and problems of biological nitrogen fixation in rice production: a critical assessment. In: Palacios R, Mora J, Newton WE (eds) New horizons in nitrogen fixation. Kluwer Academic, The Netherlands, pp 677–682
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Mandal B, Vlek PLG, Mandal LN (1998) Beneficial effect of blue-green algae and Azolla excluding supplying nitrogen, on wetland rice fields: a review. Biol Fertil Soils 27:329–342
Mehboob A, Lucas JS, Shahida H (2010) Association of non-heterocystous cyanobacteria with crop plants. Plant Soil 336:363–375
Nilsson M, Rasmussen U, Bergman B (2005) Competition among symbiotic cyanobacterial Nostoc strainsforming artificial associations with rice (Oryza sativa). FEMS Microbiol Lett 245:139–144
Nilsson M, Bhattacharya J, Rai AN, Bergman B (2002) Colonization of roots of rice (Oryza sativa) by symbiotic Nostoc strains. New Phytol 156:517–525
Nübel U, Garcia-Pichel F, Muyzer G (1997) PCR primers toamplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol 63:3327–3332
Pilar I, Susana G, Enrique D, Jorge M (2007) Cyanobacterial inoculation and nitrogen fertilization in rice. World J Microbiol Biotechnol 23:237–242
Prasanna R, Jaiswal P, Nayak S, Sood A, Kaushik BD (2009) Cyanobacterial diversity in the rhizosphere of rice and its ecological significance. Ind J Microb 49:89–97
Rai AN (1990) Handbook of symbiotic cyanobacteria. CRC Press, Boca Raton
Rai AN, Söderbäck E, Bergman B (2000) Tansley review: cyanobacterium–plant symbioses. New Phytol 147:449–481
Rao VR, Ramakrishnan B, Adhya TK, Kanungo PK, Nayak DN (1998) Review: current status and future prospects of associative nitrogen fixation in rice. World J Microbiol Biotechnol 14:621–633
Rasmussen U, Svenning MM (1998) Fingerprinting of cyanobacteria based on PCR with primers derived from short and long tandemly repeated repetitive sequences. Appl Environ Microbiol 64:265–272
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Genetic assignments, strain histories andproperties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Roger PA, Watanabe I (1986) Technologies for utilizing biological nitrogen fixation in wetland rice: potentialities current usage and limiting factors. Fertil Res 9:39–77
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Watanabe I, Roger PA (1984) Nitrogen fixation in wetland rice fields. In: Subba Rao NS (ed) Current developments in biological nitrogen fixation. Oxford–IBM, New Delhi, pp 237–276
Watanabe I (1978) Biological nitrogen fixation in rice soils. In: Soils and rice. International Rice Research Institute, Los Banos, pp 465–478
Whitton BA, Potts M (2000) The ecology of cyanobacteria. Kluwer, Dordrecht
Acknowledgments
Financial assistance from DST and UPE is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPTX 547 kb)
Rights and permissions
About this article
Cite this article
Akoijam, C., Singh, A.K. & Rai, A.N. Characterization of free-living cyanobacterial strains and their competence to colonize rice roots. Biol Fertil Soils 48, 679–687 (2012). https://doi.org/10.1007/s00374-012-0664-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-012-0664-7