Abstract
A study was undertaken to investigate the bacterial community found in metallophytic grassland soil contaminated with Zn and Pb. We hypothesised that such communities would be tolerant of additional heavy metal stress due to phylogenetic and functional adaptation. In microcosm experiments, lasting 51 days, denaturing gradient gel electrophoresis (DGGE) analyses was used to compare the total bacterial and actinobacterial communities in non-amended soils and those to which additional Pb and Zn concentrations were added. There was a decrease in total bacterial diversity with each addition of Pb and Zn; in contrast, the actinobacterial community diversity remained unaffected. The community structures were analysed using multivariate analyses of the DGGE profiles. Total bacterial community profiles showed two distinct groups sharing less than 80% similarity, irrespective of Pb and Zn addition. The first contained profiles sampled during the first 7 days of the experiment; the second contained those sampled from day 10 onwards. Actinobacterial profiles from those that were non-amended showed a similar distribution to those of the total bacterial community. However, in soil amended with fivefold additional Pb and Zn, all the profiles shared more than 80% similarity. Raup and Crick analyses suggested that total bacterial soil communities were subject deterministic selection becoming significantly similar as the experiment progressed, but this was inhibited by the highest concentration of additional Pb and Zn. Actinobacterial communities showed a similar response but were less affected by elevated Pb and Zn concentrations. These data indicate that the diversity of the actinobacterial community was not negatively affected by additional heavy metal stress in contrast to total bacterial community diversity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The metallophytic grassland habitats of the northern Pennines were formed as a result of a short period mining activity between the mid-eighteenth and mid-twentieth centuries. They comprise of spoil heaps from abandoned mine workings and alluvial deposits on the flood plains of the local surface waters that receive water from abandoned mine workings. They have high levels of heavy metal contamination, particularly Pb and Zn, and represent a unique habitat, containing a highly diverse flora.
A number of studies have shown that the presence of heavy metals in soils have had a negative impact on the diversity of the microbial community in soil (Joynt et al. 2006; Li et al. 2006) and have also led over time to the adaptation of a community more able to tolerate their presence (Schmidt et al. 2005; Frey et al. 2006). Indeed, some estimations suggest that heavy metal pollution can reduce the diversity of bacterial populations by 99.9% (Gans et al. 2005) affecting rarer taxa, whose activity may be functionally significant, most especially. Two theories have been postulated to explain how heavy metals affect microbial community diversity, the first suggests that stressed systems are more stable than those which are not, as they have acquired physiological adaptations that enable them to maintain function the face of additional stresses (Odum 1981). The second states that non-stressed systems have a greater stability, as they have more resources at their disposal that can be deployed to maintain ecosystem function in the face of stress (Tobor-Kaplan et al. 2005). These hypotheses were tested by Griffiths et al. (2001) using grassland, agricultural and industrial soil and Kuan et al. (2006) with grassland soils subject to various disturbances (biocide, sewage sludge application). Both studies challenged the soils with heat and Cu stress and measured the effects on soil functional stability. In each study, the less stressed soils had greater stability supporting the hypothesis that these soils have more resistance to stress that those previously exposed to environmental challenge.
The significance of Actinobacteria within the microbial communities of soils contaminated with heavy metals is unresolved; Gremion et al. (2003), studying the bacterial community of a soil contaminated with Cd, Cu and Zn, showed that Actinobacteria, dominated clone libraries derived from both the bulk soil and the rhizosphere of Thlaspi caerulescens, a heavy metal hyperaccumulator. Moreover, 70% of the actinobacterial clones belonged to the genus Rubrobacter, a widespread taxa in soils, that has yet few cultured representatives (Holmes et al. 2000). In contrast, other studies on the impact of heavy metal contamination on bacterial community structure have reported that there was a significant decline in the contribution of Actinobacteria to the bacterial community. In a forest soil contaminated with Cd, Cu, Zn and Pb, there was a significant decrease in the actinobacterial marker using a phospholipid fatty acid (PFLA) protocol compared to control soils (Frey et al. 2006). The microbial community also showed more general changes in response to heavy metal amendment, one that was more tolerant of heavy metals but with a compromised functional activity.
