Abstract
In one series of experiments, heart frequency (f H), blood pressure (P a), gill ventilation frequency (f R ), ventilation amplitude (V AMP) and total gill ventilation (V TOT) were measured in intact jeju (Hoplerythrinus unitaeniatus) and jeju with progressive denervation of the branchial branches of cranial nerves IX (glossopharyngeal) and X (vagus) without access to air. When these fish were submitted to graded hypoxia (water PO2 ~140, normoxia to 17 mmHg, severe hypoxia), they increased f R , V AMP, V TOT and P a and decreased f H. In a second series of experiments, air-breathing frequency (f RA), measured in fish with access to the surface, increased with graded hypoxia. In both series, bilateral denervation of all gill arches eliminated the responses to graded hypoxia. Based on the effects of internal (caudal vein, 150 μg NaCN in 0.2 mL saline) and external (buccal) injections of NaCN (500 μg NaCN in 1.0 mL water) on f R , V AMP, V TOT, P a and f H we conclude that the O2 receptors involved in eliciting changes in gill ventilation and associated cardiovascular responses are present on all gill arches and monitor the O2 levels of both inspired water and blood perfusing the gills. We also conclude that air breathing arises solely from stimulation of branchial chemoreceptors and support the hypothesis that internal hypoxaemia is the primary drive to air breathing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During periods of drought in tropical regions fish can be confined for weeks or even months to hypoxic and hypercarbic waters in marginal lakes. Aerial respiration is a common adaptation in fish species inhabiting these waters (Dehadrai and Tripathi 1976; Kramer et al. 1978; Glass et al. 1986; Graham 1997). The survival of these fish depends on, amongst other things, their ability to sense O2 change and to quickly make metabolic, cardiovascular and ventilatory adjustments that match oxygen supply and oxygen demand (Fritsche and Nilsson 1993). This, in turn depends on the central interaction of a variety of sensory inputs, including inputs from chemoreceptors that monitor internal (blood) and external (water) gas tensions and acid–base balance (Milsom 1997; Perry and Gilmour 2002).
The primary sites of peripheral O2 sensing in fish appear to be the gills (including the pseudobranch in those species that posses one) and oro-branchial cavity (Laurent and Rouzeau 1972; Randall and Jones 1973; Butler et al. 1977; Daxboeck and Holeton 1978; Smith and Davies 1984; Smatresk et al. 1986; Burleson and Smatresk 1990b; McKenzie et al. 1991; Burleson and Milsom 1993; Sundin et al. 1999, 2000; Milsom et al. 2002). Chemoreceptors in the oro-branchial cavity are innervated by branches of the fifth (trigeminal) and/or seventh (facial) cranial nerves, those on the pseudobranch by the seventh cranial nerve, and those on the gill arches by branches of the ninth (glossopharyngeal) and/or tenth (vagus) cranial nerves, (see Reid et al. 2005 for review). Some of these chemoreceptors respond only, or preferentially, to changes in external (water) O2, others respond only, or preferentially, to changes in internal (blood) O2, and some respond to both (Milsom and Brill 1986; Burleson and Milsom 1993). Histological and neurophysiological evidence suggest that O2 chemoreception in the gills, at least, may arise from neuro-epithelial cells containing vesicular serotonin (Jonz and Nurse 2003; Jonz et al. 2004; Coolidge et al. 2008).
The location (gills or oro-branchial cavity), distribution (which gill arches), and stimulus modality (external or internal sensing) of the receptors involved in reflex changes in each of the different components of the cardiorespiratory response (breathing frequency, breath amplitude, heart rate, systemic vascular resistance) varies between species of water breathing fish (see Milsom et al. 1999; Perry and Gilmour 2002; Reid et al. 2005; Coolidge et al. 2008 for reviews) and there is as yet insufficient data to determine to what extent this variability is attributable to differences in lifestyle (active versus sluggish fish) and habitat (hypoxia tolerant versus intolerant fish) (Milsom et al. 1999; Perry and Gilmour 2002; Reid et al. 2005; Coolidge et al. 2008). There is even lesser data concerning the source of the drives for gill versus air breathing in facultative air-breathing fish and the cardiovascular adjustments that accompany them.
Most facultative air breathers shift the emphasis from gill ventilation to air breathing as temperature increases or aquatic O2 levels fall (Johansen et al. 1970; Singh 1976; Randall et al. 1981; Smatresk and Cameron 1982; Smatresk 1986; Shelton et al. 1986; McKenzie et al. 1991). Once air breathing is initiated, gill ventilation usually decreases (Johansen et al. 1970; Smatresk and Cameron 1982; Graham 1997). Bradycardia, which is a hallmark of hypoxia in most fish, gives way to a ventilation tachycardia during air breathing, often accompanied by increases in cardiac output and perfusion of the air-breathing organ (Johansen 1966 Axelsson et al. 1989; Skals et al. 2006; McKenzie et al. 2007). In gar these responses are eliminated by complete branchial denervation (gills and pseudobranch (Smatresk 1988) while in the bowfin, complete branchial denervation eliminates air breathing, but does not completely eliminate the gill ventilation response (McKenzie et al. 1991).
NaCN injections, which mimic local hypoxia, have been used to elucidate the relative roles of externally versus internally sensing chemoreceptors in these responses. In the African lungfish (Protopterus) (Johansen and Lenfant 1968) and the bowfin (Amia) (McKenzie et al. 1991), both internal and external injections of nicotine or NaCN stimulated gill breathing, while in the gar (Lepisosteous), only internal injections of NaCN stimulated gill breathing (Smatresk 1986). In the African lungfish, and the gar, both internal and external nicotine or NaCN injections stimulated lung breathing (Johansen and Lenfant 1968; Smatresk 1986) while in the bowfin neither injection had any effect on air breathing (McKenzie et al. 1991). In no instance did stimulation of either group of receptors in this manner produce a decrease in gill ventilation.
Trying to reconcile these results with those arising from prolonged exposure to hypoxia is not easy. The NaCN and nicotine injections produce brief transient responses from stimulation of a single receptor site, while the exposure to hypoxia produces simultaneous stimulation at both sites and the air breathing that ensues most likely reduces the strength of the stimulus at the internal receptors. Thus, hypoxia produces air breathing in Amia but NaCN injections do not and aquatic hypoxia decreases gill breathing in gar (regardless of internal levels of PO2) while internal NaCN stimulates and external NaCN has no effect on gill breathing. This has led several authors to conclude that differences in the effects of various stimuli in different studies resulted primarily from differences in central integration of peripheral chemosensory information and the reliance of the species on air breathing. In the case of Amia it was concluded that stimulation of both sets of receptors was probably necessary to elicit air breathing (McKenzie et al. 1991). In the case of the gar it led to the conclusion that the internal receptors set the level of hypoxic drive (i.e., stimulate both gill and air breathing) while the external receptors shift the emphasis from water to air breathing; that is it inhibits gill breathing (but only if there is internal hypoxia) and stimulates air breathing further (Smatresk et al. 1986).
