Abstract
Mechanisms responsive to hypercapnia (elevated CO2 concentrations) and shaping branchial energy turnover were investigated in isolated perfused gills of two Antarctic Notothenioids (Gobionotothen gibberifrons, Notothenia coriiceps). Branchial oxygen consumption was measured under normo- versus hypercapnic conditions (10,000 ppm CO2) at high extracellular pH values. The fractional costs of ion regulation, protein and RNA synthesis in the energy budgets were determined using specific inhibitors. Overall gill energy turnover was maintained under pH compensated hypercapnia in both Antarctic species as well as in a temperate zoarcid (Zoarces viviparus). However, fractional energy consumption by the examined processes rose drastically in G. gibberifrons (100–180%), and to a lesser extent in N. coriiceps gills (7–56%). In conclusion, high CO2 concentrations under conditions of compensated acidosis induce cost increments in epithelial processes, however, at maintained overall rates of branchial energy turnover.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antarctic ecosystems with very stable biotic and abiotic factors have evolved after Antarctic waters were isolated by formation of the circum-Antarctic current in the late Oligocene (about 25 Mio years ago). The Antarctic fish fauna is dominated by the suborder Notothenioidei, with about 50% of all individuals belonging to the family Nototheniidae (Eastman 2005). No fossil record is available, but the Notothenioidei probably have appeared in the Eocene [38 Mio years ago (Eastman 1993)] and began to diversify in isolation on the Antarctic continental shelf (Di Prisco 2000). These fish have adapted to permanently cold temperatures and are considered to be highly stenothermal (Pörtner 2006; Somero and De Vries 1967; Somero et al. 1998). Unless acclimation occurs, they may prove sensitive to rising temperatures as associated with global change (Pörtner et al. 2004). Specialization on other environmental factors like salinity or CO2 and oxygen levels and thus sensitivity to fluctuations may be similarly high.
In marine animals, sensitivity to variable CO2 tensions may be mirrored in the capacity for transepithelial ion and acid–base regulation (Pörtner 2008). In both marine invertebrates and fishes, cell and tissue functions are sensitive to acidotic acid–base parameters (Pörtner et al. 2000; Langenbuch and Pörtner 2003). In a study of hypercapnia effects on isolated hepatocytes of two Antarctic fish species, elevated CO2 levels (10,000 ppm) per se did not change cellular energy turnover, however, a reduction in extracellular pH below control values led to an almost complete shutdown of protein synthesis (Langenbuch and Pörtner 2003). It is well known however, that marine fishes are capable of restoring acid–base balance during exposure to hypercapnia in vivo (Heisler 1993; Larsen et al. 1997; Michaelidis et al. 2007; Perry 1982; Toews et al. 1983) and are thereby able to circumvent pH induced metabolic depression. The ion regulation mechanisms involved in acid–base regulation have been investigated in a number of fish species (Claiborne and Evans 1992; Claiborne and Heisler 1983; Edwards et al. 2005; Jensen et al. 2000).
For a more detailed study of the functional response of the gills of Antarctic fishes to hypercapnia we chose two demersal members of the family Nototheniidae, Gobionotothen gibberifrons and Notothenia coriiceps. G. gibberifrons reaches up to 55 cm total body length and is found around the islands of the Scotia Arc and at the northern part of the Antarctic Peninsula between water depths of 5–800 m (DeWitt et al. 1990, Takahashi and Iwami 1997). N. coriiceps reaches up to 62 cm total length (Burchett et al. 1983). The distribution of this species is most likely circum-Antarctic (it is known from Scotia Arc Islands, western Antarctic Peninsula and sub-Antarctic Islands of the Indian Ocean (DeWitt et al. 1990). Both species are more or less absent from the high-Antarctic zone but dominate the demersal (benthic) fish community in shelf areas west of the Antarctic Peninsula, in particular in water depths less than 300 m (Takahashi and Iwami 1997, Casaux et al. 2003). G. gibberifrons is a typical benthos feeder with a rather sluggish life style (e.g., Kunzmann 1990). N. coriiceps is an opportunistic feeder preying mainly upon benthic invertebrates and fish (Takahashi and Iwami 1997; Casaux et al. 1990, 2003).
