Abstract
The symbiotic efficiency of coastal sand dune rhizobial isolates on four cultivated legumes, cowpea (Vigna unguiculata), green gram (Vigna radiata), black gram (Vigna mungo) and horse gram (Macrotyloma uniflorum), was assessed. Among the isolates of Someshwara (S1–S5), inoculation of S5 resulted in the highest increase of shoot biomass in cowpea (control vs experimental, 1:6), while inoculation of P1 among the Padubidri isolates (P1–P5) induced the highest shoot biomass in cowpea (1:14.4). Inoculation of the isolate P2 induced higher shoot biomass against uninoculated controls of horse gram (12.6:1), green gram (11.2:1) and black gram (6.1:1). One-way ANOVA revealed significant difference in the shoot biomass between uninoculated and inoculated cowpea plants with ten rhizobial isolates (P <0.05). Cultivation of surface-sterilized green gram seeds on unsterilized dune sand resulted in profuse flowering as well as nodules within 6 weeks indicating possibilities for isolating efficient rhizobial strains through cultivating edible legumes on coastal sand dune soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal sand dune (CSD) vegetation in the Tropics is dominated by plant species belonging to the families Asteraceae, Convolvulaceae, Leguminosae and Poaceae (Rao and Meher-Homji 1985; Moreno-Casasola and Espegel 1986; Mohankumar et al. 1988; Kulkarni et al. 1997; Moreno-Casasola 1998; Arun et al. 1999; Beena et al. 2001). These plants have adapted to the dune habitat (low nutrients, drought, high salinity and sand erosion/accretion) and facilitate dune stabilization (Rao and Meher-Homji 1985; Moreno-Casasola and Espegel 1986; Moreno-Casasola 1998). Plant species established on the CSDs of the west coast of India are known to harbor a variety of arbuscular mycorrhizal (AM) fungi (Mohankumar et al. 1988; Kulkarni et al. 1997; Beena et al. 1997, 2000a, 2000b, 2001). About 35 species of legumes have been recorded on the dunes of the Karnataka coast (Arun et al. 1999; Arun 2002). Several dune legumes showed profuse nodulation particularly during the post-monsoon season (Arun et al. 1999) and some of the legumes showed nitrogen fixation (Arun 2002). In addition to physiological and biochemical adaptations of the dune plants, symbiotic associations also provide additional strength to withstand the environmental perturbations of dunes and facilitate dune stabilization.
About one-third of the world’s irrigated land is salt affected (400–950×106 ha; Shannon et al. 1984), up to 40% of the world’s land surface shows potential salinity problems and a large segment of these areas are confined to tropical and Mediterranean regions (World Resources 1987). Native rhizobial strains are well adapted to stress conditions in association with legumes and help in the reclamation of marginal lands as they enrich soil through symbiotic nitrogen fixation (Alexander 1984). Grain legumes are often cultivated as alternate crops with rice in coastal locations in southwest India (George et al. 1988). Legumes in rotation with rice are also known to increase the soil organic matter and reduce pest problems. A literature search revealed no studies on the importance of wild rhizobia of CSDs. In view of the importance of wild rhizobia in agriculture, the present study aimed at testing the symbiotic performance of CSD wild rhizobia of selected dune legumes on commonly cultivated edible legumes along the coast of Karnataka, India.
Materials and methods
Rhizobia
Rhizobial isolates were obtained from wild legumes, which are common and abundant on the dunes of Someshwara and Padubidri, Karnataka coast, India (Table 1). The host plant species are subjected to low to moderate natural disturbance and human interference (Arun 2002). Rhizobial isolates were maintained on yeast extract mannitol (YEM) agar medium (mannitol, 10 g; yeast extract, 1 g; K2HPO4, 0.5 g; MgSO4 7H2O, 0.2 g; NaCl, 0.1 g; Congo Red, 0.025% (w/v), agar agar, 18 g; distilled water, 1,000 ml; pH 7.2; Hahn 1966) at a low temperature (8°C) in the laboratory. The isolates selected showed relatively wide tolerance to temperature, salinity and pH and some were efficient phosphate solubilizers (Table 1; Arun 2002). These isolates were assessed for their symbiotic efficiency and suitability as inoculants for cultivated edible legumes.