Based on this background, the aim of the present study was to elucidate the location, distribution and orientation of the O2-chemoreceptors involved in the respiratory [both gill (water) and ABO (air) breathing] and heart rate responses to hypoxia in jeju and to compare these to the discrepant responses found in bowfin and gar. We particularly wanted to test the hypothesis that the effect of stimulation of externally oriented receptors in the gills could vary as a function of the level of stimulation of the internally oriented receptors with respect to the balance between gill breathing and air breathing (Smatresk et al. 1986).
For this study, we chose the facultative air-breathing jeju, Hoplerythrinus unitaeniatus (Stevens and Holeton 1978; Kramer et al. 1978). Jeju is an active, predaceous fresh water fish distributed throughout South America. This species is normally found in streams and shallow waters in tropical and sub-tropical areas where it frequently encounters periods of environmental hypoxia. When waters become severely hypoxic this species begins to air breath using its swim bladder as an air-breathing organ (ABO) (Carter and Beadle 1931; Kramer 1988; Stevens and Holeton 1978). This species maintains its aquatic O2 uptake (\( \dot{V}{\text{O}}_{ 2} \)) constant down to a critical PO2 (PcO2) of 40 mmHg, below which \( \dot{V}{\text{O}}_{ 2} \) declines linearly with further reductions of PiO2 (Oliveira et al. 2004). Just below PcO2, ventilatory tidal volume (V T), gill ventilation (\( \dot{V}_{\text{G}} \)) and arterial blood pressure (P a) increase significantly while heart rate falls. While respiratory frequency changes little, the water convection requirement (\( \dot{V}_{\text{G}} \)/\( \dot{V}{\text{O}}_{ 2} \)) increases steeply. Aerial respiration is initiated once water PO2 falls below 44 mmHg and breathing episodes and time at the surface increase linearly with more severe hypoxia. At the lowest water PO2 (20 mmHg) examined, the time spent at the surface accounted for 50% of total time (Oliveira et al. 2004). Following each air breath, there is a tachycardia, and an increase in air-breathing organ perfusion, most likely due to preferential perfusion of gill arches 3 and 4 that supply the coeliac artery which perfuses the air-breathing organ (Farrell 1977; McKenzie et al. 2007).
Materials and methods
Experimental animals
Adult jeju, H. unitaeniatus, weighing 184 ± 1 g (mean ± SEM) were collected in the Paraná River Basin, near Bataguaçu, Mato Grosso do Sul, Brazil. In the laboratory, fish were maintained in 1,000-L holding tanks supplied with a continuous flow of dechlorinated and aerated water [normoxic conditions, PwO2 ≥ 130 mmHg (17.29 kPa)] at a constant temperature (25°C). Fish were fed with live food (smaller fish of various species) ad libitum. Food, however, was withheld for 2–3 days before trials.
Animal preparation
For surgical procedures fish were initially anesthetized by immersion in a solution of benzocaine (0.1 g L−1). They were then transferred to a surgical table where they were held in air with their gills irrigated with a second solution of benzocaine (0.05 g L−1) that was constantly aerated to maintain adequate levels of O2.
In series one, two polyethylene cannulae (PE 100 and PE 60) were introduced into the buccal cavity through a hole in the dorsal palate. One was used to record buccal pressure to determine ventilatory frequency (f R ) and amplitude (V AMP). The other was used for intrabuccal administration of water and NaCN. In series two and three, only one buccal catheter was used for both functions. The caudal vein and artery were also cannulated (PE 50) (Axelsson and Fritsche 1994) for intravenous injections of saline and NaCN, and to record arterial pressure (P a) and heart rate (f H) in series one and two. In series three only the caudal vein was cannulated. The intrabuccal and intravenous injections of NaCN were used to selectively stimulate external and internal O2 chemoreceptors, respectively.
Denervation of cranial nerves IX (glossopharyngeal) and X (vagus)
Fish were anesthetized and placed on a surgical table where they were artificially ventilated as described above. The denervation followed the same protocol described by Sundin et al. (2000). Under a stereoscopic microscope (Opto SM 2001, Opto Electronics, São Carlos, SP, Brazil), the operculum was reflected forward, and a small incision (2 cm) was made in the epithelium above the first and second gill arches where they meet the roof of the opercular cavity. The incision allowed access to cranial nerve IX and the branchial branches of cranial nerve X. The branchial nerves of all gill arches were carefully dissected free of connective tissue and cut with fine iris scissors to make up four experimental groups: the Intact group (I), consisted of non-operated animals (n = 7) and sham-operated animals (n = 3) that had their nerves exposed but not sectioned. The latter were included in the intact group since they produced responses that were no different from those of non-operated animals. The IX group (n = 8) consisted of animals having the branchial branch of the IXth cranial nerve sectioned. The G1 group (n = 10) consisted of animals having the branchial branch of the IXth and the first branch of the Xth cranial nerve sectioned. In this group the first gill arch was isolated from the central nervous system. Finally, the G4 group (n = 10) consisted of animals having the branchial branch of the IXth and the branchial branches of the Xth nerves, sectioned. The visceral and cardiac branches of the vagus were kept intact. The healing process in jeju was rapid, and the incisions were covered with “scar tissue” within about 24 h. This species does not have a pseudobranch. All denervations were confirmed “postmortem”.
Experimental protocol
Series one
After recovering from the surgery, animals (N = 38) were placed in individual plastic tubes with mesh at both ends and transferred to dark plastic chambers covered with lids where they remained undisturbed for a minimum of 24 h before beginning the experimental protocol. The water level in the larger chambers was set such that the plastic tubes were totally submerged and thus fish had no access to air. The cannulae were then connected to pressure transducers to record buccal (P buccal) and arterial (P a) pressures and to evaluate f R , V AMP, P a and f H, respectively. The pressure transducers were connected to pre-amplifiers and the outputs monitored and registered with a data acquisition system (Dataq DI-194) recording at 120 Hz per channel. The water PO2 (PWO2) was constantly monitored with an O2 electrode (FAC 001) connected to an O2 analyzer (FAC 204, FAC Instruments, São Carlos, SP. Brazil), calibrated with a borax solution (0.01 N) saturated with sodium sulphite, and aerated water (PWO2 = 0 and 140 mmHg, respectively).
