Abstract
Olive-tree leaves (OL) were mixed with olive press cake (OPC) and extracted olive press cake (EPC) at 1:1 dw/dw ratios to prepare two composting mixtures (OL+OPC and OL+EPC). Both CO2–C evolution and fluorescein diacetate (FDA) hydrolysis, determined as estimates of the microbial activity during composting, were related to temperature fluctuations in the compost piles, showing greater values at the temperature peaks, compared to the end, of each thermophilic phase. This, however, was only shown after handling and incubating samples at the temperatures of the compost mixtures at the sampling times and not at a low standard temperature. Incubating samples from thermophilic phases at low standard temperatures resulted in underestimation of the microbial activity occurring during composting. The effect of incubation temperature was less dramatic for FDA hydrolysis compared to CO2–C evolution measurements, probably reflecting the reduced dependence of enzymes involved in FDA hydrolysis on the respective temperatures. However, FDA hydrolysis was a less sensitive indicator of microbial activity, probably due to extracellular cleavage of fluorescein by persistent esterases, at lowered microbial activity phases. Total microbial biomass, estimated by the fumigation–extraction method, was not consistently related to temperature fluctuations during composting and showed a clear increase at the end of composting, probably resulting from a large slow-growing mycelial community colonising the end products. Since high temperatures did not induce significant non-microbial CO2–C release and FDA degradation, we propose the performance of microbial activity measurements during thermophilic composting phases at the actual temperatures evolving in the composts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Composting is a widely used environment-friendly method for treating organic wastes and by-products before recycling them into soils. It consists of an aerobic self-heating biodegradation process of organic materials performed at high rates by a variety of mesophilic/thermotolerant and thermophilic micro-organisms, predominantly of soil origin (Finstein and Morris 1975; de Bertoldi et al. 1983). Therefore, methodologies for monitoring both microbial diversity and activity have been used to understand and control composting processes.
Respiration measurements have been used for a long time as standard methods for estimating microbial activity in composts measuring either CO2 evolution or O2 depletion (Shulze 1962; Suler and Finstein 1977; Manios and Balis 1983; Nakasaki et al. 1985). Their advantage is that they are broad evaluators of the spectrum of metabolic processes, integrated under the term “microbial activity”, and they are directly related to decomposition. Low specificity enzyme assays have also been used for estimating microbial activity, detecting omnipresent classes of enzymes as dehydrogenases (Forster et al. 1993; Benito et al. 2003), phosphatases (Vuorinen 1999) and esterases (Craft and Nelson 1996). They are sensitive and easy to perform assays, but they are more difficult to interpret in complex environmental samples, as they monitor enzymatic processes indirectly related to overall energy release and decomposition and do not distinguish intracellular enzyme activities from extracellular enzyme activities from potentially stabilized enzymes not reflecting current microbial activity (Insam 2001; Nannipieri et al. 2002). Moreover, their results may depend on the taxonomic structure of the microbial community and the interference of other physico-chemical processes in complex environmental media, affecting measurement precision. Among enzyme assays, the fluorescein diacetate (FDA) hydrolysis technique, evaluating the potential activity of ester-cleaving enzymes, frequently appears in recent studies of composting processes (Levanon and Pluda 2002; Garcia-Gomez et al. 2003; Ryckeboer et al. 2003; Smith and Hughes 2004).
Both respiration and FDA hydrolysis methods were adapted from techniques developed for environment as soils (Birch and Friend 1956; Stotzky 1965; Anderson 1982; Schnürer and Rosswall 1982; Frankenberger and Dick 1983) or litter and container media (Swisher and Carroll 1980; Schnürer and Rosswall 1982; Inbar et al. 1991) and were also applied in aquatic systems (Fontvieille et al. 1992) and sediments (Meyer-Reil and Köster 1992). These environments, however, do not show the large temperature increases observed during a typical composting process, which induce not only a dramatic microbial activity rise, up to temperatures around 50–60°C (McKinley and Vestal 1984; Derikx et al. 1990; Weppen 2002), but also physiological adaptation of the microbial community at higher temperature optima (McKinley and Vestal 1984; Lasaridi et al. 1996). Therefore, applying the methods above at pre-set low temperatures (usually around 20–30°C) may not be appropriate for estimating the microbial activity during thermophilic composting phases.