In this study, we wished to test the hypothesis that microbial diversity and structure would be resistant to additional heavy metal input in to the soil due to adaptation and functional changes within the community predisposing it to cope with the increased stress (Odum 1981). Although previous studies have identified that short-term addition of heavy metals do not model long-term effects on microbial activity (Renella et al. 2002), our aim was to explore the effects of sudden acute exposure on communities subjected to long-term heavy metal concentrations in order to understand how bacterial and actinobacterial communities responded.
Materials and methods
Field sites and sampling strategy
The site at Bardon Mill, grid reference 779 644 (OS Landranger 87) was a former spoil heap. The site was predominantly bare earth, but there were patches of sparse vegetation, comprised of grass (Festuca ovina). Soil was collected from non-vegetated areas; triplicate samples (1 kg) were excavated from the top 10 cm of the soil.
Immediately after collection, soil was transported to the laboratory and stored at 4°C until required. Prior to being used in microcosms, soil was sieved (2 mm) and air-dried overnight (Baxter et al. 2006).
Analyses of soil samples
Total metal content was determined in duplicate. Samples (0.5 g) were air-dried and digested in 10 ml of boiling aqua regia (HNO3/HCl, 1:3, v/v).Once cool, solutions were filtered (Whatman no. 1 filter paper) to remove any insoluble sediment or organic material. The solution was then made up to 100 ml with distilled water. Bioavailable concentrations of Pb and Zn were determined using ethylenediaminetetraacetic acid (EDTA) and acetic acid extraction (Quevauviller 1998). Samples were analysed for total metal content using a flame atomic absorption spectroscope (Perkin Elmer/AAnalyst 100), with “Calib” software. Samples were calibrated against Pb and Zn solutions. Bioavailable metal analyses were performed using inductively coupled plasma mass spectrometry (ICP mass spectrometer XSeries II Thermo Electron) according to the protocol of Intawongse and Dean (2008).
Carbon and nitrogen analyses were done with duplicate samples of soil (1 g) using a Carlo Erba 1108 Elemental Analyser controlled with CE Eager 200 software, run in accordance with the manufacturer’s instructions and weighed using a Mettler MT 5 Microbalance by the Advanced Chemical and Material Analysis, University of Newcastle-upon-Tyne, Newcastle, UK.
Soil microcosms
For each treatment, three microcosms, consisting of 500 g of soil in each replicate, were established in 600-ml glass beakers. The soil was loamy sand (84.2% sand, 10.5% silt and 5.3% clay) of pH 6.0. Elemental analyses showed that the soil contained 0.57% organic carbon and 0.12% nitrogen. The concentration of extractable Pb and Zn in Bardon Mill soil was 3 g kg−1 and 1 g kg−1, respectively. In experimental microcosms, these levels were supplemented with PbNO3 and Zn ZnSO4 to give a twofold and fivefold increase over those concentrations. A carrier system was used to ensure consistent metal application. Appropriate concentrations of PbNO3 and ZnSO4 dissolved in dH2O were applied to 1-g aliquots of sterile soil; these were incubated overnight to allow evaporation of the water. The aliquots were cut into the rewetted soil by thorough mixing. The bioavailable concentrations of Pb and Zn were then determined. In the non-supplemented soil, these were found to be 60 ± 3 and 57 ± 2 mg kg−1 of Pb and Zn, respectively. After the two- and fivefold supplementation with PbNO3 and ZnSO4, the bioavailable concentrations were 147 ± 20 and 369 ± 24 mg kg−1 for Pb and 110 ± 9 and 203 ± 15 mg kg−1 for Zn, respectively.
Soil water content was measured and found to be constant during the experiment at 20% of the water-holding capacity of the soil. Initial samples (day 0) were taken immediately after the microcosms were set up. Between samplings microcosms were incubated at 18°C in a water-saturated atmosphere (Rasmussen and Sorensen 2001). Samples of soil (1 g) were collected immediately after setting up the microcosms (day 0) and then on days 2, 7, 10, 16, 23, 30, 37, 44, and 51.