After waiting a minimum of 30 min to ensure that the levels of all recorded variables had returned to stable resting levels, injections were made in the following sequence: (1) intravenous injection of (a) 0.5 mL saline followed by (b) 0.2 mL of NaCN solution (150 μg NaCN mL−1 saline) and (2) intrabuccal injection of (c) 1.0 mL water followed by (d) 0.5 mL of NaCN solution (500 μg NaCN mL−1 water). The cardiorespiratory variables (f R , V AMP, f H and P a) were recorded continuously and sufficient time was allowed between injections for all cardiorespiratory variables to return to their starting levels.
Subsequent to the injections, the animals were exposed to progressive hypoxia by equilibrating the water with N2 rather than air. By controlling the N2 flow, the PWO2 was slowly decreased from 140 to 10 mmHg holding O2 tensions steady for 10 min at each of 133, 94, 64, 44, 36, 27 and 17 mmHg.
All four groups of fish (I, IX, G1 and G4) underwent all procedures.
Series two and three
In these series of experiments, an experimental setup similar to that described by Rantin and colleagues (Rantin and Kalinin 1996; Rantin et al. 1998) was used. The system consisted of two chambers: an upper compartment, where the fish was kept during the experiment, and a lower part, serving to gas the water with N2. The water was continuously recirculated from the lower to the upper compartment. The shape of the upper chamber allowed fish to remain on the bottom or move up to the surface to air breathe while restricting lateral movements. All cannulae were connected and instruments calibrated as described above.
In series two, after waiting a minimum of 30 min to ensure that the levels of all recorded variables had returned to stable resting levels, NaCN injections were made into the buccal cavity and/or the caudal vein of intact fish (N = 6) in an attempt to elicit air breathing. Intrabuccal injections of 1.0 mL water were followed by 0.25–1.0 mL of 500 μg to 2 mg NaCN mL−1. Then, intravenous injections of 0.5 mL saline were followed by 0.2 mL of 150 μg to 2 mg NaCN mL−1. Respiratory variables (f R , V AMP) were recorded continuously and sufficient time was allowed between injections for all respiratory variables to return to their starting levels. While intravenous injections of progressively larger amounts of NaCN always ultimately produced air breathing, intrabuccal injections rarely did. To test the hypothesis that the effect of stimulation of externally oriented receptors in the gills on air breathing varies as a function of the level of stimulation of the internally oriented receptors, intravenous injections of NaCN sufficient to produce air breathing were followed by intrabuccal injections of NaCN as soon as the effects of the intravenous injection on air breathing had subsided, but while the effect on gill ventilation was still present (i.e., while respiratory drive was still elevated above resting levels).
In series three, individuals of all four groups of fish (I, IX, G1 and G4; N = 10, 8, 10 and 10, respectively) were subjected to progressive hypoxia, as described above, and their air-breathing frequency and time spent in aerial respiration were quantified at each of the step changes in O2 tension (133, 94, 64, 44, 36, 27 and 17 mmHg).
Sodium cyanide is not a naturally occurring substance in the bodies of fish. It is routinely used in studies such as this, however, to mimic local hypoxia and produce a strong, transient stimulus to O2-sensitive chemoreceptors. To be able to accurately determine the extent to which gill denervation reduced or eliminated responses to hypoxia, concentrations of NaCN were chosen that would produce large, significant effects. Two things of note stem from this: one is that in some instances the effects of the NaCN were far more profound than the effects of the graded hypoxia (see for instance the effects on arterial blood pressure). The other is that in the case of gill breathing amplitude, attenuated effects of the NaCN injections remained even after gill denervation had removed all of the effects of the progressive hypoxia.
Data analysis
The relative role of O2 chemoreceptors at different branchial sites was evaluated by comparing the cardiorespiratory responses to the NaCN injections and hypoxia between intact and denervated animals.
The cardiovascular and respiratory variables were measured 30 s before each injection and at 10, 20, 30, 40, 50, 60, 120, 180 and 240 s after each injection. For the progressive hypoxia trial we analyzed the cardiorespiratory variables over 1 min in normoxia (PWO2 = 140 mmHg) and for the final minute at each of the 10-min steps in O2 tension.
The measurements of f R and f H are expressed as breaths min−1 and beats min−1, respectively. The total ventilation (V TOT) was determined as V TOT = V AMP × f R . V AMP, P a and V TOT are expressed as percentage change from basal resting levels.
Data are presented as mean ± standard error. In series 1, to compare changes in each variable, over time for the NaCN injections, or at each respective O2 tension in the hypoxia trials, a one-way repeated-measures analysis of variance (ANOVA) was performed followed by a Tukey–Kramer test where appropriate. To evaluate the effect of selective denervations on the responses to the different treatments, a two-way repeated-measures ANOVA was used followed by a Dunnett’s multiple-comparison test. The commercial package SigmaStat v3.0 (SPSS Inc.) was used to carry out statistical analyses, and the fiducial limit of significance in all cases was 5%.
Results
Series 1
Effects of denervation on cardiorespiratory variables in normoxia
Complete gill denervation did not have a significant effect on any of the cardiorespiratory variables in normoxia. There was a tendency for f H to increase with progressive denervation (Table 1) but this trend did not reach significance.
Effects of hypoxia and NaCN on gill respiratory frequency (f R), ventilation amplitude (V AMP) and total ventilation (V TOT)
In fish with no access to air, progressive hypoxia led to an increase in f R (Fig. 1), V AMP (Fig. 2) and V TOT (Fig. 3) in the intact group that became significant once the PwO2 fell below the critical O2 tension for this species (PcO2 = 40 mmHg, Oliveira et al. 2004). While bilateral denervation of the first gill arch (G1 group) alone abolished the increase in f R (Fig. 1), complete denervation of all gill arches (G4 group) was required to eliminate the increase in V AMP (Fig. 2).
