Abstract
Plant growth-promoting bacteria (PGPB) were reported to influence the growth, yield, and nutrient uptake by an array of mechanisms. We selected seven different plant growth-promoting traits and antagonistic ability to screen 207 bacteria isolated from composts. Fifty-four percent of PGPB were from farm waste compost (FWC), 56% from rice straw compost (RSC), 64% from Gliricidia vermicompost (GVC), and 41% from macrofauna associated with FWC. Twelve isolates based on different plant growth-promoting traits and seed vigor index were evaluated at glasshouse for plant growth-promoting activity on pearl millet. Seven isolates significantly increased shoot length and ten isolates showed significant increase in leaf area, root length density, and plant weight. Maximum increase in plant weight was by Serratia marcescens EB 67 (56%), Pseudomonas sp. CDB 35 (52%), and Bacillus circulans EB 35 (42%). Plant growth-promoting activity of composts and bacteria (EB 35, EB 67, and CDB 35) was studied together. All the three composts showed significant increase in growth of pearl millet, which was 77% by RSC, 55% by GVC, and 30% by FWC. Application of composts with bacteria improved plant growth up to 88% by RSC with EB 67, 83% with GVC and EB 67. These results show the synergistic effect of selected bacteria applied with composts on growth of pearl millet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Current trends in agriculture are focused on reduction in the use of chemical pesticides and inorganic fertilizers, compelling the search for alternatives that enhance environmental quality (Haggag 2002). The management of soil organic matter requires inputs of composts, crop residues, green manure, and other organic wastes (Beri et al. 2003; Manici et al. 2004). The role of earthworms in maintaining soil fertility is well known and recently, emphasis is laid on their use for breaking down and stabilizing the organic wastes (Atiyeh et al. 2000). Compost is rich in microbes and when applied, the soil will be enriched with a variety of nutrients that will become available for the indigenous microflora and hence, plants (Postma et al. 2003). Plant growth-promoting bacteria (PGPB) directly stimulate growth by nitrogen fixation (Han et al. 2005), solubilization of nutrients (Rodriguez and Fraga 1999), production of growth hormones, 1-amino-cyclopropane-1-carboxylate (ACC) deaminase (Correa et al. 2004); and indirectly by antagonizing pathogenic fungi by production of siderophores, chitinase, β-1,3-glucanase, antibiotics, fluorescent pigments, and cyanide (Renwick et al. 1991; Pal et al. 2001). Majority of known plant growth-promoting rhizobacteria (PGPR) are isolated from rhizosphere (Khalid et al. 2004). However, their ability to colonize roots and survive in soil is often limited (Normander and Prosser 2000). Identification of more potent bacteria from composts can broaden the spectrum of PGPB that can survive and perform well in soil conditions.
Pearl millet [Pennisetum glaucum (L.) R. Br] is the main coarse grain crop in semiarid regions and is grown in India as rain-fed or irrigated crop on 10 million hectares producing 7.01 million tons (Bhatnagar et al. 2002). Improving the soil fertility and millet production without the use of chemical fertilizers is a difficult task (Raj et al. 2003). PGPR-mediated plant growth and control of plant diseases of pearl millet is an alternative eco-friendly approach (Kloepper 1993). Processing of crop biomass for preparation of composts rich in nutrients and PGPB provides a greater scope for development of bioinoculants for millet production. In the present study, bacteria were isolated and screened from three different composts for plant growth-promoting traits. Selected bacterial isolates were evaluated for their growth promotion of pearl millet along with composts.
Materials and methods
Composts used for isolating bacteria
Composts were prepared using three different agricultural wastes at International Crops Research Institute for Semi-Arid Tropics (ICRISAT), Patancheru, AP, India. Farm waste compost (FWC) was prepared in an above-soil surface brick chamber (150 cm×90 m×100 cm) that received farm and kitchen waste. A small population of macrofauna (10–15/kg compost) such as earthworms, centipedes, slugs, and snails were naturally present in FWC. Macrofauna were each placed separately in sterilized high-density polyethylene bags (20 cm in width and 11 cm in length) after washing with sterile distilled water to isolate bacteria from their excreta. One gram of excreta was mixed in 9 ml saline (0.9% NaCl) and appropriate dilutions were plated on nutrient agar (NA). Ten macrofauna were placed in Petri dish, desiccated to death, and appropriate dilutions of surface washings in saline were plated on NA for isolating bacteria from body surface.