DNA extraction and PCR amplification of 16S rRNA genes from soil microcosms
DNA was extracted from 1 g of soil with an UltraClean Soil DNA kit (MoBio) and stored at −20°C. Total bacterial diversity was monitored by polymerase chain reaction amplification of V3 region of the bacterial 16S rRNA gene performed using primers 1and 2 as described by Muyzer et al. (1993). Selective amplification of Actinobacteria were performed using Primer F243 (5′-GGATGAGCCCGCGGCCTA-3′) and primer R513 (5′-CGGCCGCGGCTGCTGGCACGTA-3′) (Heuer et al. 1997).Amplification of DNA and the thermocycling conditions used were as described by Baxter and Cummings (2006). All reactions were carried out using the Eppendorf Mastercycler Gradient PCR machine. PCR products were analysed by agarose (1.5% w/v) gel electrophoresis and ethidium bromide staining.
Denaturing gradient gel electrophoresis
Denaturing gradient gel electrophoresis (DGGE) analysis was performed using the D-Code DGGE system (Bio-Rad) as described by Baxter and Cummings (2006). For analysis of bacterial populations, PCR products were loaded on to polyacrylamide gels (12%) with a denaturant gradient ranging from 35% to 55% denaturant (with 100% denaturant corresponding to 7 M urea plus 40% v/v formamide). Gels were run at 60°C for 10.5 h at 80 V. Gels were stained in SYBR Green I (1:10,000 dilution in 1× Tris-acetate-EDTA buffer; Molecular Probes) for 30 min and viewed with UV transillumination and photographed using the Gel Doc 2000 gel documentation system (Bio-Rad).
Statistical analysis of DGGE profiles
For each treatment, PCR products, derived from DNA extracted from three independently derived communities, were analysed by DGGE. The patterns obtained from these replicates were highly reproducible. To facilitate sample processing, PCR products were pooled for each treatment. The pooled PCR products were then subjected to triplicate DGGE analyses to confirm that the band patterns were reproducible with those derived from the independently derived community replicates (Sigler and Turco 2002; Brodie et al. 2003).
DGGE profiles were analysed using Quantity One 4.1.1 software (Bio-Rad). The lane background was subtracted by using a rolling disc algorithm, and the lanes were ‘normalised’ to ensure that all lanes contained the same amount of total signal. Lane profiles were corrected for differences in migration rate by manually assigning R f lines to marker lane bands (Griffiths et al. 2003). To facilitate the efficient handling of samples and data, each sample from each replicate was screened with PCR-DGGE to assess replicate variability in DGGE profiles. When the reproducibility of PCR profiles within a treatment had been ensured, all PCR products derived from a common treatment were then pooled for the final analysis in order to accommodate the electrophoresis of samples on a single gel.
Band patterns were analysed using three methods. Correspondence analysis (CA) was performed to visualise the relationships among band patterns using Statistica 6.0 software (StatSoft). Cluster analyses of band patterns were also performed with the unweighted pair group method using averages based on the Sorensen index of similarity. Band pattern data were analysed using Raup and Crick’s index of similarity The Raup and Crick index of similarity was used to test if similarities observed between soil cultures profile patterns were significantly higher or lower than would be observed by chance (Rowan et al. 2003). Raup and Crick and cluster analyses were calculated using the PAST statistical analysis software. Student’s t test was used for statistical difference between means (P < 0.05)
Results and discussion
DGGE analyses of microcosm soil
Visual comparison of the total bacterial DGGE profiles and Shannon–Weaver diversity index (H′) calculated for each treatment indicated that, in non-amended microcosms soil, there were 70 individual bands (H′ = 3.8 ± 0.06), significantly more (P < 0.05) than the 46 (H′ = 3.3 ± 0.1) and 30 (H′ = 2.8 ± 0.13) bands in soils amended with two- and fivefold additional Pb and Zn, respectively. The actinobacterial profiles showed no significant difference with 36 bands in non-amended soils (H′ = 2.9 ± 0.16) and 33 bands (H′ = 3.0 ± 0.06) in soils amended with fivefold more Pb and Zn. However, straightforward measures of diversity do not resolve community structure and diversity, due to primer bias, resulting in preferential amplification of more abundant sequences and multiple taxa being represented by a single band or a single taxa by several bands (Duarte et al. 2001; Joynt et al 2006). Therefore, the use of multivariate analysis provides a more sensitive means of evaluating the overall changes in microbial community profiles, enabling a higher resolution by exploring both pattern and intensity of bands within profiles (Ogino et al. 2001).