Changes in respiratory frequency (f R ) of jeju, Hoplerythrinus unitaeniatus, following a internal (150 μg) and b external (500 μg) injections of NaCN as well as c following exposure to graded hypoxia. filled circle intact group (n = 10), filled triangle IX group (cranial nerve IX to the first gill arch sectioned, n = 8), filled square G1 group (cranial nerves IX and branches of cranial nerve X to the first gill arch sectioned, n = 10), filled diamond G4 group (all gill arches denervated, n = 10). Open symbols represent significant differences from initial values (normoxia) in that group. Points are mean ± SEM
Changes in ventilation amplitude (V AMP) of jeju, Hoplerythrinus unitaeniatus, following a internal (150 μg) and b external (500 μg) injections of NaCN as well as c following exposure to graded hypoxia. Filled circles intact group (n = 10), filled triangle IX group (cranial nerve IX to the first gill arch sectioned, n = 8), filled square G1 group (cranial nerves IX and branches of cranial nerve X to the first gill arch sectioned, n = 10), filled diamond G4 group (all gill arches denervated, n = 10). Open symbols represent significant differences from initial values (normoxia) in that group. Points are mean ± SEM
Changes in total ventilation (V TOT) of jeju, Hoplerythrinus unitaeniatus, following a internal (150 μg) and b external (500 μg) injections of NaCN as well as c following exposure to graded hypoxia. Filled circle intact group (n = 10), filled triangle IX group (cranial nerve IX to the first gill arch sectioned, n = 8), filled square G1 group (cranial nerves IX and branches of cranial nerve X to the first gill arch sectioned, n = 10), filled diamond G4 group (all gill arches denervated, n = 10). Open symbols represent significant differences from initial values (normoxia) in that group. Points are mean ± SEM
Intact fish (control) responded to both internal and external injections of NaCN with increases in f R (Fig. 1), V AMP (Fig. 2) and, consequently, V TOT (Fig. 3). The increase in f R was relatively brief; f R returned to initial values within the first minute after the injection. The increase in V AMP, and hence in V TOT, was more prolonged; V AMP did not return to initial values until 3 min (external injection) or more (internal injection) post-injection (Fig. 2).
There were also increases in f R and V AMP in the IX and G1 groups following both external and internal injections of NaCN. In both groups, the increase in V AMP was briefly delayed and the magnitude of the increase was less (Fig. 2). Complete gill denervation (G4 group) abolished the increases in f R (Fig. 1) but not the increases in V AMP (Fig. 2). The net result was an increase in V TOT after both internal and external injections of NaCN in all experimental groups (intact, IX, G1 and G4) although the increases were diminished compared to the intact group (Fig. 3).
Effects of hypoxia and NaCN on heart rate (f H) and arterial blood pressure (P a)
Once the O2 levels fell below the critical O2 tension (~40 mmHg) during progressive hypoxia, intact jeju exhibited a significant bradycardia (from 43 ± 4.5 to 21 ± 1.8 bpm) (Fig. 4) accompanied by a significant hypertension (increase in P a of 75%) (Fig. 5). The bradycardia was eliminated following complete bilateral denervation of the first gill arch (G1 group) while the hypertension was eliminated by denervation of only the glossopharyngeal nerve (Fig. 5).
Changes in heart rate (f H) of jeju, Hoplerythrinus unitaeniatus, following a internal (150 μg) and b external (500 μg) injections of NaCN as well as c following exposure to graded hypoxia. Filled circle intact group (n = 10), filled triangle IX group (cranial nerve IX to the first gill arch sectioned, n = 8), filled square G1 group (cranial nerves IX and branches of cranial nerve X to the first gill arch sectioned, n = 10), filled diamond G4 group (all gill arches denervated, n = 10). Open symbols represent significant differences from initial values (normoxia) in that group. Points are mean ± SEM
Changes in blood pressure (P a) of jeju, Hoplerythrinus unitaeniatus, following a internal (150 μg) and b external (500 μg) injections of NaCN as well as c following exposure to graded hypoxia. Filled circle intact group (n = 10), filled triangle IX group (cranial nerve IX to the first gill arch sectioned, n = 8), filled square G1 group (cranial nerves IX and branches of cranial nerve X to the first gill arch sectioned, n = 10), filled diamond G4 group (all gill arches denervated, n = 10). Open symbols represent significant differences from initial values (normoxia) in that group. Points are mean ± SEM
Both internal and external injections of NaCN induced a pronounced bradycardia in the intact group (Fig. 4). Gill denervation reduced the magnitude of the effect until the bradycardia was completely abolished after complete gill denervation (G4 group) (Fig. 4). Despite the bradycardia, both external and internal injections of NaCN produced significant increases in P a (Fig. 5). There was a progressive decrease in the magnitude of the response with progressive gill denervation. The response to external NaCN injections was abolished in the G1 group (complete bilateral denervation of the first gill arch) while the response to internal NaCN injections was only abolished in the G4 group (complete bilateral denervation of all gill arches).
Series 2
Aerial respiration of intact fish following NaCN injections
Intravenous injections of 0.5 mL saline did not have a significant effect on gill ventilation but injections of 0.2 mL of increasing concentrations of NaCN led to increases in gill ventilation frequency and amplitude (Fig. 6). Similarly, intrabuccal injections of 1.0 mL water had no significant effect on gill ventilation but injections of 0.25–1.0 mL of 2,000 μg NaCN/mL generally led to increases in gill ventilation frequency and amplitude (Fig. 7). Interestingly, in one fish external injections of NaCN led to a decrease in both respiratory variables and for this we have no explanation.
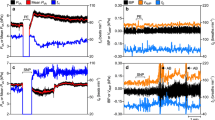
Representative traces showing respiratory responses of one representative fish to internal (caudal vein) injections of saline (0.2 mL 0.9% NaCl added at arrow, upper panel) and two different doses of NaCN (0.2 mL of 150 μg mL−1, middle panel and 0.2 mL of 500 μg mL−1, lower panel). Note the progressive increase in gill ventilation frequency and amplitude with increasing doses of NaCN as well as the air breathing at the highest concentration. Traces to the right are expanded versions of the segments of trace indicated indicated by the bars above and below the traces on the left
Representative traces showing respiratory responses of one fish to external (buccal cavity) injections of water (0.5 mL added at arrow, upper panel) and two different doses of NaCN (1.0 mL of 500 μg mL−1, middle panel and 0.5 ml of 2,000 μg mL−1, lower panel). Traces to the right are expanded versions of the segments of trace indicated by the bars above and below the traces on the left (The flat lines in the middle of the traces indicate the time during which the water or NaCN was being injected. To make these injections the catheter had to be closed to the pressure transducer. The arrows mark the exact time in the process when the injection was made.)