We established an isolated perfused gill model for these species to study the impacts of hypercapnia on energy turnover associated with transepithelial ion and acid–base regulation as well protein synthesis and RNA synthesis. Gills are multifunctional and metabolically very active organs, not only enabling gas exchange, but also covering over 90% of the fishes’ ion and acid–base regulation and taking part in the elimination of nitrogenous waste [for review see (Evans et al. 2005)]. The calculated energetic costs comprise about 7–10% of whole animal energy turnover (Boeuf and Payan 2001; Gibbs and Somero 1990; Mommsen 1984; Perry and Walsh 1989). These energetic costs may be influenced by temperature such that the response to hypercapnia may also be temperature dependent.
In the light of the putative relevance of acid–base regulation for defining the sensitivity of metabolic processes and the whole organism to hypercapnia (Pörtner 2008), the isolated perfused gill was chosen as a tissue model in accordance with its major role in ion- and acid–base regulation. Isolated or perfused gill arches have been used to study gill ion transport processes for more than 40 years (Bellamy 1961; Richards and Fromm 1969; Shuttleworth 1972). Lyndon (1994) has developed a method to measure oxygen consumption of isolated gills and Morgan and Iwama (1999) used this technique to evaluate the costs of salt transport in the gills through application of specific inhibitors. Based on these studies, we have developed a system for measuring the oxygen consumption of isolated gills by monitoring oxygen levels in the respiration chamber as well as in the perfusate of the gill arch in real time. The setup has been constructed in a way that it can also be used on board research vessels and on research stations. By application of specific inhibitors, we were able to calculate the contribution of ion transport rates (through Na+/K+-ATPase) or distinct metabolic processes like protein synthesis and RNA synthesis to the energy budget of the gills under normocapnic and hypercapnic conditions. Exposure to high CO2 concentrations (10,000 ppm) was chosen for clearly identifiable responses of key processes and mechanisms under conditions when extracellular pH remains high. At the same time, we are not aware of further publications addressing the consequences of hypercapnia for the energy budget of teleost gills. Comparative interpretation of the present data must thus await availability of information for other fish species.
Materials and methods
Animals
Specimens of Gobionotothen gibberifrons (Loennberg; 672–1,169 g, 39.5–46.0 cm, n = 16) were collected during an Antarctic summer expedition with RV ‘Polarstern’ in 2006/07 [ANT XXIII/8; (Gutt 2008)] from bottom trawls at depths of 60–490 m at the Antarctic Peninsula near Elephant and Joinville Island. Notothenia coriiceps (Richardson; 211–557 g, 26.0–34.5 cm, n = 11) were caught with fish traps at Jubany station (King-George-Island) 3–4 weeks prior to experimentation and fed once a week with fish meat. On board, all the animals were kept in an air-conditioned container in aquaria systems with aerated (100% air saturation) natural seawater at 0.0 ± 0.5°C. They were closely observed for 2–5 days prior to experimentation to ensure that they were in a healthy condition. The fish were not fed during that time. Commom eelpout (Zoarces viviparus, Linnaeus) were caught with bottom traps in the German bight near Helgoland in April 2004 and April 2005 and kept in an flow-through aquarium system in aerated seawater (100% air saturation) at 10 ± 0.5°C at the Alfred-Wegener Institute (Bremerhaven, Germany) under a 12:12-h day–night cycle. They were fed twice a week with North Sea shrimps ad libitum but not during the last 5 days before sampling. Animals were raised to overall weights of 170.8 ± 86.4 g and lengths of 30.1 ± 3.6 cm before experimentation.