Residues/by-products from the olive-mill agro-industry, i.e. olive-tree leaves (OL), olive press cake (OPC) and extracted olive press cake (EPC), are produced in large amounts in olive cultivation regions and can be successfully composted (Madejon et al. 1998; Filippi et al. 2002; Garcia-Gomez et al. 2003). They are typical xenobiotic-free organic materials of vegetative origin and were used to make the two compost mixtures (OL+OPC and OL+EPC) employed in this work.
The aim of the study was to investigate how sample incubation conditions affect the estimation of microbial activity occurring during composting and determined by respiration and FDA hydrolysis. Measurements at temperatures resembling those developing in situ during the composting process were compared to low and standard temperature measurements.
Materials and methods
Composting materials and processes
Agricultural residues/by-products from the olive oil agro-industry, specifically OL, OPC and EPC, were mixed at 1:1 dw/dw ratios to prepare two compost mixtures, OL+OPC and OL+EPC. The characteristics of the materials are shown in Table 1.
The composting process was performed in static perimetrically insulated containers (0.16-m3 volume) for a period of 3 months. Aeration was passive from bottom to top, and a 2-cm-thick layer of polyurethane was used to cover the sides of the containers to minimize temperature losses. The moisture content was kept between 40 and 60% of the water-holding capacity throughout the composting process. Turnings were performed and water was added at the end of each thermophilic phase.
Microbiological analysis
FDA hydrolysis was performed according to Adam and Duncan (2001), following the addition of 6 g of compost sample to 60 ml of 60 mM potassium phosphate buffer, pH 7.6. The enzymatic hydrolysis started by adding 0.4 ml FDA solution (1 mg ml−1). The sample was shaken for 10 min in an orbital incubator, 15 ml sub-samples were obtained at 0 and 10 min and the hydrolysis reaction was terminated by adding 15 ml CHCl3/CH3OH (2:1 vol vol−1). Following centrifugation (700×g) and filtration of the aqueous phase, the absorbance of filtrates was measured at 490 nm. Blanks, without addition of FDA, were also included to correct for background absorbance, and the concentration of fluorescein release was determined against a calibration curve, produced by 0-, 1-, 2-, 3-, 4- and 5-μg ml−1 fluorescein standards.
Carbon dioxide evolution was measured after CO2 entrapment in alkali (Anderson 1982). A dish with 7 g of compost mixture and a beaker containing 15 ml 0.5 N NaOH were placed in a 2-l screw-sealed bottle. The sample was incubated for 21 h, and subsequently, the alkali trap was removed and titrated with dilute HCl in the presence of phenolphthalein, following the addition of 6 ml 3 N BaCl2. Blanks containing no compost were also included.
At the peaks of the thermophilic phases, sample treatment and incubation for FDA degradation and CO2 evolution analysis were performed at two temperatures: one equal to the temperature of the compost at the sampling time and the other at room temperature (25°C). At the end of each thermophilic phase, FDA degradation and CO2 evolution analysis were performed at 25°C only, which matched the temperatures of the composts at the sampling time (±3°C).
FDA hydrolysis determinations were also performed in the absence of compost samples following shaking for 10 min in the orbital incubator at a range of temperatures (25, 30, 40, 50 and 60°C) to check for thermal non-enzymatic FDA degradation, potentially induced by high temperatures. Fluorescein absorbance in the presence of compost and soil samples was measured at 490 nm from 6 g samples suspended in 60 ml of 60 mM potassium phosphate buffer, pH 7.6, containing 0.32 ml fluorescein solution (1 mg ml−1), following shaking for 10 min.
The occurrence of any CO2 release induced by chemical oxidation of organic compounds at high temperatures (non-microbial CO2 release) was determined by measuring CO2 evolution as described above after incubating sterilized compost samples (121°C for 20 min) from both composts under sterile conditions at 25, 40, 50 and 60°C. The effect of sample pre-incubation (conditioning) on respiration was examined by pre-incubating samples obtained from a thermophilic phase (50°C in situ) from OL+OPC for 48 h at 25°C before performing the CO2 release determinations at the same temperature. Determination of microbial biomass was performed by the fumigation–extraction technique (Vance et al. 1987). Briefly, compost samples (10 g fresh wt) were extracted with 0.5 M K2SO4, shaken for 30 min and filtered, either directly or after fumigation (incubation for 5 days at 25°C in a vacuum desiccator, containing 30 ml alcohol-free chloroform). The extracts were further analysed for total C by a wet oxidation–titration procedure (Anderson and Ingram 1996).