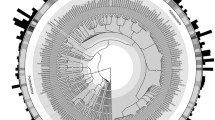
Cluster analyses were combined with ordination using CA to aid in visualising the relationship between different community profiles generated by DGGE analyses (Ramette 2007). The cluster analysis of the total bacterial community in non-amended soil demonstrated that samples taken between days 0 and 16 and those derived from the later sampling dates (day 23 onwards) formed two clusters (Fig. 1a), and indicated that the similarity between the two clusters was less than 80%. Correspondence analysis indicated that 46.2% of the variance in the total bacterial community patterns could be explained by the first two dimensions (Fig. 1a). The first axis that explained 27.8% of the inertia separated the earlier from the later profiles and reflected the groupings observed in the cluster analysis (Fig. 1b). In microcosms amended with an extra two- or fivefold concentration of Pb and Zn, CA showed that there was again a differentiation of the total bacterial community profiles into those derived from samples from days 0, 2 to 7 and those from the later time points (Fig. 1b,c). Cluster analyses indicated that the differentiation of sample profiles from the early sample points (days 0, 2 and 7) compared to the later samples was robust in soils amended with both two- and fivefold additional heavy metal and that the profiles from the these early samplings shared less than 75% similarity with the later profiles (Fig. 1b,c).
Cluster and ordination analyses of bacterial and actinobacterial DGGE community profiles identifying the relationships between the band patterns. Correspondence analyses of the total bacterial community profiles of microcosm soils subjected to a no amendment or with b twofold c fivefold additional extractable Pb and Zn. Similarly, d actinobacterial community profiles for soils subjected to no additional Pb and Zn or with e fivefold additional Pb and Zn added. Dashed lines enclose those profiles that share >80% similarity in using cluster analyses with Sorensen coefficient. Numbers identify the sample day
The actinobacterial profiles derived from microcosms without supplementation and with an additional fivefold Pb and Zn were also analysed. In non-amended microcosm soil, three distinct clusters were seen in the CA; the profiles derived from days 0 to 2 were separated from the other profiles along the first axis that explained 40.3% of the inertia. The profiles from days 23 and 30, were then separated from the remaining patterns (days 7, 10, 16, 34–51) along the second axis (Fig. 1d), explaining 16.6% of the variation. Analyses using the Sorensen coefficient demonstrated that the similarity between the three clusters was less than 80% (Fig. 1d). In contrast to the total bacterial community profiles, it appeared that adding fivefold more Pb and Zn to the soil increased the similarity between profiles. Although CA indicated that the initial two samples, days 0 and 2, were separated from the remaining community profiles (Fig. 1e), cluster analyses demonstrated that all the profiles shared a greater than 80% similarity, and indeed, all but the initial day 0 sample had population patterns with similarities of 88% or more (Fig. 1e).
The use of the Raup and Crick (S RC) index to quantify the relationships between the total bacterial community profiles indicated that, from day 23 in non-amended soils and day 16 in soils of twofold additional Pb and Zn, profiles showed a similarity to each other, greater than could be explained by the chance matching of bands. This suggests that deterministic selection was acting on the community (Table 1, non-amended soil and soil amended with a twofold increase in Pb concentration; Rowan et al. 2003). However, the trend towards significantly similar population profiles was much less pronounced in microcosms amended with an additional fivefold Pb and Zn, occurring only from day 37 onwards (Table 1, soil amended with a fivefold increase in Zn concentration). The Raup and Crick analyses showed that actinobacterial communities under both treatments showed a drift towards significantly similar community profiles, such that the comparison of profiles from day 10 onwards under both treatments showed high similarity between the community profiles (Table 2).
