Abstract
This study evaluated the response of pea (Pisum sativum cv. Trapper) to arbuscular mycorrhizal fungi (AMF) and Rhizobium leguminosarum bv. viceae strains varying in their effectiveness on pea. Plants were inoculated with the AMF species Glomus clarum NT4 or G. mosseae NT6 and/or ten Rhizobium strains, and grown for 90 days in soil containing indigenous AMF and rhizobia. The effectiveness of the Rhizobium strains on the growth (P <0.046; r =0.64) and N nutrition (P <0.04; r =0.65) of 6-week-old pea grown under gnotobiotic conditions was correlated with the growth and N nutrition of 90-day-old pea grown in natural soil for all strains except LX48. The growth and yield response of pea to co-inoculation with AMF and Rhizobium strains depended on the particular AMF-Rhizobium strain combination. In some cases, the yield and N nutrition of pea inoculated with a superior Rhizobium strain was significantly (P <0.05) enhanced by an apparently compatible AMF species compared to the Rhizobium treatment. On the other hand, an apparently incompatible AMF species significantly (P <0.05) reduced the performance of an effective Rhizobium strain. In general, treatments with effective Rhizobium strains or co-inoculation treatments with effective Rhizobium strains and a compatible AMF species produced the best results. Changes in total shoot dry matter production was significantly (P <0.05) correlated with the total shoot N (P <0.0001; r =0.95) and P content (P <0.0001; r =0.87), indicating that this response was mediated by enhanced N and P nutrition. Growth, yield and nutrition of pea were not related to AMF colonization of roots. Our results suggest that careful co-selection of AMF species and Rhizobium strains can enhance pea yield and nutrition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Legumes form intimate associations with arbuscular mycorrhizal fungi (AMF) and rhizobia. These associations are referred to as tripartite symbioses. The significance of the tripartite association between AMF, rhizobia and legumes is twofold, the accrued plant benefits and the C drain on the host. In general, plant benefits include plant growth and yield increases, improved N and P nutrition, drought resistance, disease control and P solubilization. Various workers have assessed the influence of many AMF species on the growth of nodulated legumes (Ianson and Linderman 1993; Ibijbijen et al. 1996; Saxena et al. 1997). For example, Ianson and Linderman (1993) suggested that a specific interaction occurs between AMF and the Rhizobium strain which influences nodulation and AMF colonization of roots, but not host P nutrition. Saxena et al. (1997) reported that the nodulation and growth of Vigna radiata inoculated with a Bradyrhizobium sp. varied significantly depending upon the co-inoculated AMF species. Several workers have examined the interactions between different AMF species and Rhizobium spp. strains (Ames et al. 1991; Azcon et al. 1991; Ruiz-Lozano and Azcon 1993; Ahmad 1995; Redecker et al. 1997; Xavier and Germida 2002). The basis for selecting these Rhizobium strains was strain availability (Azcon et al. 1991), effectiveness on host (Ames et al. 1991), or not specified (Ruiz-Lozano and Azcon 1993; Ahmad 1995; Redecker et al. 1997). In all cases, growth and productivity of the legumes were dependent on the specific combination of AMF and rhizobia, indicating that synergistic interactions between compatible microsymbionts resulted in growth and yield increases.
Despite the reality that most if not all legume crops are inoculated with appropriate Rhizobium sp. strains and readily colonized by AMF in the field, factors that regulate this tripartite association are yet to be clearly defined. In a recent study (Xavier and Germida 2002) we found that the response of lentil (Lens culinaris L.) to two dominant AMF species isolated from Saskatchewan soils and nine R. leguminosarum bv. viceae strains isolated from field-grown lentil varied significantly depending on Rhizobium strain. Furthermore, we found that for lentil an incompatible AMF association could reduce the efficacy of an effective Rhizobium strain, and that a compatible AMF association could enhance the efficacy of an ineffective Rhizobium strain. Other studies examining tripartite relationships between legumes, AMF and rhizobia have not reported this phenomenon. It was unclear from our study whether this response is unique to lentil or similar in other legumes. To answer this question we hypothesized that the tripartite response noted in lentil to AMF and effective and ineffective Rhizobium strains would be similar in pea. Here, we assessed the interactions between the AMF species Glomus clarum and G. mosseae and R. leguminosarum bv. viceae strains varying in efficacy on pea, on the growth, yield and nutrient content of pea grown in soil containing indigenous AMF and rhizobia.