Statistical analysis
Standard errors of means (±SE) were calculated, and analysis of variance and linear regression analysis were performed using SPSS software (version 10 for Windows OS).
Results
The composting of the OL+OPC and OL+EPC mixtures was carried out by performing successive turnings at the end of each of the respective three and four thermophilic phases (Fig. 1), respectively. The C/N ratios in the original materials decreased during the composting process, whereas the ash content increased (Table 1), indicating significant C losses. The total N, P and K contents, the concentration of NH4 +–N and the bulk density of the final compost products also increased during the process.
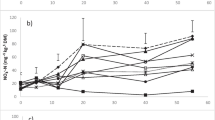
The CO2 evolution and the degradation of FDA during composting of OL+OPC and OL+EPC were related to temperature fluctuations in the compost piles, showing greater values of fluorescein and CO2 release when the composts were at their peak temperatures as compared to the values observed at the end of each thermophilic phase. This, however, was only shown when sample incubations were performed directly after sampling at the temperatures occurring in the compost piles (Figs. 2 and 3). The CO2 evolution and the degradation of FDA from the OL+OPC and OL+EPC mixtures were not related to temperature fluctuations in the compost piles when the samples were incubated at room temperature (Figs. 2 and 3). The differences between the two sample treatment processes were greater for the CO2 compared to FDA determinations.
No CO2 evolution was observed at either low or high incubation temperatures from any of the sterilized compost samples, where the microbial biomass had been killed, indicating lack of chemical oxidation at temperatures up to 60°C. Pre-incubation of samples obtained during a thermophilic phase (50°C in situ) from OL+OPC at 25°C resulted in only a marginal increase in the following respiration measurements, compared to direct respiration measurements at 25°C. In contrast, respiration measurements performed at 50°C showed dramatically greater values (Fig. 4).
A limited FDA hydrolysis was observed at temperatures up to 40°C (Fig. 5), which was doubled at temperatures between 50 and 60°C. The compost materials used in this study showed either no direct fluorescein absorbance (OL+EPC) or 10% fluorescein absorbance (OL+OPC) during incubations containing fluorescein instead of FDA. A correction was therefore applied on the FDA hydrolysis values obtained for OL+OPC.
The microbial biomass pattern was not consistent; however, at the end of the composting process when microbial activity values were low, high microbial biomass was found in both composts (Fig. 6). This indicates changes in the metabolic quotient (Crespired/Cmicrobial) probably related to differences in C availability and/or the composition of the microbial community.
Discussion
Higher fluorescein and CO2 release at the peak compared to the end of the thermophilic phases are expected, since the temperature increases during composting result from extensive aerobic microbial activity (Finstein and Morris 1975). Micro-organisms show their greatest bio-oxidative potential for a wide range of materials between 45 and 60°C (Wiley 1957; Jeris and Regan 1973; Suler and Finstein 1977; MacGregor et al. 1981; Derikx et al. 1990; Lopez-Real and Vere 1992; Stentiford 1996). This pattern, however, was only obtained in our microbial activity measurements when sample incubations were carried out directly after sampling at temperatures similar to those occurring at the respective composting phases (Figs. 2 and 3). Incubating compost samples obtained during the thermophilic phases at low standard temperatures led to significant underestimation of the microbial activity, probably as a result of (1) physiological adaptation shock and/or (2) poor metabolic activity at low temperature. Short pre-incubations to allow for physiological adaptation of the microbial community to the low-temperature incubations showed only a marginal effect on respiration activity, indicating that reduced metabolic rates at lower temperatures were the main reason for the observed reduction in microbial activity. Incubation temperatures at 37°C have been suggested as a compromise (Iannotti-Frost et al. 1992; Iannotti et al. 1993). They could be acceptable for certain materials; however, CO2 release was still underestimated as indicated by the measurements from the last thermophilic phases in our study (Fig. 2). Similar observations were also made by Mari et al. (2003) for incubations at 35°C.