The total bacterial community diversity decreased in response to additional heavy metal application in a dose-dependent manner. This response does not support the hypothesis that pre-exposed communities would be more tolerant of additional heavy metal stresses but rather supports the observation by Tobor-Kaplan et al. (2005) that ecosystem stability to heavy metals was not enhanced by long-term exposure to this stress. Other studies have identified negative impacts on bacterial community diversity exposed to elevated heavy metal concentrations that agree with these observations (Sandaa et al. 1999; Joynt et al. 2006; Li et al 2006; Wu et al. 2006). Frey et al. (2006) analysed the impact on community structure of heavy metal contamination and observed a shift in the community structure measured using both PLFA and terminal restriction fragment length polymorphism suggesting that the resulting community had a reduced functional diversity compared to control soils. They speculated that the changes caused by the heavy metal application were to shift to more metal tolerant species. However, in studies of hyporheic (sediment) communities from heavy metal contaminated watercourses, no correlation between microbial diversity and metal concentration was apparent nor that the presence of heavy metals led to a more resistant bacterial community (Feris et al. 2003).
In contrast to the total bacterial community, the actinobacterial diversity was not significantly affected by the addition of fivefold more extractable heavy metal, a response that would appear to support the original hypothesis. There are conflicting data on the specific changes of soil microbial communities subjected to heavy metal stress (Giller et al. 1998). For example, PFLA and DGGE data have indicated that fungi, Proteobacteria and Gram-negative bacteria increase under such conditions, whereas Actinobacteria, rhizobia and Firmicutes decrease (Kelly et al. 1999; Hinojosa et al. 2005; Lorenz et al. 2006; Renella et al. 2007). However, a study of forest soil indicated an increase in Gram-positive bacteria and a decrease in Gram-negative organism (Åkerblom et al. 2008). In contrast, in some heavily contaminated soils, Actinobacteria have been shown to dominate clone libraries, high numbers have also been identified in serpentine soils (Gremion et al. 2003; DeGrood et al. 2005). The observations of the effects of additional Pb and Zn addition to actinobacterial community structure may be an artefact of the analyses; for example, it has be demonstrated that Actinobacteria are dominant with micro-aggregates of soil (Mummey and Stahl 2004), where they may be protected from additional heavy metal addition. However, in metallophytic soils, it seems that Actinobacteria are a diverse taxa that are resistant to exposure to additional Pb and Zn. Whether this reflects a community that has become functionally adapted to this soil environment (Almås et al. 2004; Schmidt et al. 2005) or simply an artefact of their dominance within protected micro-environments requires further study.
The findings that the most common bacterial taxa present in the metallophytic soils are adversely affected by increasing Pb and Zn levels while actinobacterial community diversity is not suggest that studies on the impact of heavy metals in soil need to account for these differences between different functional and phylogenetic groupings in order to draw robust conclusions.
References
Åkerblom S, Bååth E, Bringmark L, Bringmark E (2008) Experimentally induced effects of heavy metal on microbial activity and community structure of forest mor layers. Biol Fertil Soils 44:79–91 doi:10.1007/s00374-007-0181-2
Almås AR, Bakken LR, Mulder J (2004) Changes in tolerance of soil microbial communities in Zn and Cd contaminated soils. Soil Biol Biochem 36:805–813 doi:10.1016/j.soilbio.2004.01.010
Baxter J, Cummings SP (2006) The impact of bioaugmentation on metal cyanide degradation and soil bacteria community structure. Biodegradation 17:207–217 doi:10.1007/s10532-005-4219-6