Isolated perfused gill preparations
Experiments with gills from both Nototheniidae were carried out onboard RV ‘Polarstern’; similar experiments were performed with Z. viviparus gills at the Alfred-Wegener-Institute. For a maximal sample number, several gill arches from one fish were used for isolated perfused gill preparations as described in earlier studies (Decostere et al. 2002; Smith et al. 2006). In all fish species, the first three gill arches of each side were used, as their sizes and fractional masses of the respective whole basket mass were similar (see Table 1). Due to limited time available for the preparation and in order to retain some gill tissue for reference and further analyses, the filaments of each fourth gill arch were frozen and stored at −80°C. Fish were stunned by a blow to the head and infused intravenously with heparin (500 U 100 g−1). After about 10 min of heparin exposure, animals were taken out of the aquarium and killed by cutting their spine. For preparation, the gill arches were dissected quickly and placed in ice-cold saline, where they were cleared of blood and cannulated by use of polyethylene tubing (inner diameter 0.38–0.86 mm, Portex Ltd., Smiths Industries Medical Systems, Kent, UK). To enable easier cannulation, the outer ends of the arches were cut off. They were blotted dry and their weight was determined immediately or, in case of the Antarctic gill arches, frozen in liquid nitrogen and stored at −80°C for later weight determination at the Alfred Wegener Institute in Bremerhaven. The tubing fed through the gill arch had an opening over the length of the arch to enable perfusion (Fig. 1). To prevent leakage, cannulae were sutured to both sides of the gill arch with perma-hand silk suture (4-0, Ethicon Inc., Johnson & Johnson, Somerville, NJ, USA). Preparation of up to six gill arches per fish was completed within 1 h. Prepared gill arches were used for respiration measurements directly after preparation (see below), or were kept in saline at habitat temperature, while being perfused with a pulsatile flow of saline provided by a peristaltic pump (Reglo Digital MS-4/8, Ismatec Laboratoriumstechnik GmbH, Wertheim-Mondfeld, Germany). After measurements, the weight of the gill arches was determined again, or they were stored for later weight determination (see above). Saline used for gill preparations was composed as follows: 148.4 mM Na+, 131 mM Cl−, 5.2 mM K+, 2 mM Ca2+, 1.3 mM Mg2+, 0.3 mM SO4 2−, 27 mM HCO3 −, 0.2 mM H2PO4 −, 0.7 mM HPO4 2−, 5.6 mM d-Glucose (Gey’s Balanced Salt Solution: CaCl2 2 mM, MgCl2·6H2O 1 mM, MgSO4 (anhyd) 0.3 mM, KCl 5 mM, KH2PO4 (anhyd) 0.2 mM, NaHCO3 27 mM, NaCl 120 mM, Na2HPO4 (anhyd) 0.7 mM, d-Gluose 5.6 mM, Sigma–Aldrich, Taufkirchen, Germany). For Z. viviparus the saline contained 160 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 2 mM Mg2SO4, 30 mM NaHCO3, 0.05 mM NaPO4, 0.7 mM Na2HPO4 The perfusate (ice-cold in case of Antarctic fishes, 10°C in case of the zoarcid) was briefly gassed with N2 to lower its oxygen content to 50–70% air saturation. As it is not possible to finely tune medium composition onboard of a ship HCO3 − concentrations were maintained at 25 ± 0.5 mM (determined at the Alfred Wegener Institute in a gas chromatograph, 6890 N, Agilent Technologies, Waldbronn, Germany) at a pH of 7.85 ± 0.05. The bicarbonate concentration was chosen to match pH compensated hypercapnic conditions in the blood, when bicarbonate has accumulated to compensate for the hypercapnia induced acidosis (Heisler 1993). The applied perfusate pH value was within the range of 7.5–8.1 reported for nothothenioid blood (Kunzmann 1991; Egginton and Davison 1998). Based on previous experience a shift of pH in an alkaline direction does not modify the cellular rate of oxygen consumption. For the sake of feasibility we have therefore tolerated such shifts. Similar rates of oxygen consumption under normocapnia and during pH compensated hypercapnic conditions are in line with these previous findings, and confirm the hypothesis that only a drop in extracellular pH below a certain threshold value causes metabolic depression. All pH measurements were performed with a pH-Meter (340i, WTW, Weilheim, Germany) equipped with a SenTix 81 electrode (WTW) calibrated between pH 7 and 10 according to NBS scale (National Bureau of Standards).