In a study of vegetable and garden waste composting, Ryckeboer et al. (2003) observed a positive correlation between compost temperature and oxygen depletion but a weak negative correlation between temperature- and the log-transformed FDA hydrolysis data. This seems contradictory as both variables estimate microbial activity; it is, however, less surprising according to our findings since oxygen concentration measurements were made at the outlet gasses of the composter (produced at the actual composting temperatures), whereas FDA hydrolysis measurements were performed in compost samples incubated at 25°C. Relatively small respiration increases were reported during thermophilic composting phases by other researchers, applying respiration measurements at low temperatures (Tiquia et al. 1996; Levanon and Pluda 2002; Paredes et al. 2002), and small FDA hydrolysis values were shown (Levanon and Pluda 2002). Moreover, in recent studies where respiration was monitored throughout the composting process by CO2 evolution measurements at standard low-temperature incubations, only a part of the reported C losses seems to be accounted for by CO2–C evolution (Benito et al. 2003; Garcia-Gomez et al. 2003). Assuming adequate oxygenation levels necessary for obtaining the characteristic CO2 flush at the initial composting stages (Michel and Reddy 1998), an underestimation of microbial activity during thermophilic composting phases may have contributed to the results above. On the contrary, large increases are shown at thermophilic composting phases when measurements are performed directly in the gas outlet of composters (Shulze 1962; Jeris and Regan 1973; Nakasaki et al. 1985; Komilis and Ham 2000). This respiration flush is particularly evident at the initial stages of thermophilic phases (Michel et al. 1993; Atkinson et al. 1996; Hellmann et al. 1997; Wong and Fang 2000) when the microbial activity flush results in rapid self-heating of the compost mass.
To perform CO2 evolution and FDA hydrolysis measurements at high incubation temperatures, however, significant chemo-oxidation of C substrates and thermal degradation of FDA, respectively, must be excluded. Indeed, the killing of the microbial biomass resulted in the lack of any CO2 evolution in all sterilized samples incubated at temperatures up to 60°C, indicating no chemo-oxidation phenomena. Therefore, the higher CO2 evolution values obtained at higher temperature incubations should be attributed to higher microbial activity alone. Our data also indicate that chemical hydrolysis of FDA at high temperatures was limited, reaching less than 3% of the FDA hydrolysis observed in compost incubations at temperatures up to 40°C and less than 6% for temperatures up to 60°C (Figs. 3 and 5). This is in line with Adam and Duncan (2001), who showed no spontaneous hydrolysis of FDA in the range from 20 to 40°C. At higher temperatures, however, considerable spontaneous hydrolysis of FDA could occur, making comparisons with lower temperature incubations difficult, except if a reference FDA hydrolysis blank is used. Significant absorption of fluorescein may occur in humic materials. Their presence in solution would therefore reduce the fluorescein absorbance readings by the FDA hydrolysis assay. Our preliminary tests showed that the presence of different compost materials and soils in control fluorescein solution standards resulted in 0 to 29% lower fluorescein absorbance for a range of five compost materials and 4 to 13% for a range of seven soils (data not shown). Similarly, variable effects observed by Adam and Duncan (2001) indicate the need for preliminary tests before applying the FDA hydrolysis method in new environmental samples. Correction for lower fluorescein absorbance values in the presence of the compost materials was indeed needed for OL+OPC, but not for OL+EPC, which did not affect fluorescein absorbance values.
Much greater differences in microbial activity between the peak and the end of each thermophilic phase were shown for the CO2 evolution compared to the FDA degradation. It appears that total respiration measurements are better evaluators of differences in microbial activity at each composting stage, compared to the fluorescein release, since microbial activity may be reduced towards the end of each thermophilic phase (limited availability of labile-C substrates which directly affects bio-oxidative processes and respiration). Indeed, enzymes, such as extracellular thermotolerant esterases, reactive to FDA may still maintain their structure and activity when microbial activity is low (Insam 2001; Nannipieri et al. 2003). Remarkably stable and persistent extracellular esterases have been isolated from complex environmental media like soils (Satyanarayana and Getzin 1973). This may lead to relatively high fluorescein values following the thermophilic composting phases. FDA is degraded by a broad range of enzymes capable of hydrolysing the ester bond, and therefore, any significant preservation of enzyme structure and activity could lead to methodological overestimation of the active bio-oxidative community in periods of lowered metabolic activity. The long lag phase in the drop in FDA hydrolysis, shown by Ryckeboer et al. (2003) following the thermophilic composting phase, is in accordance with this hypothesis. The smaller effect of the incubation temperature on FDA hydrolysis compared to CO2 evolution in our samples obtained from thermophilic phases may be due to similar reasons.