Legumes
The seeds of cowpea [Vigna unguiculata (L.) Walp.], black gram [Vigna mungo(L.) Hepper], green gram [Vigna radiata (L.) R.] and horse gram [Macrotyloma uniflorum (Lam.) Verdc.] were collected during May 2001 from the local farmers of Padubidri region. These legumes are commonly grown after the harvest of paddy along the coastal belts during late monsoon-early summer regimes (December–February). Seeds were surface-sterilized by sequential exposure to 96% ethanol (v/v; for 30 s) and 12% sodium hypochlorite (w/v; for 7 min) and rinsed in sterile distilled water until neutrality (Rodriguez-Navarro et al. 1999).
Symbiotic performance
Sand samples collected from Someshwara were steam sterilized (1.05 kg/cm2) for 1 h and allowed to cool. Each sterile earthen pot (diameter, 15 cm; depth, 20 cm) was filled with 5 kg sterile sand and kept closed until further use. Sterilized seeds were coated with carboxymethyl cellulose (4%), containing 1 ml 5-day-old rhizobial culture (approx. 108 cells/ml) in YEM broth. Seeds were then sown in pots containing sterile sand and allowed to grow under greenhouse conditions with a 12 h photoperiod at temperatures of 28/23±2°C (day/night). Plants were trimmed to four per pot after 3 days of emergence. After 3–4 days of emergence, 1 ml 5-day-old rhizobial suspension was used as second inoculum. Pots were watered daily and saturated once a week with N-free sterile nutrient solution (KH2PO4, 200 mg; MgSO4 7H2O, 200 mg; KCl, 200 mg; CaSO4 2H2O, 120 mg; Na2FeEDTA, 25 mg; Na2MoO4 2H2O, 4 mg; MnSO4 2H2O, 2 mg; CuSO4 5H2O, 2 mg; ZnSO4 7H2O, 3 mg; H3BO3, 18 mg and CoCl2 4H2O, 120 mg per liter distilled water; Rigaud and Puppo 1975). After 6 weeks, nodule numbers were enumerated, and shoot, root and nodule biomasses were determined on drying at 80°C for 48 h. Surface-sterilized green gram seeds were cultivated in unsterilized dune sand to test the performance.
Statistical analyses
One-way analysis of variance (ANOVA) and the Student t test (post-hoc analysis) were employed to assess the difference between treated and control plants in biomass of shoot, root, nodule and nodule numbers (Stat Soft 1995).
Results
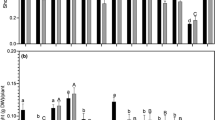
Variations in plant biomass, nodule biomass and nodule number was found between control and treated plants with different isolates of rhizobia. Among the isolates of Someshwara, S5 showed the highest increase of shoot biomass of cowpea (1:6; Fig. 1). Similarly, S4 in black gram (1:1.5), S1 in green gram (1:2.8) and S2 and S5 in horse gram (1:5.5 each) showed elevated shoot biomass. Among the Padubidri isolates, P1 induced the highest shoot biomass in cowpea (1:14.4) followed by P2 (1:7.5; Fig. 2). In the rest of the plants, isolate P2 performed well by inducing shoot biomass (black gram, 1:6.1; green gram 1:11.2; horse gram, 1:12.6). One-way ANOVA revealed significant difference in the shoot biomass of cowpea on inoculation of ten rhizobial isolates against the control (P <0.05). Post-hoc analysis also revealed significant increase in shoot biomass of cowpea on inoculation of isolates S1, S4, P1, P2, P4 and P5 compared to control plants (P <0.05). However, plants inoculated with isolate P1 showed the highest increase in shoot biomass (1:14.4). In almost all rhizobial treatments, shoot biomass as well as root biomass was increased. One-way ANOVA also revealed significant increase in root biomass (P <0.05), nodule biomass (P <0.01) and nodule number (P <0.01) of cowpea plants, as did post-hoc analysis of nodule biomass (P <0.01) and nodule number (P <0.01). The isolate P2 was the most successful isolate due to its ability to bring about significant increase in all plant parameters (shoot biomass, root biomass, nodule biomass and nodule number) of four plant species, except for shoot biomass in cowpea. On cultivation of surface-sterilized green gram seeds in unsterilized dune sand resulted in profuse flowering and nodule formation within 6 weeks.