Baxter J, Garton NJ, Cummings SP (2006) The impact of acrylonitrile and bioaugmentation on the biodegradation activity and bacterial community structure of a topsoil. Folia Microbiol (Praha) 51:591–598
Brodie E, Edwards S, Clipson N (2003) Soil fungal community structure in a temperate upland grassland soil. FEMS Microbiol Ecol 45:105–114 doi:10.1016/S0168-6496(03)00126-0
DeGrood SH, Claassen VP, Scow KM (2005) Microbial community composition on native and drastically disturbed serpentine soils. Soil Biol Biochem 37:1427–1435 doi:10.1016/j.soilbio.2004.12.013
Duarte GF, Rosado AS, Seldin L, de Araujo W, van Elsas JD (2001) Analysis of bacterial community structure in sulphurous-oil-containing soils and detection of species carrying dibenzothiophene desulfurization (dsz) genes. Appl Environ Microbiol 67:1052–1062 doi:10.1128/AEM.67.3.1052-1062.2001
Feris K, Ramsey P, Frazar C, Moore JN, Gannon JE, Holbert WE (2003) Differences in hyporheic-zone microbial community structure along a heavy-metal contamination gradient. Appl Environ Microbiol 69:5563–5573 doi:10.1128/AEM.69.9.5563-5573.2003
Frey B, Stemmer M, Widmer F, Luster JC, Sperisen C (2006) Microbial activity and community structure of a soil after heavy metal contamination in a model forest ecosystem. Soil Biol Biochem 38:1745–1756 doi:10.1016/j.soilbio.2005.11.032
Gans J, Wolinsky M, Dunbar J (2005) Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 309:1387–1390 doi:10.1126/science.1112665
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30:1389–1414 doi:10.1016/S0038-0717(97)00270-8
Gremion F, Chatzinotas A, Harms H (2003) Comparative 16S rDNA and 16S rRNA sequence analysis indicates that Actinobacteria might be a dominant part of the metabolically active bacteria in heavy metal-contaminated bulk and rhizosphere soil. Environ Microbiol 5:896–907 doi:10.1046/j.1462-2920.2003.00484.x
Griffiths BS, Bonkowski M, Roy J, Ritz K (2001) Functional stability, substrate utilisation and biological indicators of soils following environmental impacts. Appl Soil Ecol 16:49–61 doi:10.1016/S0929-1393(00)00081-0
Griffiths RI, Whitely AS, O’Donnell AG, Bailey MJ (2003) Influence of depth and sampling time on bacterial community structure in an upland grassland soil. FEMS Microbiol Ecol 43:35–43 doi:10.1111/j.1574-6941.2003.tb01043.x
Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH (1997) Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 63:3233–3241
Hinojosa MB, Carreira JA, Garcia-Ruiz R, Dick RP (2005) Microbial response to heavy metal-polluted soils: Community analysis from phospholipid-linked fatty acids and ester-linked fatty acids extracts. J Environ Qual 34:1789–1800 doi:10.2134/jeq2004.0470
Holmes AJ, Bowyer J, Holley MP, O’Donoghue M, Montgomery M, Gillings MR (2000) Diverse, yet-to-be-cultured members of the Rubrobacter subdivision of the Actinobacteria are widespread in Australian arid soils. FEMS Microbiol Ecol 33:111–120 doi:10.1111/j.1574-6941.2000.tb00733.x
Intawongse M, Dean JR (2008) Use of the physiologically-based extraction test to assess the oral bioaccessibility of metals in vegetable plants grown in contaminated soil. Environ Poll 152:60–72
Joynt J, Bischoff M, Turco R, Konopka A, Nakatsu CH (2006) Microbial community analysis of soils contaminated with lead, chromium and petroleum hydrocarbons. Microb Ecol 51:209–219 doi:10.3354/ame01202
Kelly JJ, Häggblom MM, Tate RL (1999) Changes in soil microbial communities over time resulting from one time application of zinc: a laboratory microcosm study. Soil Biol Biochem 31:1455–1465 doi:10.1016/S0038-0717(99)00059-0
Kuan HL, Fenwick C, Glover LA, Griffiths BS, Ritz K (2006) Functional resilience of microbial communities from perturbed upland grassland soils to further persistent or transient stresses. Soil Biol Biochem 38:2300–2306 doi:10.1016/j.soilbio.2006.02.013