The fluctuations in microbial activity and heat production were not necessarily accompanied by similar fluctuations in the microbial biomass, indicating changes in the metabolic quotient (qCO2–C) during the composting process. At the end of the composting process, the high microbial biomass and low CO2 evolution (reduced metabolic quotient) could be related to the depletion of labile-C and/or changes in the composition of the microbial community: Increased microbial biomass is observed at the end of the thermophilic composting stages (Hellmann et al. 1997; Tiquia et al. 1996), and fungi and actinomycetes dominate (Levanon and Pluda 2002; Tiquia et al. 2002; Ryckeboer et al. 2003). Indeed, changes in the microbial community structure may affect, among other factors, the metabolic quotient (Wardle and Ghani 1995; Insam et al. 1996), and the increase in the fungal-to-bacterial biomass ratio has been directly related to qCO2 decline (Sakamoto and Oba 1994; Blagodatskaya and Anderson 1998). A reduced metabolic quotient has been proposed as a sensitive indicator of compost stability (Kostov et al. 1994); as fungi release less CO2 per unit cell mass compared to bacteria (Alexander 1977), the qCO2 reduction may derive from a shift towards filamentous micro-organisms rather than directly from the depletion of labile-C that occurs at the end of composting.
In conclusion, since (1) respiration is greatly underestimated when samples from thermophilic composting phases are treated and incubated at lower temperatures, (2) increased temperature incubations for respiration measurements are free of non-biological CO2 release and (3) respiration measurements are direct indicators of microbial oxidative activity, we propose the incubation and respiration measurements at the temperatures occurring during the composting process as the best evaluators of the microbial activity occurring in situ. The FDA hydrolysis method, even when applied at the relevant temperatures, is probably a less sensitive evaluator of the oxidative microbial activity, especially when the latter is rapidly declining during the final stages of the process.
References
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951
Alexander M (1977) Introduction to soil microbiology, 2nd edn. Wiley, New York
Anderson JPE (1982) Soil respiration. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis: chemical and microbiological properties, Part 2. Agronomy Monograph No. 9, ASA-SSSA, Madison WI, USA, pp 831–871
Anderson MJ, Ingram JSI (1996) Microbial biomass. In: Tropical soil biology and fertility: a handbook of methods (2nd print). CAB International, Wallingford, UK, pp 68–70
Atkinson CF, Jones DD, Gauthier JJ (1996) Biodegradabilities and microbial activities during composting of municipal solid waste in bench-scale reactors. Compost Sci Util 4:14–23
Benito M, Masaguer A, Moliner A, Arrigo N, Palma RM (2003) Chemical and microbiological parameters for the characterisation of the stability and maturity of pruning waste compost. Biol Fertil Soils 37:184–189
Birch HF, Friend MI (1956) Humus decomposition in East African soils. Nature 178:500–501
Blagodatskaya E, Anderson TH (1998) Interactive effects of pH and substrate quality on the fungal-to-bacterial ratio and qCO2 of microbial communities in forest soils. Soil Biol Biochem 30:1269–1274
Craft CM, Nelson EB (1996) Microbial properties of composts that suppress damping-off and root rot of creeping bentgrass caused by Pythium graminicola. Appl Environ Microbiol 62:1550–1557
de Bertoldi M, Vallini G, Pera A (1983) The biology of composting: a review. Waste Manag Res 1:156–176
Derikx PJL, Op Den Camp HJM, van der Drift C, Van Griensven LJLD, Vogels GD (1990) Biomass and biological activity during the production of compost used as a substrate in mushroom cultivation. Appl Environ Microbiol 56:3029–3034
Filippi C, Bedini S, Levi-Minzi R, Cardelli R, Saviozzi A (2002) Cocomposting of olive oil mill by-products: chemical and microbiological evaluations. Compost Sci Util 10:63–71