Materials and methods
AMF inocula
Monospecific cultures of the AMF species G. clarum Nicolson and Schenck (INVAM no. SA101) NT4 and G. mosseae (Nicolson and Gerdemann) Gerdemann & Trappe (INVAM no. SA103) NT6 which are dominant in Saskatchewan soils (Talukdar and Germida 1993), were produced in a (1:1) soil:sand mix using maize as host. The AMF strain G. clarum NT4 has been recently renamed (Kennedy et al. 1999), but the original nomenclature has been retained here for cross-reference to our published work. The AMF inocula consisting of spores, external mycelium and AMF-colonized roots were stored at 4°C. The G. clarum NT4 inoculum contained 330 propagules per 50 g, whereas the G. mosseae NT6 inoculum contained 390 propagules per 50 g, as determined using the "Most probable number" assay of Porter (1979).
R. leguminosarum bv. viceae strains
The Rhizobium strains and isolates used in this study are listed in Table 1. The Rhizobium isolates LX1, LX43, LX48 and LX57 enhanced the growth and N content of 6-week-old pea under gnotobiotic conditions, whereas isolates LX13, LX36, LX51 and strain 175P4 were ineffective on pea. The Rhizobium cultures were prepared by inoculating a loopful of cells from a stock culture into 100 ml YEM broth and growing on a gyrotory shaker (150 rev min-1) at 28°C for 72 h. The Rhizobium cultures contained around 108 colony forming units per ml (cfu ml-1).
Soil
The loamy sand soil used in this study was collected from Bradwell, Saskatchewan. The soil was air-dried, passed through a 4-mm sieve, and the nutrient content determined at the Enviro-Test Laboratories, Saskatoon, Saskatchewan, using the following chemical extractants: N and S, 0.001 M CaCl2; P and K, 0.25 N ammonium acetate, 0.015 N ammonium fluoride and 0.25 N acetic acid; micronutrients (except B), 0.005 M EDTA, 0.01 M CaCl2 and 0.1 M triethylamine; B, 0.01 M CaCl2, organic matter, concentrated H2SO4, 0.4 N potassium dichromate; pH and conductivity, 1:2 soil:water slurry. Select nutrient characteristics of the soil were as follows (µg g-1): N 10; P 13; K 300; S 9.4; B 0.72; Cu 0.56; Fe 11.2; Mn 9.6; Zn 3.18; pH 8.1; EC 0.2 mS/cm, organic C 1.1%; organic matter 1.9%. The soil was mixed 1:1 (w/w) with silica sand and 2 kg potted into 15-cm diameter pots. About 200 ml distilled water were added to each pot and the soil:sand mixture was thoroughly mixed. The soil:sand mix was allowed to equilibrate for 10 days before planting. The number of indigenous rhizobia and AMF propagules in the soil:sand mix were 5.5 per g and 170 per 50 g, respectively, as determined using the most probable number technique of Somasegaran and Hoben (1994) for rhizobia and Porter (1979) for AMF.
Inoculation and plant growth
For all the treatments, four pea seeds (cv. Trapper) were planted at 5-cm depth from the soil surface. For the Rhizobium treatments, 2.0 ml Rhizobium culture were added over the seeds. For the AMF treatments, 10 g appropriate AMF inoculum (i.e., NT4 or NT6) was placed at 5-cm depth over which pea seeds were placed. For the AMF + Rhizobium treatments, 10 g AMF inoculum were placed at 5-cm depth over which pea seeds were planted, and 2.0 ml appropriate Rhizobium culture was added. After seedling emergence, plants were thinned to two per pot. Autoclaved polypropylene beads were applied on the soil surface to prevent cross contamination and top soil drying. Plants were grown in a growth chamber with the following conditions: 25°C, 16 h day and 20°C, 8 h night; 375–400 µm m-2 sec-1 of irradiance and around 60% relative humidity. Soil was maintained at around 60% moisture holding capacity, and pots randomized and repositioned once a week.
Parameters
Plants were grown for 90 days and harvested. The above-ground plant material was separated into shoot and seed, dried (65°C, 48 h) and weighed. Roots were washed thoroughly in running tap water, dried (65°C, 48 h) and weighed. The %AMF root colonization was determined using a modified gridline intersect method (Giovanetti and Mosse 1980). The N and P concentration of the shoot and seed material was determined using a mixture of H2SO4-H2O2 (Thomas et al. 1967). The P use efficiency was expressed as grams shoot or grain dry weight per gram P absorbed.