Perfusion of isolated gill arches. a Tubing with suitable diameters and a certain length of openings was used for the respective gill arches. Tubes were fixed and tightened with suture (s) on both ends. Afferent (a) and efferent (e) endings were led through gas tight connectors in the chamber lid and attached to the perfusion tubings. b N. coriiceps gill arch during perfusion with colored saline. The picture was taken directly after removing the gill arch from the respiration chamber. The dye (0.01% Trypan Blue) had reached the filament tips after 20–30 min. Perfusion was not complete in the saline entrance region and saline could not reach outer filaments, where the tubing had to be fixed with suture. However, the main part of the perfused area shows homogenous supply with saline
Oxygen consumption measurements
The respiration measurements used here were essentially those described by Lyndon (1994) and Morgan and Iwama (1999) with the following modifications: Gas tight, custom-made cylindrical respiration chambers with an adjustable volume of 60–120 ml were used (Construction by E. Dunker, Alfred Wegener Institute, Bremerhaven, Fig. 2). They were filled with 0.2 μm filtered, freshly aerated seawater. Air provided by a membrane pump (HP-100, Hagen Deutschland GmbH, Holm, Germany) and humidified was used for normocapnic controls. A gas mixture of 1% CO2 in air, provided by a gas-mixing pump (Woesthoff Messtechnik GmbH, Bochum, Germany) generated hypercapnic conditions. The specific role of CO2 as a modulator of the physiological machinery can best be studied by applying levels at the upper end of what can be expected in the whole animal, both for a clear response and for later investigations of intermediate CO2 accumulation scenarios. The respective pH values at 0°C were 8.0 ± 0.05 for normocapnic and 6.9 ± 0.05 for hypercapnic seawater. At 10°C, values were 8.15 ± 0.05 and 6.85 ± 0.05, respectively (NBS scale, pH determination see above).
Technical drawing of the respiration chamber. A cooling jacket (CJ), supplied with circulating cooling fluid via tubing connectors (incoming, IC and outgoing, OC), enabled maintenance of low, stable temperatures in the respiration chamber (RC). The chamber lid consisted of a plunger (P) on a threaded spindle (TS) and was adjustable by a collar (AC). It was fitted with an o-ring (OR) for tightness to gas and fixed by a metal ring (MR) with six screws (SH screw holes). The gill arch was suspended in the chamber by the perfusion tubing leading through holes in the chamber lid. The tubes could be constricted in the gastight connectors (GC) by compressing rubber seals (RS) with screwable end caps (EC). The oxygen micro-sensor could be inserted through a third hole in the lid (MH)
After cannulation, the gill arch was placed into the chamber. The polyethylene tubing was fed through holes in the lid and sealed with gas tight connector pieces (Microelectrode Holder Half-Cells, World Precision Instruments Inc., Sarasota, FL, USA), which had been screwed into the chamber lid (see Fig. 2). An oxygen micro-sensor (needle type, 140 μm, PreSens, Regensburg, Germany) used for oxygen consumption measurements in the chamber was inserted through a third hole in the lid. It was sealed with a rubber gasket, but still allowed extrusion of air bubbles while closing the chamber and checking for leakage (Figs. 2, 3). Leaking gill arches were discarded.
Respiration setup [modified after (Lyndon 1994; Morgan and Iwama 1999)]. S saline, P peristaltic pump, T a/e afferent/efferent flow-through temperature-sensor, O 2a/e afferent/efferent flow-through oxygen sensor, O oxygen micro-sensor, PT pressure transducer, RG rubber gasket, CP gas tight perfusion tubing connector piece, OR o-ring, SW sea water, EPH efferent pressure head
A cooling jacket around the respiration chambers ensured a constant temperature of 0 ± 0.1°C in Antarctic preparations by cooling with a thermostat (Lauda GmbH, Lauda-Koenigshofen, Germany). A second thermostat set to −16°C was used to keep the temperature of the perfusion system at 1 ± 1.0°C by means of a cooling coil. The respiration chamber was equipped with a magnetic stir bar to enable consistent mixtures of the water bath (Fig. 3). In additional sets of experiments, G. gibberifrons gill respiration rates were evaluated at a temperature of 6°C and compared to control conditions. These measurements allowed the evaluation of Q 10 values from Van’t Hoff’s law. Studies in Z. viviparus gills were carried out at 10°C.