Rice-straw compost (RSC) was prepared in heaps (5-m-long, 1.5-m-wide, and 1.5-m-high) of 500-kg capacity. Five to 10 kg bundles of air-dried rice-straw were tied up in plastic nets and soaked in water (every 1 kg dry straw soaked in 1.5 l of water) for 2 to 3 min and then allowed to drain for 5 min. Moistened straw was allowed to stay in a heap and covered with polythene sheet to reduce evaporation and to prevent it from getting extra moisture from rain during the composting period.
Gliricidia vermicompost (GVC) was prepared in cement cylinder (90 cm diameter and 50 cm height) with leaves and twigs of Gliricidia sepium (Jacq.) by soaking in 1% cow dung slurry. After 2 weeks, about 500 earthworms (Eisenia foetida) were released into the same tank. Seven layers of foliage of 10-cm thickness of each were added after decomposition of the lower layer.
Each compost preparation took about 45–50 days at 70% moisture. Four to 8 weeks after maturity, 10 g of the compost was taken and appropriate dilutions were plated on NA to isolate bacteria. Isolated colonies were subcultured from each sample and preserved as glycerol stock at −13°C and/or lyophilized ampoules. The isolates from FWC and/or the macrofauna associated, RSC and GVC were designated as EB, CDB, and BWB, respectively.
Screening of bacterial isolates for plant growth-promoting traits
A total of 207 bacterial isolates were collected and initially tested for Gram staining and morphology. About 250 μl of inoculum of each isolate grown in Luria–Bertani broth was added to the well of sterilized multiple pin inoculator that transferred ∼103 cells to media plates (as given below) for simultaneous inoculation of 25 isolates (Josey et al. 1979). Modified M9 medium per liter (MgSO47H2O, 0.5 g; Na2HPO4.7H2O, 12.8 g; KH2PO4, 3 g; NaCl, 0.5 g; NH4Cl, 1 g; CaCl2, 0.15 g, FeCl3, trace) with 10 g cellulose (Hi-Media, Mumbai) as sole carbon source was used for cellulose degradation. Pseudomonas sp. was screened on Pseudomonas isolation agar (Hi-Media). Phosphate (P) solubilization was determined on rock phosphate-buffered medium (Gyaneshwar et al. 1998). Phytase production was determined on plates with phytic acid (inositol hexaphosphate sodium salt) as sole P source (Richardson and Hadobas 1997). The presence of ACC deaminase activity in bacteria was detected on plates with DF salts (Dworkin and Foster 1958) minimal medium with ACC as sole nitrogen source (Jacobson et al. 1994). Indole acetic acid (IAA) production was detected on nitrocellulose membrane disk (Millipore, USA; Bric et al. 1991). Siderophore production was detected on chromeazurol S agar plates (Bernhard and Neilands 1987). Hydrolysis of chitin was studied on defined medium amended with 1.5% colloidal chitin as sole carbon source (Reid and Ogrydziak 1981). Hydrocyanic acid (HCN) production was tested on 35-mm petri dish containing Kings B agar medium amended with 4.4 g glycine/l with filter paper dipped in picric acid in the upper lid and sealed with Parafilm. After inoculation, plates were incubated at 30±1°C for 24 to 144 h as required based on the trait under study. Rating the bacterial isolates positive for plant growth-promoting trait was recorded based on growth and/or zone size accordingly. Screening was done thrice with two replications each.