Finstein MS, Morris ML (1975) Microbiology of municipal solid waste composting. In: Perlman D (ed) Advances in applied microbiology, vol 19. Academic, New York, pp 113–151
Fontvieille DA, Outaguerouine A, Thevenot DR (1992) Fluorescein diacetate hydrolysis as a measure of microbial activity in aquatic systems—application to activated sludges. Environ Technol 13:531–540
Forster JC, Zech W, Würdinger E (1993) Comparison of chemical and microbiological methods for characterization of the maturity of composts from contrasting sources. Biol Fertil Soils 16:93–99
Frankenberger WT, Dick WA (1983) Relationships between enzyme-activities and microbial-growth and activity indexes in soil. Soil Sci Soc Am J 47:945–951
Garcia-Gomez A, Roig A, Bernal MP (2003) Composting of the solid fraction of olive mill wastewater with olive leaves: organic matter degradation and biological activity. Bioresour Technol 86:59–64
Hellmann B, Zelles L, Palojärvi A, Bai QY (1997) Emission of climate-relevant trace gases and succession of microbial communities during open-window composting. Appl Environ Microbiol 63:1011–1018
Iannotti DA, Pang T, Toth BL, Elwell DL, Keener HM, Hoitink HAJ (1993) A quantitative respirometric method for monitoring compost stability. Compost Sci Util 1:52–65
Iannotti-Frost D, Toth BL, Hoitink HAJ (1992) Dissolved oxygen respirometry method monitors progress in MSW composting and helps predict potential for odor generation as well as end product value. Biocycle 33:62–66
Inbar Y, Boehm MJ, Hoitink HAJ (1991) Hydrolysis of fluorescein diacetate in sphagnum peat container media for predicting suppressiveness to damping-off caused by Pythium ultimum. Soil Biol Biochem 23:479–483
Insam H (2001) Developments in soil microbiology since the mid 1960s. Geoderma 100:389–402
Insam H, Hutchinson TC, Reber HH (1996) Effects of heavy metal stress on the metabolic quotient of the soil microflora. Soil Biol Biochem 28:691–694
Jeris JS, Regan RW (1973) Controlling environmental parameters for optimum composting. I. Experimental procedures and temperature. Compost Sci 14:10–15
Komilis DP, Ham RK (2000) A laboratory method to investigate gaseous emissions and solids decomposition during composting of municipal solid wastes. Compost Sci Util 8:254–265
Kostov O, Petkova G, Van Cleemput O (1994) Microbial indicators for sawdust and bark compost stability and humification processes. Bioresour Technol 50:193–200
Lasaridi KE, Papadimitriou EK, Balis C (1996) Development and demonstration of a thermogradient respirometer. Compost Sci Util 4:53–61
Levanon D, Pluda D (2002) Chemical, physical and biological criteria for maturity in composts for organic farming. Compost Sci Util 10:339–346
Lopez-Real J, Vere A (1992) Composting control parameters and compost product characteristics. In: Jackson DV, Merillot JM, L'Hermite P (eds) Composting and compost quality assurance criteria. Commission of the European Communities, pp 131–141
MacGregor ST, Miller FC, Psarianos KM, Finstein MS (1981) Composting process-control based on interaction between microbial heat output and temperature. Appl Environ Microbiol 41:1321–1330
Madejon E, Galli E, Tomati U (1998) Composting of wastes produced by low water consuming olive mill technology. Agrochimica 42:135–146
Manios V, Balis C (1983) Respirometry to determine optimum conditions for the biodegradation of extracted olive press-cake. Soil Biol Biochem 15:75–83
Mari I, Ehaliotis C, Kotsou M, Balis C, Georgakakis D (2003) Respiration profiles in monitoring the composting of by-products from the olive oil agro-industry. Bioresour Technol 87:331–336
McKinley VL, Vestal JR (1984) Biokinetic analyses of adaptation and succession: microbial activity in composting municipal sewage-sludge. Appl Environ Microbiol 47:933–941
Meyer-Reil LA, Köster M (1992) Microbial life in pelagic sediments: the impact of environmental parameters on enzymatic degradation of organic material. Mar Ecol, Prog Ser 81:65–72