Gills were perfused with a pulsatile flow of saline (flow rate 0.27 for Antarctic and 0.035 ml/min for North Sea fish) provided by a peristaltic pump (Reglo Digital MS-4/8, Ismatec) using gas tight Tygon-tubing. The efferent pressure head could be adjusted with a lab stand set to 15–25 cm above the respiration chamber to maintain a pressure of 2–3 kPa. The pressure head was monitored by use of a pressure transducer (MLT 0699, AD Instruments GmbH, Spechbach, Germany) inserted into the efferent cannula. Online monitoring of oxygen content in the perfusate occurred before and after the gill arch, using two flow-through cell oxygen mini-sensors coupled with temperature sensors (FTC-PSt3, PreSens; Fig. 3). Oxygen consumption was recorded by use of oxygen meters (Microx TX3 for needle type and Fibox 2 AOT for flow-through oxygen sensors, PreSens). All data were recorded using a PowerLab system (AD Instruments) and transferred to a PC using the software Chart 4.0 (AD Instruments). Prior to measurements, oxygen sensors were calibrated to 0% with a saturated sodium-dithionite-solution and to 100% in humid air.
Gill oxygen consumption was calculated as described by Lyndon (1994) with the following formula:
where, M(O2) is the oxygen consumption rate [μmol (O2) g−1 h−1], P a and P e are the afferent and efferent oxygen partial pressures of the perfusate [kPa]; α(O2) is the oxygen capacity of the water [μmol (O2) l−1 kPa−1] at the respective salinity and temperature after Boutilier et al. (1984), vfl is the flow rate [l h−1], ΔP ch is the gradient of oxygen decrease over time in the chamber [kPa h−1], V is the chamber volume [l], and w is the fresh weight of the gill [g].
Viability tests
After preparation of the gill arches, viability was determined through analyses of respiration measurements over 24 h. In an additional experiment blue colored saline (0.01% Trypan Blue, Sigma–Aldrich, Taufkirchen, Germany) was used to validate homogeneous supply of saline to the filaments in one exemplary gill arch from each species.
Analyses of branchial energy budget
In the gill arches from Antarctic fish energy budget components were evaluated from inhibition of protein synthesis by use of cycloheximide, RNA synthesis by actinomycin D and ion regulation through Na+/K+-ATPase by ouabain. (The eelpout gill preparation did not allow the application of inhibitors due to the small sizes of the gill arches and, therefore, too small changes in respiration rates upon inhibitor application.) All substances were dissolved in dimethyl sulfoxide (DMSO) and added to the perfusate. In preliminary experiments inhibitor levels were determined as needed to achieve a maximal effect. Final DMSO concentration did not exceed 1.6% and exclusive addition of DMSO to the saline did not affect oxygen consumption of the gills (data not shown). Cycloheximide specifically inhibits eukaryotic protein synthesis by inactivating peptidyl transferase activity of the cytosolic ribosomal 60S subunit (Obrig et al. 1971). As in previous studies (Casey et al. 2002; Mark et al. 2005; Smith and Houlihan 1995), the concentration used was 5 mM to avoid unspecific effects. Actinomycin D was adjusted to 3.2 μM (Morgan et al. 1991; Smith and Houlihan 1995) to block DNA-primed RNA synthesis by forming a stable complex with double stranded DNA (Kirk 1960; Sobell 1985). Ouabain, which specifically inhibits Na+/K+-ATPase activity (Wheeler and Whittam 1962; Whittam 1962) was set to 5 mM as in earlier studies (Krumschnabel et al. 1994; Mark et al. 2005). Preliminary experiments with ouabain concentrations of 1 mM led to insufficient inhibition (data not shown).
Data analysis and statistics
Oxygen consumption rates were determined in gill arches from all three species under normocapnic and hypercapnic conditions. If not depicted differently, data are given as mean values ± standard error of the mean (SEM). In case of G. gibberifrons, effects of inhibitors were determined in individual gill arches. Inhibitor-sensitive components of respiration rates were evaluated by subtracting the value of oxygen consumption under inhibited conditions from the control value for both normocapnic and hypercapnic conditions. For the determination of control respiration rates several gill arches from each of 12 individual fish were used, and the normocapnic and hypercapnic groups each comprised 19 or 20 measurements, respectively. For the evaluation of inhibitor-sensitive respiration rates 7 to 11 gill arches (=n numbers) from 3 to 4 individual fish were measured for each inhibitor, cycloheximide, actinomycin D, and ouabain, respectively.