Discussion
The present study was performed to assess the symbiotic efficiency of native CSD rhizobia of the Tropics since no such studies are known to date. Rhizobial isolates employed are fast-growing and well adapted to the CSD environment and showed optimum growth at elevated temperature, high salinity and acidic/alkaline pH (Table 1; Arun 2002). While screening the symbiotic performance of rhizobial strains of different geographical origin on Phaseolus vulgaris, local isolates of rhizobia showed higher nitrogen-fixing efficiency than the strains from the culture collections, indicating the importance of native rhizobia (Rodriguez-Navarro et al. 1999). Fast-growing rhizobial strains effectively nodulated Vigna unguiculata and showed significant increase over uninoculated control (Gandhi and Godbole 1990). The fast-growing rhizobial strains were also of great interest in generating sufficient amounts of rhizobial inoculum, having easy establishment in soils and easy manipulation of genes (Chatterjee et al. 1990; Buendía-Clavería et al. 1994; Cregan and Keyser 1998; Hungria et al. 2001). In legumes, salt stress at 50–200 mM NaCl significantly limits productivity by interfering with plant growth (Bekki et al. 1987; Delgado et al. 1993). Salinity reduces shoot and root weights in several legumes (Cordovilla et al. 1999); shoot growth was more affected by salt stress than root growth (Cordovilla et al. 1999). In our study, among the ten rhizobial isolates tested, isolates of Padubidri (P1–P5) performed better than the isolates of Someshwara (S1–S5), which can be linked to their efficient phosphate solubilization (phosphate solubilization index, 1.36–4.16; see Table 1). The salinity of the dune sand used for cultivation of legumes was about 188 μg/g. Overall performance of Padubidri isolates was encouraging at such saline conditions. Cultivation of green gram (Vigna radiata) on unsterilized dune soil picked up the native rhizobia and fixed atmospheric nitrogen. This shows the possibilities for evolving a suitable technique to isolate efficient strains of native CSD rhizobia by employing specific legumes as “bait” in unsterilized dune soils of different geographical origin.
Environmental stress affects the process of root hair colonization and nodulation by rhizobia (Alexander 1984). A suitable rhizosphere environment is important for interaction between rhizobia and root hairs (Cordovilla et al. 1999). Generally such conditions exist on the CSDs of Karnataka during the post-monsoon season and allow rhizobia to colonize most of the dune legumes (Arun 2002). Our study emphasizes the importance of screening native rhizobial isolates of CSDs, which are adapted to high temperature, salinity, pH and low moisture regimes as inoculants for cultivated legumes. In view of AM fungal colonization of the CSD legumes (Beena et al. 2001; which facilitates phosphate solubilization), it is worth screening the efficient strains of AM fungi compatible with dune rhizobia as suitable inoculants for cultivated legumes. Interestingly, of the legumes found on CSDs of the southwest coast of India, some are cultivated (e.g. Cajanus sp., Vigna spp.; Arun 2002). Vigna radiata and Vigna sp. grow on the mid-dunes and/or hind-dunes. This suggests that some cultivated legumes are also adapted to dune habitats and such plant species might be of great importance for the development of improved varieties. Being a major vegetable crop grown in the coastal belt of Karnataka, the genetic improvement and symbiotic performance with CSD rhizobia of cowpea (V. unguiculata) needs further exploration.
References
Alexander M (1984) Ecology of Rhizobium. In: Alexander M (ed) Biological nitrogen fixation: ecology, technology and physiology. Plenum, New York, pp 39–50
Arun AB (2002) Studies on coastal sand dune legumes of Karnataka (India). Ph.D. Thesis, Mangalore University, India
Arun AB, Beena KR, Raviraja NS, Sridhar KR (1999) Coastal sand dunes—a neglected ecosystem. Curr Sci 77:19–21
Beena KR, Raviraja NS, Sridhar KR (1997) Association of arbuscular mycorrhizal fungi with Launaea sarmentosa on maritime sand dunes of west coast of India. Kavaka 25:53–60
Beena KR, Raviraja NS, Sridhar KR (2000a) Seasonal variations of arbuscular mycorrhizal fungal association with Ipomoea pes-caprae of coastal sand dunes, Southern India. J Environ Biol 21:341–347