Statistics
Percentage values were arcsine-transformed before statistical analysis. The general linear models procedure and least significant test in SAS were used for data analysis. Correlation coefficients were obtained using Pearson's correlation analysis in SAS (SAS Institute 1997). Unless indicated otherwise, all treatment means were tested for significant differences at P <0.05.
Results
Dry matter production
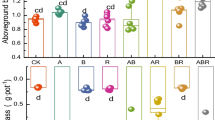
The shoot dry weight of pea was not affected by G. clarum NT4 compared to the control, and was significantly lower than that of G. mosseae NT6-inoculated plants, irrespective of the Rhizobium strain, i.e., AMF main effect (Tables 2, 6). The effect of the Rhizobium sp. strains on the shoot growth of plants varied depending upon the strain, irrespective of the AMF species, i.e., Rhizobium main effect (Tables 2, 6). For example, the Rhizobium strains 175P4 and LX48 were significantly less effective at stimulating shoot growth compared to other inoculants such as LX43 and LX57. The efficacy of most Rhizobium strains under gnotobiotic conditions (Table 1) was similar to that in soil, except for strain LX48. The effectiveness of the Rhizobium strains on the shoot dry weight (P <0.046; r =0.64) and N nutrition (P <0.04; r =0.65) of 6-week-old pea grown under gnotobiotic conditions was correlated with the growth and N nutrition of 90-day-old pea grown in natural soil for all strains except LX48. Co-inoculation of pea with NT4 or NT6 resulted in significantly different effects on the shoot growth of plants inoculated with the same Rhizobium strain for half the number of Rhizobium treatments (Table 2). For example, the shoot growth of pea inoculated with the NT4 + PB101 combination was significantly greater than the NT6 + PB101 combination. In contrast, the NT6 + LX57 combination increased the shoot growth of pea by 30% relative to the NT4 + LX57 combination, indicating that interactions between pea, the Rhizobium strains and the AMF species were specific.
Irrespective of the Rhizobium strain used, the total root dry weight of plants inoculated with NT4 or NT6 was not significantly different from each other (Tables 2, 6). Most of the Rhizobium treatments except 175P4 significantly increased the total root dry weight of plants relative to the control, irrespective of the AMF species (Tables 2, 6). Co-inoculation of pea with AMF and rhizobia significantly increased (NT4 + LX51, NT6 + LX51), decreased (e.g., NT6 + LX43, NT6 + LX1), or had no effect on the total root dry weight of pea inoculated with the same Rhizobium strain (Table 2).
The effect of the NT4 inoculant on the seed yield of pea was inferior to the native AMF and NT6 (Tables 3, 6). Inoculation of pea with the Rhizobium strains significantly altered the yield of pea plants, and regardless of the AMF species, most inoculants (7 of 10 Rhizobium strains) increased grain yield relative to the uninoculated control (Tables 3, 6). The AMF species had very different effects on the yield response of pea to inoculation with the same Rhizobium strain (Table 3). For example, the NT4 + LX43 combination resulted in higher yields than the NT6 + LX43 combination (Table 3). This yield increase was around 116% higher than the control plants, 34% higher than plants inoculated with LX43, and 48% higher than the NT6 + LX43 combination. In contrast, the NT6 + 175P4 combination produced a higher grain yield than the NT4 + 175P4 combination. However, the yield of plants inoculated with NT6 + LX43 was around 43% higher than the NT6 + 175P4 combination, indicating that although AMF inoculation increased the yield of an inferior Rhizobium-inoculated pea, the yield was higher for pea co-inoculated with a superior Rhizobium strain.
Regardless of the Rhizobium strain, there were no significant differences between the proportion of dry matter allocated to seed or harvest index (HI) in any of the AMF treatments (Tables 3, 6). However, there were significant differences between the Rhizobium strains, irrespective of the AMF species used (Tables 3, 6). For example, the HI of pea plants inoculated with LX1 or LX43 was significantly higher than that of plants inoculated with 175P4. Co-inoculation with the AMF species NT4 or NT6 caused significant changes in the HI for plants inoculated with the same Rhizobium strain (e.g., 175P4, LX43 and LX51; Table 3). It appeared that the effectiveness of the Rhizobium strain had no impact on the HI of the co-inoculated plants. It was noteworthy that the NT4 + LX43 combination significantly increased the HI by around 28% compared to the NT6 + LX43 combination and by around 17% compared to LX43. The HI was dependent on the specific combination of AMF and Rhizobium strain, and not on the efficacy of either microsymbiont, as noted for other parameters. A significant positive relationship was noted between HI and total shoot dry weight (P <0.0003; r =0.59), total shoot N (P <0.0001; r =0.64) and total shoot P (P <0.0011; r =0.54).