For N. coriiceps each gill arch was used as its own control prior to application of one inhibitor. Mean values ± SEM were derived from percent inhibition data per group. Control respiration rates were evaluated from the gill arches of 11 individual fish in 27 and 28 measurements under normo- and hypercapnia, respectively. Thereby, each of the six inhibitor groups comprised 8 to 10 gill arches (=n numbers) from 4 to 5 individual fish. Statistical significance of differences between control and hypercapnic groups was tested at the P < 0.05 level using Students’ t tests.
Results
The long-term experiment confirmed the viability of gill arches in the respiration chamber: After 20 min of equilibration the respiration rate remained stable for at least 23 h (Fig. 4). Generally, keeping isolated gill arches in the “waiting loop” for up to 2 h prior to measurements did not diminish their respiratory performance. Homogeneous perfusion of the gills was controlled with trypan blue added to the perfusate. The dye reached the filament tips within 20–30 min after infusion. Only at the entrance region of the saline and at the outer filaments (beyond the tied suture) perfusion remained incomplete (This applied to about 25% of gill wet weight, Fig. 1).
Test of gill viability. One gill arch had been stored in the waiting loop during measurements of 1 day and was placed into the respiration system 8 h after its preparation. Seawater and saline were repeatedly exchanged during the measurement. Decreases in oxygen tensions in the chamber were following a linear regression over the whole time course [9–12 h (before water change): r 2 = 0.9854; 13–22.5 h (after water change): r 2 = 0.9986. According to Run’s test there was no significant deviation from linear regression]. The gill arch showed a stable respiration rate until 23 h after gill preparation, when the experiment was abandoned
Oxygen consumption rates of gills from N. coriiceps at 0°C and from G. gibberifrons at 0° and after warming to 6°C, are depicted in Table 2 for both normo- and hypercapnic conditions. Rates were normalized to the weight of the complete individual gill arches, including cartilaginous arch and the non-perfused area. Mean oxygen consumption of G. gibberifrons isolated perfused gills at 0°C did not change significantly under hypercapnia, (Table 2). Upon warming, oxygen consumption rose under normocapnia and hypercapnia following Q 10 values of 1.54 and 1.43, respectively. No significant difference could be observed between Q 10 values under control and hypercapnic conditions. Gills of N. coriiceps had a significantly higher mean respiration rate of than those of G. gibberifrons at 0°C, which was also not affected by hypercapnic exposure. Data for temperate Z. viviparus ranged somewhat higher than those of the notothenioids (whole animal and gills). Gill oxygen consumption also remained unaffected by the hypercapnia (Table 2).
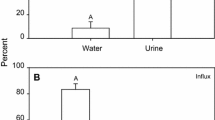
Figure 5 shows the percent fractions of energy allocation to RNA and protein synthesis, as well as ion regulation in the isolated perfused gills. Oxygen uptake was usually measured over a time course of 30 min. As respiration rates were only evaluated when they were showing a constant slope, the rates recorded were considered as steady state rates under the maximum effects of inhibitors. The time course of inhibition did not differ significantly between species or inhibitors. Generally, each of the three inhibited processes shared equal fractions of the energy budget under control conditions, between 6.9 and 11.8% in G. gibberifrons (Fig. 5a) and between 18.5 and 23.1% in N. coriiceps (Fig. 5b), leaving 71.4 and 39.0%, respectively, of the energy consumption unexplained. Under hypercapnic acidosis, the fractions of the individual processes increased significantly—with the exception of actinomycin D sensitive respiration in N. coriiceps—to values between 16.3 and 28.1% in G. gibberifrons (Fig. 5a) and between 20.8 and 29.7% in N. coriiceps (Fig. 5b). With unchanged metabolic rates, this increase occurred at the expense of the unexplained fraction, which was halved to 32.2 and 20.6%, respectively. In summary, all three processes increased by a factor of 2 to 2.8 in G. gibberifrons. In N. coriiceps, oxygen demand of RNA synthesis remained constant, whereas it increased for protein synthesis and Na+/K+-ATPase-dependent ion exchange by a factor of 1.6 and 1.3, respectively.