Screening bacteria for in vitro antagonism
All the 207 isolates were screened for in vitro antagonism against Sclerotium rolfsii, Macrophomina phaseolina, Fusarium solani, and Fusarium oxysporum on potato dextrose agar plates using dual culture technique. An agar plug (5 mm in diam) was cut from an actively growing (96 h) fungal culture and placed at the center of the agar plate. Simultaneously, the bacterium (24 h grown) to be tested was streaked 2 cm away from the agar plug. Plates with only fungus without bacterial culture were used as controls. Plates were incubated at 30±1°C until fungal mycelia covered the agar surface of the control plate. Growth of pathogen was recorded and percent inhibition of fungus was calculated by using the formula:
where I is the percent inhibition of mycelial growth, C is the radial growth of fungus in the control plate (mm), and T is the radial growth of fungus on the plate inoculated with bacterium (mm).
Identification of selective bacterial isolates
Twelve bacterial isolates were identified by staining, morphology, cultural, growth, and biochemical characters as per Bergey’s manual of determinative bacteriology (Krieg and Holt 1984). Three potential isolates were identified at Microbial Type of Culture Collection, Chandigarh, India for confirmation.
Seed bacterization
Pearl millet seeds were surface sterilized with 1% sodium hypochlorite for 5 min and washed five times with sterilized distilled water. Seeds were coated with peat (Biocare Technology Pvt., Australia) based inoculum of bacteria (108−109/g peat) using 1% carboxymethylcellulose as adhesive, dried in air and the cell count was 106−107 CFU per seed before sowing.
Evaluation of bacteria and/or composts on plant growth in glasshouse conditions
Based on plant growth-promoting traits, percent germination and seed vigor, 12 bacterial isolates were selected and evaluated separately for growth of pearl millet cultivar ICMV 155. For plant growth studies, unsterilized vertisol soil was used as potting medium in 15-cm diameter plastic pots. Ten bacterized seeds were sown in each pot and thinning was done to five per pot 1 week after germination. Seeds coated with Azotobacter chroococcum HT-54 was used as positive control (Alka et al. 2001) and only with peat served as control. Three composts (FWC, RSC, and GVC) each independently and in combination with three bacterial strains (EB 35, EB 67, and CDB 35 selected based on plant growth studies) were separately evaluated for growth of pearl millet. Compost was added to the soil before filling the pots at the rate of 5 tons per hectare (on dry weight basis) and ten bacterized seeds were sown in each pot and thinned to five per pot as above.
Plant growth measurements
Plants were irrigated once every 2 days with 20 ml sterilized distilled water. All pots were watered by weight once a week to achieve field capacity of the potting mix. Temperature in the greenhouse ranged from 22 to 32°C (average 26°C). After 30 days, shoot length, leaf area (using Leaf Area meter Licor model, NE, USA), root length (using Rhizo Regent Win Mac Instruments, USA), and dry matter of both root and shoot samples (oven-dried at 70°C to constant weight) were recorded.
Data analysis
All glasshouse experiments were arranged in completely randomized block design with three replications in each treatment and repeated twice. The data were subjected to analysis of variance using Genstat 6.1 statistical package (Lawes Agricultural Trust, Rothamsted, UK). Mean values in each treatment were compared using least significant differences at 5% probability (P=0.05).