Michel FC Jr, Reddy CA (1998) Effect of oxygenation level on yard trimmings composting rate, odor production, and compost quality in bench-scale reactors. Compost Sci Util 6:6–14
Michel FC Jr, Reddy CA, Forney LJ (1993) Yard waste composting: studies using different mixes of leaves and grass in a laboratory scale system. Compost Sci Util 1:85–96
Nakasaki K, Sasaki M, Shoda M, Kubota H (1985) Change in microbial numbers during thermophilic composting of sewage-sludge with reference to CO2 evolution rate. Appl Environ Microbiol 49:37–41
Nannipieri P, Kandeler E, Ruggiero P (2002) Ezyme activities and microbiological and biochemical processes in soil. In: Burns RG, Dick R (eds) Enzymes in the environment. Marcel Dekker, New York, pp 1–33
Nannipieri P, Ascher J, Ceccherini L, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670
Paredes C, Bernal MP, Cegarra J, Roig A (2002) Bio-degradation of olive mill wastewater sludge by its co-composting with agricultural wastes. Bioresour Technol 85:1–8
Ryckeboer J, Mergaert J, Coosemans J, Deprins K, Swings J (2003) Microbiological aspects of biowaste during composting in a monitored compost bin. J Appl Microbiol 94:127–137
Sakamoto K, Oba Y (1994) Effect of fungal to bacterial biomass ratio on the relationship between CO2 evolution and total soil microbial biomass. Biol Fertil Soils 17:39–44
Satyanarayana T, Getzin LW (1973) Properties of a stable cell-free esterase from soil. Biochemistry 12:1566–1572
Schnürer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 43:1256–1261
Shulze KL (1962) Continuous thermophilic composting. Appl Microbiol 10:108–122
Smith DC, Hughes JC (2004) Changes in maturity indicators during the degradation of organic wastes subjected to simple composting procedures. Biol Fertil Soils 39:280–286
Stentiford EI (1996) Composting control: principles and practice. In: de Bertoldi M, Sequi P, Lemmes B, Papi T (eds) The science of composting. Blackie Academic and Professional, Glasgow, Scotland, pp 49–59
Stotzky G (1965) Microbial respiration. In: Methods of soil analysis, Part 2. American Society of Agronomy Inc., Madison, WI, pp 1550–1572
Suler DJ, Finstein MS (1977) Effect of temperature, aeration, and moisture on CO2 formation in bench-scale, continuously thermophilic composting of solid waste. Appl Environ Microbiol 33:345–350
Swisher R, Carroll GC (1980) Fluorescein diacetate hydrolysis as an estimator of microbial biomass on coniferous needle surfaces. Microb Ecol 6:217–226
Tiquia SM, Tam NFY, Hodgkiss IJ (1996) Microbial activities during composting of spent pig-manure sawdust litter at different moisture contents. Bioresour Technol 55:201–206
Tiquia SM, Wan JHC, Tam NFY (2002) Microbial population dynamics and enzyme activities during composting. Compost Sci Util 10:150–161
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707
Vuorinen AH (1999) Phosphatases in horse and chicken manure composts. Compost Sci Util 7:47–54
Wardle DA, Ghani A (1995) A critique of the microbial metabolic quotient (qCO2) as a bioindicator of disturbance and ecosystem development. Soil Biol Biochem 27:1601–1610
Weppen P (2002) Determination of compost maturity: evaluation of analytical properties. Compost Sci Util 10:6–15
Wiley JS (1957) Progress report on high-rate composting studies. In: 12th Purdue Industrial Waste Conference Proceedings. Ann Arbor Press Inc., Chelsea, MI, pp 596–603
Wong JWC, Fang M (2000) Effects of lime addition on sewage sludge composting process. Water Res 34:3691–3698
Acknowledgements
This work was carried out in the frame of the RECOVEG E.U. project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ntougias, S., Ehaliotis, C., Papadopoulou, K.K. et al. Application of respiration and FDA hydrolysis measurements for estimating microbial activity during composting processes. Biol Fertil Soils 42, 330–337 (2006). https://doi.org/10.1007/s00374-005-0031-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-005-0031-z