Intravenous injections of 0.2 mL of increasing concentrations of NaCN always led to air breathing (Fig. 6). Intrabuccal injections of 0.25–1.0 mL of 2,000 μg NaCN/mL generally did not (Fig. 7). In two instances only, the highest concentrations of NaCN instantly led to a single air breath (Fig. 9, left panel). In all animals tested, however, when intravenous injections of NaCN that produced air breathing were followed by intrabuccal injections of NaCN, after the effects of the intravenous injection on air breathing had subsided, air breathing was greatly stimulated (Figs. 8, 9). At the time of these latter external NaCN injections, gill frequency had returned to normal but gill ventilation amplitude, and hence total gill ventilation, had not (Fig. 9d–f). Now bouts of roughly six air breaths lasting over 40 s would be generated by levels of external NaCN that failed to have any effect previously (Fig. 9).
Representative trace showing the respiratory response of the fish in Fig. 7 (Jeju F) to an external (buccal cavity) injection of NaCN (0.5 mL of 2 mg ml−1) following an internal (caudal vein) injection of NaCN (0.2 mL of 2 mg ml−1). While an external injection of this concentration alone did not produce air breathing in this fish, it did following the internal injection
The effects of internal NaCN injections (white bars), external NaCN injections alone (grey bars), and external NaCN injections immediately following internal injections (black bars) on a the time following injection until the first air breath, b the number of air breaths taken and c the length of time sent air breathing in jeju. Also shown are the levels of d gill ventilation frequency, e gill amplitude and f total gill ventilation immediately before external NaCN injections that did not cause air breathing (black bars) before internal injections that did cause air breathing (light grey bars) and immediately before external injections that did cause air breathing (dark grey bars). Values are mean ± SEM. Values not denoted by the same letter are significantly different
Series 3
Aerial respiration of denervated fish exposed to graded hypoxia
On exposure to progressive hypoxia, jeju started to breathe air at a PiO2 of about 44 mmHg, again, just below the PcO2 of this species (40 mmHg). Progressive denervation of the gills reduced both f RA and T RA and total bilateral gill denervation completely abolished air breathing (Fig. 10).
Changes in air-breathing frequency (f RA) and time spent in aerial respiration (duration, T RA) of jeju, Hoplerythrinus unitaeniatus, following exposure to graded hypoxia. Filled circle intact group (n = 10), filled triangle IX group (cranial nerve IX to the first gill arch sectioned, n = 8), filled square G1 group (cranial nerves IX and branches of cranial nerve X to the first gill arch sectioned, n = 10), filled diamond G4 group (all gill arches denervated, n = 10). Open symbols represent significant differences from initial values (normoxia) in that group. Points are mean ± SEM
Discussion
The results of the present study indicate that denervation of the first gill arch alone will eliminate the fall in heart rate and increase in Gill breathing frequency associated with graded hypoxia. Denervation of all gill arches, however, was required to eliminate the hypoxia-induced increases in gill ventilation amplitude, air breathing, and blood pressure. Comparison of the results of injections of NaCN into the buccal cavity and caudal vein suggest that all responses resulted from the involvement of both externally and internally oriented chemoreceptors, sensing water and blood, respectively. The results further suggest that while sufficient stimulation of the internal receptors can give rise to air breathing even in normoxic water, stimulation of external receptors only gives rise to air breathing if the internal receptors are co-activated.
Effects of branchial denervation on cardiorespiratory variables in normoxia
While there was a trend for f H in jeju to increase with progressive gill denervation in normoxia, this was not statistically significant. This suggests that input from branchial O2 chemoreceptors does not contribute to resting cardiac vagal tone in normoxia. Similar results have been documented for bowfin (McKenzie et al. 1991; Hedrick and Jones 1999), traira and tambaqui (Sundin et al. 1999, 2000). In pacu, on the other hand, f H increased significantly following complete gill denervation (Leite et al. 2007). Gill ventilation frequency (f R ) was also unaffected by gill denervation. Again, similar results were obtained in bowfin, traira and tambaqui (McKenzie et al. 1991; Hedrick and Jones 1999; Sundin et al. 1999, 2000) although in longnose gar and pacu resting f R was decreased following complete afferent branchial denervation (Smatresk 1988; Leite et al. 2007). The similarities between studies are more striking than the differences and indicate that removal of all afferent information arising from the gills along with efferent branchial vascular control and postural motor control of the gill arches does not alter the resting (normoxic) physiological control of the heart or the buccal and opercular pumps.
Effects of branchial denervation on cardiorespiratory responses to hypoxia
Cardiovascular responses
Heart rate
In intact jeju, hypoxic bradycardia was not induced until the PwO2 reached tensions near the PcO2 of this species (40 mmHg; Oliveira et al. 2004), as has been shown for other species as well (Sundin et al. 2000). Below this critical tension, in the present study, a significant bradycardia was recorded in both the intact and glossopharyngeal nerve denervated groups, but was absent following complete denervation of the first gill arch. Elimination of the bradycardia induced by both external and internal NaCN injections, on the other hand, did not occur until complete bilateral denervation of all gill arches suggesting that the O2 chemoreceptors mediating the bradycardia were distributed on all gill arches with the majority situated on the first gill arch.
A pronounced bradycardia is the common response of teleost fish to environmental hypoxia. In studies of other facultative air-breathing fish the reflex bradycardia was mediated exclusively by externally oriented receptors in the bowfin (Amia calva) (McKenzie et al. 1991) while in the gar (Lepisosteus osseus) it could be initiated by both externally and internally oriented receptors (Smatresk et al. 1986).This was also the case in the jeju in the present study. This is the first study to examine the distribution of these receptors in the different gill arches of a facultative air breather and we found that they were distributed across all gill arches. This is similar to the situation seen in tambaqui and pacu (Sundin et al. 2000; Leite et al. 2007) although significant variation has been found in various species of exclusively aquatic breathing teleosts (Daxboeck and Holeton 1978; Smith and Jones 1978; Smith and Davie 1984; Fritsche and Nilsson 1989; Burleson and Smatresk 1990a; McKenzie et al. 1995; Sundin et al. 1999). A parsimonious explanation for differences in location (first versus other gill arches) and orientation (external versus internal) of the O2 chemoreceptors involved in producing the hypoxic bradycardia remains elusive.
Blood pressure
In the present study, the reflex bradycardia was accompanied by a hypertension. As a consequence, blood pressure was elevated during the graded hypoxia and following both external and internal injections of NaCN and this hypertension was no longer statistically significant following complete gill denervation. In other studies the hypoxic bradycardia has been accompanied by a fall (dogfish, Satchell 1961; Butler and Taylor 1971; traira, Sundin et al. 1999), no change (tambaqui, Sundin et al. 2000) or an increase in blood pressure (Atlantic cod, Fritsche and Nilsson 1989; trout, Sundin and Nilsson 1997). Based on the current results it is not possible to determine whether the changes in blood pressure were an indirect result of barostatic reflexes or a direct effect of O2 chemoreceptor stimulation.