Results
Isolation and characterization of bacteria for plant growth-promoting and antagonistic traits
Population of bacteria was log 7.4, 6.7, and 5.7 CFU/g in FWC, RSC, and GVC, respectively. Bacterial population on body surface of macrofauna was log 4.4 for centipedes, 5.0 for earthworm, and 5.4 for slugs CFU per macrofauna and in excreta of macrofauna (on hourly basis) were log 3.8 for centipedes, 5.7 for earthworms, 6.4 for slugs, and 5.1 for snails CFU macrofauna−1 h−1. Of 207 isolates, 24 were from FWC, 78 from macrofauna (isolates from excreta or body surface of macrofauna were pooled as one group), 55 from RSC, and 50 from GVC (Table 1). Gram-negative bacteria were least at 36% in RSC and maximum at 54% in macrofauna and Gram-positive were least at 46% in macrofauna and maximum at 64% in RSC. RSC had cellulose-degrading bacteria as a dominant class (91%) while in the other three sources, this trait was present in 24 to 31% of the bacteria. Population of P-solubilizers (2–3%), chitinase producers (6–7%), fluorescent Pseudomonas (9%), HCN (9–14%), and phytase producers (16–23%) were selectively present in the sources used in the study and comparatively lower than the other groups of bacteria. Siderophore producers were marginally similar in RSC (46%) and GVC (43%) and minimum in FWC (13%). IAA producers were 57% in GVC followed by 33% in RSC, 26% in macrofauna and 4% in FWC. ACC deaminase producers were 53% in RSC and 13% in FWC. HCN producers were between 9% in RSC and 14% in GVC (Table 1). Percent of bacterial isolates that had antagonistic activity against S. rolfsii and M. phaseolina was 17% in FWC, 28–34% in RSC, 38% in GVC, and 21% in macrofauna and against F. solani and F. oxysporum was 20% in FWC, 44% in RSC, 40% in GVC, and 25% in macrofauna (Table 1).
Glasshouse evaluation of plant growth-promoting activity
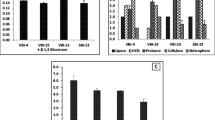
Twelve isolates (Table 2) with different plant growth-promoting traits showed significant increase in germination and seed-vigor index of pearl millet tested using paper towel technique in vitro (data not shown). Of these, seven isolates significantly (P=0.05) increased the shoot length and ten isolates increased leaf area, root length, and plant dry weight (biomass) after seed treatment in repeated experiments at glasshouse conditions. All the bacterial isolates producing ACC deaminase showed significant increase in root length (except EB 75 and BWB 36) and the increase in root length density ranged from 22 to 71% over uninoculated control. Few of the bacterial isolates (EB 35, EB 67, and CDB 47) that did not produce auxin (IAA) also increased the root length (Tables 1 and 3). Increase in biomass (plant dry weight) was with S. marcescens EB 67 (56%) isolated from macrofauna (slug body surface), followed by Pseudomonas sp. CDB 35 (52%) isolated from RSC, and B. circulans EB 35 (42%) isolated from macrofauna (centipede excreta). Most of the bacterial isolates tested for plant growth activity were at par or greater than A. chroococcum HT 54 used as positive control in the study (Table 3). Seed treatment with peat-based formulation of selected bacterial isolates applied along with compost significantly (P=0.05) increased the shoot length, leaf area, plant dry weight, and root length density. RSC applied with EB 67 showed increase in shoot length (30%) and leaf area (63%). Maximum increase in dry biomass occurred when RSC was applied along with EB 67 (88%), followed by GVC applied with EB 67 (83%), and GVC applied with CDB 35 (82%) when compared to control (Fig. 1).
Effect of three composts and potential bacteria (B.circulans EB 35, S. marcescens EB 67, and Pseudomonas sp. CDB 35) on plant growth of pearl millet ICMV 155 using unsterilized soil in glasshouse conditions (a) shoot length, (b) leaf area, (c) root length density, and (d) plant dry weight. FWC Farm waste compost, RSC rice straw compost, and GVC Gliricidia vermicompost. Compost was applied at 5 ton/ha and bacteria were applied as seed treatment. Data presented is the mean (percent increase over control) and the standard error of three replications in two glasshouse experiments with three replications each
Discussion
Bacteria associated with different composts and macrofauna had plant growth-promoting and antagonistic activity in vitro and promoted growth of pearl millet in glasshouse conditions. Thermophilic bacilli are reported to be the predominant group during composting, whereas genus Bacillus, Pseudomonas, and Pantoea spp. recolonize the compost during curing (Hoitnik and Boehm 1999). Population of Pseudomonas in composts was low in our observation (Table 1) though they are reported to be the predominant group in rhizosphere as PGPB (Dey et al. 2004). Very low percentage of bacteria were P-solubilizers, which might be due to incorporation of Tris–HCl buffer of pH 8 that allows excess organic acid producing bacteria to show P solubilization (Gyaneshwar et al. 1998).