Energy budgets of G. gibberifrons (a) and N. coriiceps (b) gills under hypercapnia at elevated medium bicarbonate levels to reflect CO2 effects at elevated extracellular pH as during compensation for the hypercapnia induced acidosis. Asterisks indicate significant differences (P < 0.05) between processes under hypercapnia and normocapnic control values. Values are mean percent fractions of gill respiration ± standard error of the mean (SEM)
Under control conditions (normocapnia, water pH 8.0 ± 0.05, 0°C) and in line with the difference between whole animal data of the species (Holeton 1974) the mass specific rate of gill oxygen consumption was 40% higher in N. coriiceps than in G. gibberifrons (P < 0.0001). By scaling MO2 to the respective mean body weights [scaling coefficient = 0.8 (Clarke and Johnston 1999; Holeton 1974)] and considering the weight fraction of the gill basket in total body weight, the expected fraction of gill MO2 in whole animal metabolic rate was evaluated (see Table 2), using whole animal MO2 for N. coriiceps and G. gibberifrons provided by Holeton (1970). The measured fraction of gill respiration in resting SMR resulted higher than expected from its weight fraction and accounts for 6.4% in G. gibberifrons and, similarly, 6.3% in N. coriiceps. Values obtained in Z. viviparus were 4.6%.
Discussion
Methodology
One has to bear certain methodological constraints in mind when using inhibitors for the determination of individual energy consuming processes. Even if effects are usually treated as specific and likely are to begin with, the targeted metabolic processes, if modified through inhibition, influence each other or further processes. For example, inhibiting transcription will also cause an inhibition of translation due to the lack of mRNA transcripts. Furthermore, any Na+-gradient set by the activity of Na+K+-ATPase drives other ion exchange processes and the concentrations reached may allosterically affect other ATPases. Cellular pH shifts resulting from a consecutive disturbance of secondary transport like Na+H+-exchange may modify the functional milieu for other ATPases, thereby reducing their functional rates. Such interactions may explain why inhibitors sometimes exert effects stronger than the initial specific effects. Unspecific side effects of inhibitors might also lead to overestimates. This has especially been discussed for cycloheximide, where such side effects are dose-dependent (Bowgen et al. 2007; Wieser and Krumschnabel 2001). Here we applied inhibitor concentrations as low as possible and minimized variability in the experimental protocol. Observed changes indicate functional shifts in energy budget, even if the exact values may remain elusive. The fractions of energy budget allocated to transcription, translation and ion regulation were similar to those reported for various tissues and cell types of fish and mammals.
Energy turnover
The present study addresses the role of elevated CO2 tensions on energy budget under conditions, when bicarbonate levels were set similar between normocapnia and hypercapnia. Some elevation of extracellular pH above control levels in vivo was tolerated, as it does not affect cellular rates of oxygen consumption (Langenbuch and Pörtner 2003 and Fig. 5 below).
Gill energy turnover in Antarctic fish at 0°C is found well below oxygen consumption rates available for gills from temperate fish at their respective habitat temperature, marine flounder and seawater acclimated cutthroat trout (Lyndon 1994; Morgan and Iwama 1999) as well as eelpout Zoarces viviparus at 10°C (Table 2). Exposure of the gills to various temperatures yielded Q 10 values for G. gibberifrons of 1.54 and 1.43, indicating low thermal sensitivity of overall oxygen demand. The variation in temperature did not influence the overall response to hypercapnia. The fractions of gill energy turnover in whole animal metabolic rate of the three species (Table 2) are comparable to values of 2.4 in seawater and 3.9% in freshwater trout (Morgan and Iwama 1999) and 6.6% in Atlantic cod (Johansen and Pettersson 1981). A much larger range between 11 and 31% was reported for seawater flounder (Lyndon 1994). As the gills are the organs primarily facing environmental conditions and disturbances, we consider them relevant indicators of the hypercapnic response of mechanisms involved in ion and acid–base regulation.