Respiratory responses
Ventilatory responses to hypoxia in fish generally consist of increases in both respiratory frequency and amplitude although the relative contribution of increases in each of these variables differs amongst species (Holeton 1977; Shelton et al. 1986). The increases in V TOT in jeju due to larger increases in V AMP than f R (by roughly 2:1). This strategy appears to be common in hypoxia-tolerant species and Rantin et al. (1992) have argued that this is an energetically more favorable way to overcome the mechanical work of breathing.
Breathing frequency
The magnitude of the increase in f R with graded hypoxia decreased progressively as the degree of gill denervation increased and, along with the responses to internal and external NaCN injections, was eliminated by complete bilateral gill denervation. These data suggest there are O2 chemoreceptors present on all gill arches that monitor the O2 levels of both inspired water and blood involved in producing hypoxia-induced increases in f R . These results are the same as those obtained for traira, Hoplias malabaricus (Sundin et al. 1999), and tambaqui, Colossoma macropmum (Sundin et al. 2000), channel catfish, Ictalurus punctatus (Burleson and Smatresk 1990a, b) gar, Lepisosteus osseus (Smatresk 1989) and bowfin, A. calva (McKenzie et al. 1991) but differ from those obtained for sea raven, Hemitripterus americanus (Saunders and Sutterlin 1971), rainbow trout, Salmo gairdneri (Bamford 1974) and pacu, Piaractus mesopotamicus (Leite et al. 2007) where complete branchial denervation did not eliminate the increase in f R .
Breathing amplitude
The magnitude of the increase in V AMP with graded hypoxia was not greatly affected by gill denervation until it was eliminated by complete bilateral gill denervation. Both external and internal injections of NaCN also stimulated increases in V AMP but neither response was eliminated by complete bilateral gill denervation. While only conjecture at this time, it is possible that the doses of NaCN chosen in the present study represented a more powerful stimulus than the final level of hypoxia. If this was the case, it suggests that the receptors responsible for the residual response to NaCN have a high threshold and would only be recruited in severe hypoxia. These data suggest that extrabranchial O2 chemoreceptors also participated in the increases in V AMP. Similar results were observed in Hemitripterus americanus (Saunders and Sutterlin 1971), traira, H. malabaricus (Sundin et al. 1999), tambaqui, Colossoma macropomum (Sundin et al. 2000; Milsom et al. 2002; Florindo et al. 2006) and pacu, Piaractus mesopotamicus (Leite et al. 2007). In the tambaqui, the extrabranchial O2 chemoreceptors were innervated by cranial nerves V and VII (Milsom et al. 2002; Florindo et al. 2006).
The effects of gill denervation on the increases in total ventilation in response to hypoxia and NaCN injections mirror the changes in V AMP closely reflecting the larger contribution of changes in V AMP to V TOT.
Air-breathing frequency (f RA) and time spent in aerial respiration (T RA)
As previously shown (Riggs et al. 1978; Oliveira et al. 2004), intact jeju initiated aerial respiration when PiO2 fell below their PcO2 (~44 mmHg). With further reductions in water PO2 beyond this point, air-breathing frequency and the time spent in aerial respiration increased progressively. While this response was reduced in the IX and G1 groups, it was only eliminated after complete bilateral gill denervation (group G4). Similar results have been obtained in the African lungfish, Protopterus aethiopicus (Lahiri et al. 1970), gar, Lepisosteus oculatus (Smatresk 1988), and the bowfin, A. calva (McKenzie et al. 1991). An increase in aerial respiration in response to graded hypoxia is a uniform response in all facultative air-breathing fish (Graham 1997) and the accumulated evidence suggests that this is uniformly mediated by O2 chemoreceptors located exclusively in the gills and distributed on all gill arches.
Location and orientation of receptors involved in producing air breathing
Internal injections of NaCN stimulated both gill and air breathing in jeju as they did in gar and African lungfish (Smatresk 1986; Lahiri et al. 1970). Similar injections had no effect on air breathing in the bowfin (McKenzie et al. 1991). External injections of NaCN into the buccal cavity stimulated only air breathing in the gar yet only gill breathing in the bowfin (Smatresk 1986; McKenzie et al. 1991). It always stimulated gill breathing in the jeju and also air breathing if internal drive was high. Thus, if the animal was normoxic, even very large concentrations of NaCN rarely stimulated air breathing but if internal drive was high following an internal injection of NaCN, then external injections of NaCN produced air-breathing episodes very similar to those produced by internal injections alone. This is similar to the situation in gar where, when internal PO2 levels were low, stimulation of external chemoreceptors increased air breathing further (Smatresk et al. 1986; Smatresk 1988). Smatresk hypothesized that it is the internally oriented receptors that primarily stimulate air breathing but that input from externally oriented receptors modulates the threshold for this response (Smatresk et al. 1986). Our data are consistent with this and suggest that in jeju, while sufficient stimulation of the internal receptors can give rise to air breathing even in normoxic water, stimulation of external receptors can only give rise to air breathing if the internal receptors are co-activated. This raises the possibility that in the two instances where external injections of NaCN alone did lead to a single air breath, the fish were not completely normoxic and at rest.
Conclusions
The complex distribution of O2 chemoreceptor populations involved in respiratory and cardiac responses to hypoxia and/or hypoxemia in fish remain difficult to explain. It has been suggested that in hypoxia-tolerant water breathers, these responses arise primarily due to hypoxemia whereas in less tolerant species they are an immediate response to aquatic hypoxia. This has not turned out to be the case. Facultative air breathers are certainly more tolerant of aquatic hypoxia as long as they have access to air. The present study suggests that for jeju at least, the cardiorespiratory responses to moderate hypoxia arise from both internally and externally oriented chemoreceptors situated exclusively in the gills. Fish that utilize bimodal respiration (water/air) must not only have adapted new structures for aerial respiration, they must also have reorganized the integration of sensory inputs to coordinate the activities at two distinct sites to promote appropriate adjustments in cardiorespiratory activities (Fritsche and Nilsson 1993). The present data suggest that as a part of this reorganization, environmental hypoxia continues to lead to increases in gill ventilation only for as long as arterial blood gases are maintained. Once hypoxaemia begins to occur, however, it leads to a reduction in gill ventilation and an increase in air breathing.
Ethics in animal experimentation
In the present study, the experiments were conducted according to the rules of the Brazilian College on Ethics in Animal Experimentation (COBEA), meeting all the regulations and ethical guidelines in Brazil.