Population of phytase producers was more or less similar in all sources studied and is comparable with earlier studies of Richardson and Hadobas (1997). Significant population of siderophore producers in this study also reveals that composts provide a conducive environment for proliferation of antagonistic bacteria that promote plant growth (Sharma and Johri 2003). Indole and ACC deaminase producers were present in all sources studied and these groups of bacteria are known to promote root elongation and plant growth (Pattern and Glick 2002; Hontzeas et al. 2004). ACC deaminase producers promote root and plant growth by hydrolyzing ACC and decreasing ethylene biosynthesis in roots. Species of Pseudomonas and Bacillus were predominant among antagonistic bacteria in this study (Table 2) and previous studies revealed that they produce a wide range of antibiotics and cell wall-degrading enzymes and promote plant growth (Pal et al. 2001; Sadfi et al. 2001).
Twelve isolates were selected based on plant growth-promoting traits for growth-promoting studies on pearl millet (Table 2). High diversity of microorganisms is reported from macrofauna and insects but very few reports of bacterial identification exist (Thimm et al. 1998). S. marcescens EB 67 isolated from slug (body surface) present in FWC and Pseudomonas sp. CDB 35 isolated from RSC exhibited multiple PGPR traits and increased root length and plant dry weight of pearl millet under glasshouse conditions (Tables 2 and 3). Various species of Serratia and fluorescent pseudomonads are potential plant growth promoters and biocontrol agents (Kalbe et al. 1996; Dey et al. 2004). Phosphorus-solubilizing bacteria in this study were positive for siderophore and ACC deaminase (Table 2) and three out of the five strains increased plant dry weight significantly (Table 3). Considering the P-availability as a limiting step in plant nutrition (Goldstein 1986), the contribution of these bacteria to plant growth promotion can be supported. Four of the five strains having phytase activity (important in P-acquisition by plant) showed significant increase in plant dry weight (Table 3). However, responses to inoculation with phytate-mineralizing bacteria were generally observed only when large amount of phytate is provided (Richardson et al. 2001). Seven of the nine strains possessing ACC deaminase activity significantly improved root length. This was in consistency with the observations in canola and other crops, which showed root elongation when ACC deaminase-producing bacteria were applied (Penrose et al. 2001). Two bacterial strains EB 35 and CDB 47 were effective in the promotion of root growth though they did not produce IAA. Recent report by Kishore et al. (2005) showed that there was no correlation between IAA production and root length.
Application of three composts FWC, RSC, and GVC along with three bacterial strains EB 35, EB 67, and CDB 35 showed enhancement in root length and dry biomass (Fig. 1a–d). Composts and vermicomposts amendment to soil stimulates growth of plants through the nutrients present in them, microbial activity, and plant growth regulators (Arancon et al. 2004). In our studies, the improvement in plant growth due to compost application could be due to the high percent of siderophore, ACC deaminase, and IAA producers and nutrients present in them. Vermicomposts are known to promote plant growth comparatively better than composts (Atiyeh et al. 2000) but in our study, application of compost (RSC) was better than vermicompost (GVC), which might be due to low proportion of IAA producers (Table 1) as the concentration of IAA above the threshold levels is deleterious for root growth (Xie et al. 1996). Application of Azospirillum spp. along with composts alleviated the noxious effect of composts and enhanced germination of wheat (Bacilio et al. 2003). In this study, PGPB stimulated germination and promoted plant growth and their application with composts synergistically enhanced plant growth. Such PGPB can be applied as additional inoculants along with composts and make a synergistic treatment for improving plant growth.