Li ZJ, Xu JM, Tang CX, Wu JJ, Muhammad A, Wang HZ (2006) Application of 16S rDNA-PCR amplification and DGGE fingerprinting for detection of shift in microbial community diversity in Cu-, Zn-, and Cd-contaminated paddy soils. Chemosph 62:1374–1380 doi:10.1016/j.chemosphere.2005.07.050
Lorenz N, Hintemann T, Kramarewa T, Katayama A, Yasuta T, Marschner P et al (2006) Response of microbial activity and microbial community composition in soils to long-term arsenic and cadmium exposure. Soil Biol Biochem 38:1430–1437 doi:10.1016/j.soilbio.2005.10.020
Mummey DL, Stahl PD (2004) Analysis of soil whole- and inner-microaggregate bacterial communities. Microb Ecol 48:41–50 doi:10.1007/s00248-003-1000-4
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Odum EP (1981) The effects of stress on the trajectory of ecological succession. In: Barrett GW, Rosenburg R (eds) Stress effects on natural ecosystems. Wiley, London, pp 43–47
Ogino A, Koshikawa H, Nakahara T, Uchiyama H (2001) Succession of microbial communities during a biostimulation process as evaluated by DGGE and clone library analyses. J Appl Microbiol 91:625–635 doi:10.1046/j.1365-2672.2001.01424.x
Quevauviller P (1998) Operationally defined extraction procedures for soil and sediment analysis—II. Certified reference materials. Trends Analyt Chem 17:632–642 doi:10.1016/S0165-9936(98)00078-8
Ramette A (2007) Multivariate analyses in microbial ecology. FEMS Microbiol Ecol 62:142–160 doi:10.1111/j.1574-6941.2007.00375.x
Rasmussen DL, Sørensen SJ (2001) Effects of mercury contamination on the culturable heterotrophic, functional and genetic diversity of the bacterial community in soil. FEMS Microbiol Ecol 36:1–9 doi:10.1111/j.1574-6941.2001.tb00820.x
Renella G, Chaudri AM, Brookes PC (2002) Fresh additions of heavy metals do not model long-term effects on microbial biomass and activity. Soil Biol Biochem 34:121–124 doi:10.1016/S0038-0717(01)00150-X
Renella G, Chaudri AM, Falloon CM, Landi L, Nannipieri P, Brookes PC (2007) Effects of Cd, Zn, or both on soil microbial biomass and activity in a clay loam soil. Biol Fertil Soils 43:751–758 doi:10.1007/s00374-006-0159-5
Rowan AK, Snape JR, Fearnside D, Barer MR, Curtis TP, Head IM (2003) Composition and diversity of ammonia-oxidising bacterial communities in wastewater treatment reactors of different design treating identical wastewater. FEMS Microbiol Ecol 43:195–206 doi:10.1111/j.1574-6941.2003.tb01059.x
Sandaa RA, Torsvik V, Enger O, Daae FL, Castberg T, Hahn D (1999) Analysis of bacterial communities in heavy metal-contaminated soils at different levels of resolution. FEMS Microbiol Ecol 30:237–251 doi:10.1111/j.1574-6941.1999.tb00652.x
Schmidt A, Haferburg G, Sineriz M, Merten D, Buchel G, Kothe E (2005) Heavy metal resistance mechanisms in Actinobacteria for survival in AMD contaminated soils. Chem Erde-Geochem 65:131–144 doi:10.1016/j.chemer.2005.06.006
Sigler WV, Turco RF (2002) The impact of chlorothalonil application on soil bacterial and fungal populations as assessed by denaturing gradient gel electrophoresis. Appl Soil Ecol 21:107–118 doi:10.1016/S0929-1393(02)00088-4
Tobor-Kaplon M, Bloem AJ, Romkens P, de Ruiter PC (2005) Functional stability of microbial communities in contaminated soils. Oikos 111:119–129 doi:10.1111/j.0030-1299.2005.13512.x
Wu SC, Luo YM, Cheung KC, Wong MH (2006) Influence of bacteria on Pb and Zn speciation, mobility and bioavailability in soil: a laboratory study. Environ Pollut 144:765–773 doi:10.1016/j.envpol.2006.02.022
Acknowledgements
The authors thank BIONET, the Sustainable Cities Institute, Northumbria University and the Centre for Excellence in Life Sciences, Newcastle-upon-Tyne for financial support for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bamborough, L., Cummings, S.P. The impact of increasing heavy metal stress on the diversity and structure of the bacterial and actinobacterial communities of metallophytic grassland soil. Biol Fertil Soils 45, 273–280 (2009). https://doi.org/10.1007/s00374-008-0323-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-008-0323-1