References
Axelsson M, Fritsche R (1994) Cannulation techniques. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes. Analytical techniques. Elsevier, Amsterdam, pp 17–36
Axelsson M, Abe AS, Bicudo JEPW, Nilsson S (1989) On the cardiac control in the South American lungfish, Lepidosiren paradoxa. Comp Biochem Physiol 93A:561–565
Bamford OS (1974) Oxygen reception in the rainbow trout (Salmo gairdneri). Comp Biochem Physiol 48A:69–76
Burleson ML, Milsom WK (1993) Sensory receptors in the first gill arch of rainbow trout. Respir Physiol 93:97–110
Burleson ML, Smatresk NJ (1990a) Effects of sectioning cranial nerves IX and X on cardiovascular and ventilatory reflex responses to hypoxia and NaCN in channel catfish. J Exp Biol 154:407–420
Burleson ML, Smatresk NJ (1990b) Evidence for two oxygen-sensitive chemoreceptor loci in channel catfish, Ictalurus punctatus. Physiol Zool 63:208–221
Butler PJ, Taylor EW (1971) Response of the dogfish (Scyliorhinus canicula L.) to slowly induced and rapidly induced hypoxia. Comp Biochem Physiol 39A:307–323
Butler PJ, Taylor EW, Short S (1977) The effect of sectioning cranial nerves V, VII, IX and X on the cardiac response of the dogfish Scyliorhinus canicula to environmental hypoxia. J Exp Biol 69:233–245
Carter GS, Beadle LC (1931) The fauna of the swamps of the Paraguayan Chaco in relation to its environment, II. Respiratory adaptations in fishes. J Linn Soc Lond Zool 37:327–368
Coolidge EH, Ciuhandu CS, Milsom WK (2008) A comparative analysis of putative oxygen-sensing cells in the fish gill. J Exp Biol 211:1231–1242
Daxboeck C, Holeton GF (1978) Oxygen receptors in the rainbow trout, Salmo gairdneri. Can J Zool 56:1254–1256
Dehadrai PV, Tripathi SD (1976) Environmental and ecology of freshwater air-breathing teleosts. In: Hughes GM (ed) Respiration of amphibious vertebrates. Academic Press, London, pp 39–72
Farrell AP (1977) Cardiovascular events associated with air breathing in two teleosts, Hoplerythrinus unitaeniatus and Arapaima gigas. Can J Zool 56:953–958
Florindo LH, Leite CAC, Kalinin AL, Reid SG, Milsom WK, Rantin FT (2006) The role of branchial and orobranchial O2 chemoreceptors in the control of aquatic surface respiration in the neotropical fish tambaqui (Colossoma macropomum): progressive responses to prolonged hypoxia. J Exp Biol 209:1709–1715
Fritsche R, Nilsson S (1989) Cardiovascular responses to hypoxia in the Atlantic cod, Gadus morhua. Exp Biol 48:153–160
Fritsche R, Nilsson S (1993) Cardiovascular and ventilatory control during hypoxia. In: Rankin JC, Jensen FB (eds) Fish ecophysiology. Chapman and Hall, New York, pp 180–206
Glass ML, Ishimatsu A, Johansen K (1986) Responses of aerial ventilation to hypoxia and hypercapnia in Channa argus, an air-breathing fish. J Comp Physiol 156B:425–430
Graham JB (1997) Air-breathing fishes: evolution diversity and adaptation. Academic Press, San Diego
Hedrick MS, Jones DR (1999) Control of gill ventilation and air-breathing in the bowfin Amia calva. J Exp Biol 202:87–94
Holeton GF (1977) Constancy of arterial blood pH during CO-induced hypoxia in the rainbow trout. Can J Zool 55:1010–1013
Johansen K (1966) Air breathing in the teleost Symbranchus marmoratus. Comp Biochem Physiol 18:383–395
Johansen K, Lenfant C (1968) Respiration in the African lungfish, Protopterus aethiopicus II. Control of breathing. J Exp Biol 49:453–468
Johansen K, Hanso D, Lenfant C (1970) Respiration in the primitive air breather, Amia calva. Respir Physiol 9:162–174
Jonz MG, Nurse CA (2003) Neuroepithelial cells and associated innervation of the zebrafish gill: a confocal immunofluorescence study. J Comp Neurol 461:1–17
Jonz MG, Fearon IM, Nurse CA (2004) Neuroepithelal oxygenchemoreceptors of the zebrafish gill. J Physiol (Lond) 560:737–752
Kramer DL (1988) The behavioral ecology of air breathing by aquatic animals. Can J Zool 66:89–94
Kramer DL, Lindsey CC, Moodie GEE, Stevens ED (1978) The fishes and aquatic environment of the central Amazon basin, with particular reference to respiratory patterns. Can J Zool 56:717–729
Lahiri S, Szidon JP, Fishman AP (1970) Potential respiratory and circulatory adjustments to hypoxia in the African lungfish. Fed Proc 29:1141–1148
Laurent P, Rouzeau JD (1972) Afferent neural activity from the pseudobranch of teleost. Effects of PO2, pH, osmotic pressure and Na+ ions. Resp Physiol 14:307–331
Leite CAC, Florindo LH, Kalinin AL, Milsom WK, Rantin FT (2007) Gill chemoreceptors and cardio-respiratory reflexes in the neotropical teleost pacu, Piaractus mesopotamicus. J Comp Physiol A 193:1001–1011
McKenzie DJ, Burleson ML, Randall DJ (1991) The effects of branchial denervation and pseudobranch ablation on cardio-ventilatory control in an air-breathing fish. J Exp Biol 161:347–365
McKenzie DJ, Taylor EW, Bronzi P, Bolis CL (1995) Aspects of cardioventilatory control in the Adriatic sturgeon (Acipenser naccarii). Resp Phyisiol 100:45–53
McKenzie DJ, Campbell HA, Taylor EW, Micheli M, Rantin FT, Abe AS (2007) The autonomic control and functional significance of the changes in heart rate associated with air breathing in the jeju, Hoplerythrinus unitaeniatus. J Exp Biol 210:4224–4232
Milsom WK (1997) Control of breathing in fish: role of chemoreceptors. In: Val AL, Almeida-Val VMF, Randall DJ (eds) Physiology and biochemistry of the fishes of the amazon. INPA, Manaus, pp 359–377
Milsom WK, Brill RW (1986) Oxygen sensitive afferent information arising from the first gill arch of yellowfin tuna. Respir Physiol 66:193–203
Milsom WK, Reid SG, Rantin FT, Sundin L (2002) Extrabranchial chemoreceptors involved in respiratory reflexes in the neotropical fish Colossoma macropomum (the tambaqui). J Exp Biol 205:1765–1774