Nutrient parameters
Statistical analyses revealed that, irrespective of the Rhizobium strain, pea inoculated with G. mosseae NT6 had significantly higher total aboveground dry matter N compared with plants inoculated with G. clarum NT4 (Tables 4, 6). Regardless of the AMF treatment, pea inoculated with most of the effective Rhizobium strains, except LX48, had significantly higher levels of total shoot N compared to the control plants or those inoculated with an inferior Rhizobium strain (Tables 4, 6). For the same Rhizobium strain, the shoot N content of pea co-inoculated with the two AMF species was significantly different in 7 of the 11 Rhizobium treatments, indicating a specific interaction. For example, the total shoot N of pea co-inoculated with the AMF G. clarum NT4 and the Rhizobium strain LX43 was 24% greater than pea co-inoculated with G. mosseae NT6 and LX43, and 43% greater than pea inoculated with only LX43 (Table 4). Furthermore, a significant positive correlation was noted between total shoot dry weight and total shoot N (P <0.0001; r =0.95).
Regardless of the Rhizobium strain in combination, the AMF treatments had a similar effect on total shoot P as that of total shoot N (Tables 4, 6). Similarly, regardless of the AMF treatment, the effect of the various Rhizobium treatments on total shoot P content was similar to that noted for total shoot N (Tables 4, 6). The P response of pea to co-inoculation with AMF and Rhizobium strain was not as pronounced as that noted for total shoot N content (Table 4). For example, pea co-inoculated with NT4 and PB101 had a 24% greater level of shoot P than plants inoculated with PB101 alone and 33% more total shoot P than pea co-inoculated with the NT6 + PB101 combination. A significant positive relationship was noted between total shoot dry weight and total shoot P (P <0.0001; r =0.87), similar to that noted for total shoot N.
AMF colonization
Irrespective of the Rhizobium strain, inoculation of pea with NT4 significantly increased the AMF colonization levels compared to NT6 and the control (Tables 5, 6). Inoculation of pea with some Rhizobium strains enhanced (e.g., LX43) or restricted (e.g., RGP2, LX48) AMF colonization of roots relative to plants not inoculated with rhizobia, irrespective of the AMF species (Tables 5, 6). The AMF inoculants NT4 and NT6 had different effects on the AMF colonization of plants inoculated with the same Rhizobium strain (Table 5). For example, NT4 increased the mycorrhizal colonization of pea inoculated with RGP2 and LX43, but reduced that of 175P4. None of the measured plant parameters were correlated with the AMF colonization of pea roots.
Discussion
Studies examining the link between the nod and myc symbioses show that a high level of similarity exists between both symbioses (Duc et al. 1989; Gianinazzi-Pearson 1996). Recently, Harrison (2000), using pea and alfalfa mutants blocked in the Nod signaling pathway, showed that the processes of nodulation and mycorrhizae formation do not occur in these mutants, indicating that the Nod signaling pathway is shared in both processes. Due to the intimacy of the relationship between the AMF and rhizobia, the nature of AMF-Rhizobium interactions can determine the efficacy of the tripartite symbiosis in terms of enhancing plant yield. Therefore, depending upon the AMF-Rhizobium interaction, the response of a legume host to a given set of AMF-Rhizobium sp. partners may or may not be favorable for plant growth depending on the interaction of the symbionts. This study demonstrated that there were synergistic interactions between compatible AMF species and rhizobia which were manifested as growth, yield and tissue N and P content increases. However, these growth and yield increases were not restricted to the effective AMF species or rhizobia.