Beena KR, Raviraja NS, Arun AB, Sridhar KR (2000b) Diversity of arbuscular mycorrhizal fungi on the coastal sand dunes of west coast of India. Curr Sci 79:1459–1466
Beena KR, Arun AB, Raviraja NS, Sridhar KR (2001) Association of arbuscular mycorrhizal fungi with plants of coastal sand dunes of west coast of India. Trop Ecol 42:213–222
Bekki A, Trinchant JC, Rigaud J (1987) Nitrogen fixation (C2H4 reduction) by Medicago nodules and bacteroids under sodium chloride stress. Physiol Plant 71:61–67
Buendía-Clavería AM, Rodriguez-Navarro DN, Santamaria-Linaza C, Ruíz-Sasínz JE, Temprano-Vera F (1994) Evaluation of the symbiotic properties of Rhizobium fredii in European soils. Syst Appl Microbiol 17:155–160
Chatterjee A, Balatti PA, Gibbons W, Pueppke SG (1990) Interactions of Rhizobium fredii USDA 257 and nodulation mutants derived from it with the agronomically improved soybean cultivar McCall. Planta 180:303–311
Cordovilla MDP, Ligero F, Lluch C (1999) Effects of NaCl on growth and nitrogen fixation and assimilation of inoculated and KNO3 fertilized Vicia faba L. and Pisum sativum L. plants. Plant Sci 140:127–136
Cregan PB, Keyser HH (1998) Influence of Glycine spp. on competitiveness of Bradyrhizobium japonicum and Rhizobium fredii. Appl Environ Microbiol 54:803–808
Delgado MJ, Ligero F, Lluch C (1993) Effects of salt stress on growth and N2 fixation by pea, faba bean, common bean and soybean plants. Soil Biol Biochem 26:371–376
Edi PM, Moaward AM, Vlek PLG (1996) Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. J Crop Sci 11:13–23
Gaind S, Gaur AC (1991) Thermotolerant phosphate solubilizing microorganisms and their interaction with mungbean. Plant Soil 133:141–149
Gandhi MB, Godbole SH (1990) Effect of fast-growing rhizobia from wild legumes on Vigna unguiculata (L.) Walp. Indian J Exp Biol 28:438–440
George T, Buresh RJ, Ladha JK, Punzalan G (1988) Recycling (in situ) of legume fixed and soil nitrogen in tropical lowland rice. Agron J 90:429–437
Hahn NJ (1966) The congored reaction in bacteria and its usefulness in the identification of rhizobia. Can J Microbiol 12:725–733
Hungria M, Campo RJ, Chueire LML, Grange L, Megías M (2001) Symbiotic effectiveness of fast-growing rhizobial strains isolated from soybean nodules in Brazil. Biol Fertil Soil 33:387–394
Jensen V (1951) Notes on the biology of Azotobacter. Proc Soc Appl Bacteriol 74:89–93
Kulkarni SS, Raviraja NS, Sridhar KR (1997) Arbuscular mycorrhizal fungi of tropical sand dunes of west coast of India. J Coastal Res 13:931–936
Mohankumar V, Ragupathy S, Nirmala CB, Mahadevan A (1988) Distribution of vesicular-arbuscular mycorrhizae (VAM) in the sandy beach soils of Madras coast. Curr Sci 57:367–368
Moreno-Casasola P (1998) Patterns of plant species distribution on coastal dunes along the Gulf of Mexico. J Biogeogr 15:787–806
Moreno-Casasola P, Espegel I (1986) Classification and ordination of coastal sand dune vegetation along the Gulf and Caribbean Sea of Mexico. Vegetatio 66:147–182
Rao TA, Meher-Homji VM (1985) Strand plant communities of the Indian Subcontinent. Proc Indian Acad Sci (Plant Sci) 94:504–523
Rigaud J, Puppo A (1975) Indole-3-acetic acid catabolism by soybean bacteroids. J Gen Microbiol 88:223–228
Rodriguez-Navarro DN, Santamaria C, Temprano F, Leidi EO (1999) Interaction effects between Rhizobium strain and bean cultivar on nodulation, plant growth, biomass partitioning and xylem sap composition. Eur J Agron 11:131–143
Shannon M (1984) Breeding, selection and the genetics of salt tolerance. In: Staples RC, Toenniessen GH (eds) Salinity tolerance in plants. Wiley, New York, pp 300–308
Stat Soft (1995) STATISTICA for windows. Stat Soft, Tulsa, Okla.
World Resources (1987) An assessment of the resource base for the global economy. International institute for environment and development, World Resources Institute, Basic Books, New York
Acknowledgements
Authors are grateful to Mangalore University for permission to carry out this investigation. We thank Dr. NS Raviraja for statistical analysis and reviewers for constructive suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arun, A.B., Sridhar, K.R. Symbiotic performance of fast-growing rhizobia isolated from the coastal sand dune legumes of west coast of India. Biol Fertil Soils 40, 435–439 (2004). https://doi.org/10.1007/s00374-004-0800-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-004-0800-0