References
Alka V, Kamlesh K, Pathak DV, Suneja S, Neeru N (2001) In vitro production of plant growth regulators (PGRs) by Azotobacter chroococcum. Indian J Microbiol 41:305–307
Arancon NQ, Edwards CA, Bierman P, Metzger JD, Lee S, Welch C (2004) Effects of vermicomposts on growth and marketable fruits of field-grown tomatoes, peppers and strawberries. Pedobiolgia 47:731–735
Atiyeh RM, Subler S, Edwards CA, Bachman G, Metzger JD, Shuster W (2000) Effects of vermicomposts and composts on plant growth in horticultural media and soil. Pedobiolgia 44:579–590
Bacilio M, Vazquez P, Bashan Y (2003) Alleviation of noxious effects of cattle ranch composts on wheat seed germination by inoculation with Azospirillum spp. Biol Fertil Soils 38:261–266
Beri V, Sidhu BS, Gupta AP, Tiwari RC, Pareek RP, Rupela OP, Khera R, Ahluwalia JS (2003) Organic resources of a part of Indo-Gangetic plain and their utilization. PAU
Bernhard S, Neilands JB (1987) Universal chemical assay for detection and determination of siderophores. Anal Biochem 169:46–56
Bhatnagar SK, Khairwal IS, Pareek S (2002) Pearl millet nucleus and breeder seed production. Technical bulletin. Project co-ordinator (Pearl millet). All India co-ordinated pearl millet improvement project (AICPMIP), Jodhpur, India
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Correa JD, Barrios ML, Galdona RP (2004) Screening for plant growth-promoting rhizobacteria in Chamaecytisus proliferus (tagasaste), a forage tree-shrub legume endemic to the Canary Islands. Plant Soil 266:75–84
Dey R, Pal KK, Bhatt DM, Chauhan SM (2004) Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth promoting rhizobacteria. Microbiol Res 159:371–394
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
Goldstein AH (1986) Bacterial solubilization of mineral phosphates: historical perspectives and future prospects. Am J Altern Agric 1:51–65
Gyaneshwar P, Naresh Kumar G, Parekh LJ (1998) Effect of buffering on the P-solubilizing ability of microorganisms. World J Microbiol Biotechnol 14:669–673
Haggag WM (2002) Sustainable agriculture management of plant diseases. J Biol Sci 2(4):280–284. http://www.ansinet.org/fulltext/jbs/jbs24280–284.pdf (online journal)
Han J, Sun L, Dong X, Cai Z, Yang H, Wang Y, Song W (2005) Characterization of a novel plant growth-promoting bacteria strain Delftia tsuruhatensis HR4 both as a diazotroph and a potential biocontrol agent against various pathogens. Syst Appl Microbiol 28:66–76
Hoitnik HAJ, Boehm MJ (1999) Biocontrol within the context of soil microbial communities: a substrate-dependent phenomenon. Annu Rev Phytopathol 37:427–446
Hontzeas N, Jerome Zoidakis J, Glick BR, Abu-Omar MM (2004) Expression and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the rhizobacterium Pseudomonas putida UW4: a key enzyme in bacterial plant growth promotion. Biochim Biophys Acta 1703:11–19
Jacobson BC, Pasternak JJ, Glick BR (1994) Partial purification characterization of 1-aminocyclopropane-1-carboxylate deaminase from the plant growth promoting rhizobacterium Pseudomonas putida GR 12-2. Can J Microbiol 40:1019–1025
Josey DP, Beynon JL, Johnston AWB, Beringer JE (1979) Strain indentification in Rhizobium using intrinsic antibiotic resistance. J Appl Bacteriol 46:343–350
Kalbe C, Marten P, Sastry GRK (1996) Strains of genus Serratia as beneficial rhizobacteria of oilseed rape with antifungal properties. Microbiol Res 151:433–439
Khalid A, Arshad M, Zahir ZA (2004) Screening plant growth promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96:473–480
Kishore GK, Pande S, Podile AR (2005) Phylloplane bacteria increase seedling emergence, growth and yield of field-grown groundnut (Arachis hypogeae L.). Lett Appl Microbiol 40:260–268