Oliveira RD, Lopes JM, Sanches JR, Kalinin AL, Glass M, Rantin FT (2004) Cardiorespiratory responses of the facultative air-breathing fish jeju, Hoplerythrinus unitaeniatus (Teleostei, Erythrinidae), exposed to graded ambient hypoxia. Comp Biochem Physiol 139A:479–485
Perry SF, Gilmour KM (2002) Sensing and transfer of respiratory gases at the fish gill. J Exp Biol 293:249–263
Randall DJ, Jones DR (1973) The effects of deafferenation of the pseudobranch on the respiratory response to hypoxia and hyperoxia in the trout (Salmo gairdneri). Respir Physiol 17:291–302
Randall DJ, Cameron JN, Daxboeck C, Smatresk N (1981) Aspects of bimodal gas exchange in the bowfin, Amia calva (Actinopterygii: Amiiformes). Respir Physiol 43:339–348
Rantin FT, Kalinin AL (1996) Cariorespiratory function and aquatic surface respiration in Colossoma macropomum exposed to graded and acute hypoxia. In: Val AL, Almeida-Val, Randall DJ (eds) Physiology and biochemistry of the fishes of the Amazon. INPA Manaus, Brazil, pp 169–180
Rantin FT, Kalinin AL, Glass ML, Fernandes MN (1992) Respiratory responses to hypoxia in relation to mode of life of two erythrinid species (Hoplias malabaricus and Hoplias lacerdae). J Fish Biol 41:805–812
Rantin FT, Guerra CDR, Kalinin AL, Glass ML (1998) The influence of aquatic surface respiration (ASR) on cardio-respiratory function of the serrasalmid fish Piaractus mesopotomicus. Comp Biochem Physiol 119(A):991–997
Reid SG, Sundin L, Milsom WK (2005) The cardio-respiratory system in tropical fish: structure, function and control. In: Val AL, Almeida-Val VMF, Randall DJ (eds) The physiology of tropical fish. Fish physiology. Elsevier Science, San Diego, pp 225–275
Riggs A, Fyhn HJ, Fyhn UEH, Noble RW (1978) Estudo das propriedades funcionais da hemoglobina de Hoplias malabaricus e Hoplerythrinus unitaeniatus. Acta Amazônica 8:251–257
Satchell GH (1961) The response of the dogfish to anoxia. J Exp Biol 38:531–543
Saunders RL, Sutterlin AM (1971) Cardiac and respiratory response to hypoxia in the searaven, Hemitripterus americanus, an investigation of possible control mechanism. J Fish Res Board Can 28:491–503
Shelton G, Jones DR, MilsomWK (1986) Control of breathing in ectotermic vertebrates. In: Geiger SR, Fishman AP, Cherniack NS, Widdicombe JG (eds) Handbook of physiology, section 3, the respiratory system, Vol II, control of breathing, part 2. Waverly Press, Baltimore, pp 857–909
Singh BN (1976) Balance between aquatic and aerial respiration. In: Hughes GM (ed) Respiration in amphibious vertebrates. Academic Press, London, pp 125–164
Skals M, Skovgaard N, Taylor EW, Leite CAC, Abe AS, Wang T (2006) Cardiovascular changes under normoxic and hypoxic conditions in the air-breathing teleost Symbranchus marmoratus: importance of the venous system. J Exp Biol 209:4167–4173
Smatresk NJ (1986) Ventilatory and cardiovascular responses to hypoxia and NaCN in Lepisosteus osseus, an air-breathing fish. Physiol Zool 59:385–397
Smatresk NJ (1988) Control of the respiratory mode in air-breathing fishes. Can J Zool 66:144–151
Smatresk NJ (1989) Chemoreflex control of respiration in an air-breathing fish. In: Lahiri S, Foster RE II, Davies RO, Pack AI (eds) Chemoreceptors and chemoreflexes in breathing—cellular and molecular aspects. Oxford University Press, London, pp 29–52
Smatresk NJ, Cameron JN (1982) Respiration and acid-base physiology of the spotted gar, a bimodal breather. III. Response to a transfer from fresh water to 50% sea water, and control of ventilation. J Exp Biol 96:295–306
Smatresk NJ, Burleson ML, Azizi SQ (1986) Chemoreflexive responses to hypoxia and NaCN in longnose gar: evidence for two chemoreceptive loci. Am J Physiol 251:116–125
Smith FM, Davie PS (1984) Effects of sectioning cranial nerves IX and X on the cardiac response to hypoxia in the coho salmon, Oncorhynchus kisutch. Can J Zool 62:766–768
Smith FM, Jones DR (1978) Localization of receptors causing hypoxic bradycardia in trout (Salmo gairdneri). Can J Zool 56:1260–1265
Stevens ED, Holeton GF (1978) The partitioning of oxygen uptake from air and from water by erythrinids. Can J Zool 56:965–969
Sundin L, Nilsson G (1997) Neurochemical mechanisms behind gill microcirculatory responses to hypoxia in trout: in vivo microscopy study. Am J Physiol 272:576–585
Sundin L, Reid SG, Kalinin AL, Rantin FT, Milsom WK (1999) Cardiovascular and respiratory reflexes: the tropical fish, traira (Hoplias malabaricus) O2 chemoresponses. Respir Physiol 116:181–199
Sundin L, Reid SG, Rantin FT, Milsom WK (2000) Branchial receptors and cardio-respiratory reflexes in a neotropical fish, the tambaqui (Colossoma macropomum). J Exp Biol 203:1225–1239
Acknowledgments
This study was supported by the São Paulo State Research Foundation, FAPESP, and the Brazilian National Research Council for Development of Sciences and Technology, CNPq (grants to Tadeu Rantin) and NSERC of Canada (grants to William. K. Milsom). We would like to thank Cosima Ciuhandu (Department of Zoology/UBC) and Tiago de Campos Belão (Department of Physiological Sciences/UFSCar) for their excellent assistance with these experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Lopes, J.M., de Lima Boijink, C., Florindo, L.H. et al. Hypoxic cardiorespiratory reflexes in the facultative air-breathing fish jeju (Hoplerythrinus unitaeniatus): role of branchial O2 chemoreceptors. J Comp Physiol B 180, 797–811 (2010). https://doi.org/10.1007/s00360-010-0461-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0461-2