Many workers have shown that the growth and yield of legumes are influenced by interactions between AMF species and rhizobia (Ames et al. 1991; Azcon et al. 1991; Vejsadova et al. 1992; Ianson and Linderman 1993; Ruiz-Lozano and Azcon 1993; Ahmad 1995; Redecker et al. 1997; Xavier and Germida 2002). Notably, Azcon et al. (1991) found that alfalfa growth was enhanced by the specific combination of Glomus and Rhizobium meliloti strains. Similarly, in comparing the effects of interactions between G. pallidum, G. aggregatum and Sclerocystis microcarpa and four R. phaseoli strains on the growth and yield of three kidney bean cultivars, Ahmad (1995) found that the symbiotic efficiency was "dependent on the particular combination" of the AMF species, Rhizobium strain and even the host cultivar. The growth, yield and nutrition of pea were dependent on the specific combination of AMF species and Rhizobium strain, also reported by Azcon et al. (1991) and Ahmad (1995) for alfalfa and kidney bean. However, in contrast to other studies, wherein only effective rhizobia were included, ineffective Rhizobium strains were also included in order to appreciate the role of rhizobia in the tripartite symbiosis. It was noted that the efficacy of a superior Rhizobium isolate such as LX43 was dramatically enhanced in terms of yield, HI, and N and P content when co-inoculated with an apparently compatible AMF species such as NT4. Furthermore, the yield of pea inoculated with a less effective Rhizobium strain was enhanced when combined with an apparently compatible AMF species such as NT6. However, this enhanced productivity was still significantly lower than that of an effective Rhizobium or AMF-Rhizobium combination. We noted a similar response in lentil co-inoculated with effective and ineffective Rhizobium strains and AMF (Xavier and Germida 2002).
The role of AMF in tripartite symbiosis as a mechanism for supplying the crucial levels of P may be important in soils with a low available P content as N fixation is restricted by an inadequate P supply. The growth and yield increase of legumes inoculated with AMF and rhizobia is generally due to enhanced N and/or P uptake (Manjunath et al. 1984; Pacovsky et al. 1986; Azcon et al. 1991; Ruiz-Lozano and Azcon 1993; Xavier and Germida 2002). The yield increase observed for pea was associated with increased total shoot N and P content, and therefore suggests that the yield increase noted in compatible AMF + rhizobia treatments was probably due to enhanced N and P uptake.
Correlation analyses revealed no significant relationships between the AMF colonization levels and yield or nutrient levels. Many reports suggest that AMF colonization may not be related to the ability of the fungi to absorb and translocate nutrients to the host (Azcon et al. 1991; Ruiz-Lozano and Azcon 1993). It is possible that the enhanced nutrient uptake by pea inoculated with some co-inoculation treatments was mediated by the activity of the external mycelium, the P absorbing organ of the AMF (Jakobsen et al. 1992), and therefore not directly related to the colonization of roots by AMF.
Xavier and Germida (2002) found that for lentil an incompatible AMF association could reduce the efficacy of an effective Rhizobium strain, and that a compatible AMF association could enhance the efficacy of an ineffective Rhizobium strain. This study confirms our hypothesis that this phenomenon occurs in other legumes. Nevertheless, there were subtle differences between the two legumes in terms of their response to symbionts. Most important was the fact that the magnitude of growth and yield response to co-inoculation with a compatible AMF-rhizobia association was much higher for lentil than pea.
This study demonstrated that by carefully co-selecting for AMF and rhizobia which are compatible with each other and with the host plant, the growth, yield and nutrition of the legume host can be dramatically enhanced even in non-sterile soil containing indigenous AMF and rhizobia. In addition, co-inoculation with one AMF species significantly increased plant productivity over the other for the same Rhizobium strain or isolate, irrespective of its effectiveness on the host. The application of this co-inoculation strategy for agriculture still appears to be limited due to the non-culturable nature of AMF. However, the applicability of this research strategy for legumes in various other situations, such as in phytoremediation (Wiltse et al. 1998), revegetation of disturbed land (Requena et al. 2001), and forestry (Stamford et al. 1997), is high.