Kloepper JW (1993) Plant growth-promoting rhizobacteria as biological control agents. In: Metting FB Jr (ed) Soil microbial ecology. Marcel Dekker, NewYork, pp 255–274
Krieg NR, Holt JG (1984) In: Murray RGE, Brenner DJ, Bryant MP, Holt JG, Krieg NR, Moulder JW, Pfennig N, Sneath PHA, Staley JT (eds) Bergey’s manual of systematic bacteriology, vol I. Williams and Wilkins, Baltimore, MD, USA
Manici LM, Caputo F, Babibi V (2004) Effect of green manure on Pythium spp. population and microbial communities in intensive cropping systems. Plant Soil 263:133–142
Normander B, Prosser JI (2000) Bacterial origin and community composition in the barley phytosphere as a function of habitat and presowing conditions. Appl Environ Microbiol 66:4372–4377
Pal KK, Tilak KVBR, Saxena AK, Dey R, Singh CS (2001) Suppression of maize root diseases caused by Macrophomina phaseolina, Fusarium moniliforme and Fusarium graminearum by plant growth promoting rhizobacteria. Microbiol Res 156:209–223
Pattern CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Penrose DM, Moffatt BA, Glick BR (2001) Determination of 1-aminocyclopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Can J Microbiol 47:77–80
Postma J, Montenari M, van den Boogert PHJF (2003) Microbial enrichment to enhance disease suppressive activity of compost. Eur J Soil Biol 39:157–163
Raj SN, Deepak SA, Basavaraju P, Shetty HS, Reddy MS, Kloepper JW (2003) Comparative performance of formulations of plant growth promoting rhizobacteria in growth promotion and suppression of downy mildew in pearl millet. Crop Prot 22:579–588
Reid JD, Ogrydziak DM (1981) Chitinase-overproducing mutant of Serratia marcescens. Appl Environ Microbiol 41:64–669
Richardson AE, Hadobas PA (1997) Soil isolates of Pseudomonas spp. that utilize inositol phosphates. Can J Microbiol 43:509–516
Richardson AE, Hadobas PA, Hayes JE, O’Hara CP, Simpson RJ (2001) Utilization of phosphorous by pasture plants supplied with myo-inositol hexaphosphate is enhanced by the presence of soil microorganisms. Plant Soil 229:47–56
Renwick AR, Campbell A, Coe S (1991) Assessment of in vivo screening systems for potential biocontrol agents of Gaeumannomyces graminis. Plant Physiol 40:524–532
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Sadfi N, Cherif M, Fliss I, Boudabbous A, Antoun H (2001) Evaluation of bacterial isolates from salty soils and Bacillus thuringensis strains for the biocontrol of Fusarium dry rot of potato tubers. J Plant Pathol 83:101–118
Sharma A, Johri BN (2003) Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiol Res 158:243–248
Thimm T, Hoffmann A, Borkott H, Munch JN, Tebbe CC (1998) The gut of the soil microarthropod Folsomia candida (Collembola) is a frequently changeable but selective habitat and a vector for microorganisms. Appl Environ Microbiol 64:2660–2669
Xie H, Pasternak JJ, Glick BR (1996) Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2 that overproduce indole acetic acid. Curr Microbiol 32:67–71
Acknowledgements
We thank Dr. R P Thakur of ICRISAT for providing pathogenic fungal cultures, Dr. Neeru Narula of CCS Haryana Agricultural University, Hisar, Haryana, and Dr. G Naresh Kumar of M S University of Baroda, Gujarat for providing the reference strains used in these studies. Doctoral fellowship to Ms Hameeda Bee from the Jawaharlal Nehru Memorial Fund, New Delhi is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hameeda, B., Rupela, O.P., Reddy, G. et al. Application of plant growth-promoting bacteria associated with composts and macrofauna for growth promotion of Pearl millet (Pennisetum glaucum L.). Biol Fertil Soils 43, 221–227 (2006). https://doi.org/10.1007/s00374-006-0098-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-006-0098-1