References
Ahmad MH (1995) Compatibility and co-selection of vesicular-arbuscular mycorrhizal fungi and rhizobia for tropical legumes. Crit Rev Biotech 15:229–239
Ames RN, Thiagarajan TR, Ahmad MH, McLaughlin WA (1991) Co-selection of compatible rhizobia and vesicular-arbuscular mycorrhizal fungi for cowpea in sterilized and non-sterilized soils. Biol Fertil Soils 12:112–116
Azcon R, Rubio R, Barea JM (1991) Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New Phytol 117:399–404
Duc G, Trouvelot A, Gianinazzi-Pearson V, Gianinazzi S (1989) First report of non-mycorrhizal plant mutants (Myc-) obtained in pea (Pisum sativum L.) and faba bean (Vicia faba L.). Plant Sci 60:215–222
Gianinazzi-Pearson V (1996) Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. Plant Cell 8:1871–1883
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Harrison MJ (2000) Molecular genetics of model legumes. Trends Plant Sci 5:414–415
Ianson DC, Linderman RG (1993) Variation in the response of nodulating pigeonpea (Cajanus cajan) to different isolates of mycorrhizal fungi. Symbiosis 15:105–119
Ibijbijen J, Urquiaga S, Ismail M, Alves BJR, Boddey RM (1996) Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition and nitrogen fixation of three varieties of common beans (Phaseolus vulgaris). New Phytol 134:353–360
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. I. Spread of hyphae and phosphorus inflow into roots. New Phytol 120:371–380
Kennedy LJ, Stutz JC, Morton JB (1999) Glomus eburneum and G. luteum, two new species of arbuscular mycorrhizal fungi, with emendation of G. spurcum. Mycologia 91:1083–1093
Leonard LT (1943) A simple assembly for use in the testing of cultures of Rhizobium. J Bacteriol 45:523–527
Manjunath A, Bagyaraj DJ, Gowda HSG (1984) Dual inoculation with VA mycorrhiza and Rhizobium is beneficial to Leucaena. Plant Soil 78:445–448
Pacovsky RS, Fuller G, Stafford AE (1986) Nutrient and growth interactions in soybeans colonized with Glomus fasciculatum and Rhizobium japonicum. Plant Soil 92: 37–45
Porter WM (1979) The 'Most Probable Number' method for enumerating infective propagules of vesicular-arbuscular mycorrhizal fungi in soil. Aust J Soil Res 17:515–519
Redecker D, von Berswordt-Wallrabe P, Beck DP, Werner D (1997) Influence of inoculation with arbuscular mycorrhizal fungi on stable isotopes of nitrogen in Phaseolus vulgaris. Biol Fertil Soils 24:344–346
Requena N, Perez-Solis E, Azcon-Aguilar C, Jeffries P, Barea JM (2001) Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Appl Environ Microbiol 67:495–498
Ruiz-Lozano JM, Azcon R (1993) Specificity and functional compatibility of VA mycorrhizal endophytes in association with Bradyrhizobium strains in Cicer arietinum. Symbiosis 15: 217–226
SAS Institute (1997) SAS procedures guide, version 6, 3rd edn. SAS, Cary, N.C.
Saxena AK, Rathi SK, Tilak KVBR (1997) Differential effect of various endomycorrhizal fungi on nodulating ability of green gram by Bradyrhizobium sp. (Vigna) strain S24. Biol Fertil Soils 24:175–178
Somasegaran P, Hoben HJ (1994) The handbook for Rhizobia. Methods in legume-Rhizobium technology. Springer, New York Berlin Heidelberg
Stamford NP, Ortega AD, Temprano F, Santos DR (1997) Effects of phosphorus fertilization and inoculation of Bradyrhizobium and mycorrhizal fungi on growth of Mimosa caesalpiniaefolia in an acid soil. Soil Biol Biochem 29:959–964
Talukdar NC, Germida JJ (1993) Occurrence and isolation of vesicular-arbuscular mycorrhizal fungi in cropped field soils of Saskatchewan, Canada. Can J Microbiol 39:567–575
Thomas RL, Sheard RW, Moyer, JR (1967) Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agron J 59:240–243
Vejsadova H, Siblikova D, Hrselova H, Vancura V (1992) Effect of the VAM fungus Glomus sp. on the growth and yield of soybean inoculated with Bradyrhizobium japonicum. Plant Soil 140:121–125
Wiltse CC, Rooney WL, Chen Z, Schwab AP, Banks MKT (1998) Greenhouse evaluation of agronomic and crude oil-phytoremediation potential among alfalfa genotypes. J Environ Qual 27:169–173
Xavier LJC, Germida JJ (2002) Response of lentil to co-inoculation with arbuscular mycorrhizal fungi and rhizobia varying in efficacy. Soil Biol Biochem 34:181–188
Acknowledgements
This study was supported by Natural Sciences and Engineering Research Council of Canada, Western Grains Research Foundation and Agriculture Development Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xavier, L.J.C., Germida, J.J. Selective interactions between arbuscular mycorrhizal fungi and Rhizobium leguminosarum bv. viceae enhance pea yield and nutrition. Biol Fertil Soils 37, 261–267 (2003). https://doi.org/10.1007/s00374-003-0605-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-003-0605-6