Energy allocation
While translation, transcription and ion regulation accounted for 60% of the energy budget of N. coriiceps gills, those of G. gibberifrons used 30% of their total energy turnover for the same processes (Fig. 5). The remaining energy fraction likely reflects other ATPases, anabolism as well as the mitochondrial proton leak. Unfortunately, no comprehensive data on energy budget are available for teleost fish epithelia. In mammalian cells, substrate bound energy is allocated to protein synthesis (25–30%), Na+/K+-ATPase (19–28%), Ca2+-ATPase (4–8%), gluconeogenesis (7–10%), ureagenesis (3%) and myosin ATPase (2–8%), leaving between 13 and 40% for processes like RNA synthesis, substrate cycling (e.g., glucose/glucose-6-phosphate, acetate/acetylCoA) and signal transduction (Rolfe and Brown 1997), as well as about 25% for proton leak (Nobes et al. 1990; Rolfe and Brand 1996). Gills may also use proton ATPases, as demonstrated for salmonid fish in freshwater (Lin et al. 1994; Perry et al. 2000; Seidelin et al. 2001; Sullivan et al. 1995, 1996). In cutthroat trout, bafilomycin-sensitive H+-ATPase contributed significantly to the oxygen consumption of excised gill tissue (Morgan and Iwama 1999). In a recent study, we found two isoforms of V-type H+-ATPases upregulated in the gills of Z. viviparus after 24 h of hypercapnia (unpublished data), also indicating a role for branchial proton pumps in marine fish.
Ion regulation, protein and RNA synthesis require larger fractions of the total energy budget in N. coriiceps than in G. gibberifrons gills, While G. gibberifrons is a more sluggish, benthic fish (e.g., Kunzmann 1990), N. coriiceps as a benthopelagic species preying on smaller fish (Takahashi and Iwami 1997, Casaux et al. 1990, 2003) probably displays a higher activity pattern. This more active lifestyle is reflected in a higher standard metabolic rate (Holeton 1974; Morris and North 1984; Zimmerman 1997), which according to present data is also mirrored in a higher energy turnover of the gills. Furthermore, the lower baseline ion exchange rate and the larger unexplained gap in normocapnic gills from the sluggish G. gibberifrons may imply a higher “idling” of the proton leak rate than in N. coriiceps. The shrinkage of the unexplained gap, associated with a drastic rise in energy turnover of the tested processes under hypercapnia in G. gibberifrons, at maintained overall branchial oxygen turnover, would be in line with these considerations. This pattern indicates a shift from a high rate of proton leakage due to futile idling of metabolic pathways and mitochondria under control conditions to a lower rate as a mechanism of rapid response to changing ATP demand (Rolfe and Brand 1997), e.g., in ion or acid–base regulation.
The fractions of processes contributing to standard metabolic rate usually differ between tissues. In G. gibberifrons and N. coriiceps gills, the highest energy fraction was consumed by Na+/K+-ATPase, 11.8 and 23.1% of gill metabolic rate, respectively (Fig. 5) which compares to the fraction of 25% in gills of freshwater acclimated trout (Morgan and Iwama 1999), and of seawater adapted flounder (Stagg and Shuttleworth 1982). As mentioned above, the low value for G. gibberifrons might be associated with the lower metabolic rate and the sluggish life style of these fish.
Fractions elaborated here were within the range reported for isolated cell preparations. In rat thymocytes and temperate fish hepatocytes RNA synthesis ranked second after protein synthesis and higher than Na+/K+-ATPase (Buttgereit and Brand 1995; Wieser and Krumschnabel 2001). Na+/K+-ATPase comprised 22% of cellular energy turnover in rat cardiomyocytes (Casey et al. 2002) and 24% in goldfish hepatocytes (Krumschnabel et al. 1994). Antarctic fish hepatocyte respiration due to Na+/K+-ATPase even comprised between 40 and 45% of energy budget (Mark et al. 2005), indicating a slightly higher energy demand of ion exchange than of either protein or RNA synthesis (Mark et al. 2005).
The fraction of energy used by RNA synthesis (12.8 and 19.4% in G. gibberifrons and N. coriiceps, respectively, Fig. 5) under control conditions was also within the range reported for isolated cells: 8% in human blood cells (Schmid et al. 2000), 15% in rat thymocytes (Buttgereit and Brand 1995), 20% in rat cardiomyocytes (Casey et al. 2002). Again, the highest values of 24–35% were reported in Antarctic fish hepatocytes (Mark et al. 2005).
The estimated fractional costs of protein synthesis determined from cycloheximide sensitive oxygen consumption (6.9% and 18.5% in G. gibberifrons and N. coriiceps, respectively, Fig. 5) were lower than reported for isolated liver cells, where values varied between 27% in rat cardiomyocytes (Casey et al. 2002), 20–37% in Antarctic fish hepatocytes (Langenbuch and Pörtner 2003; Mark et al. 2005), 50% in goldfish hepatocytes (Krumschnabel et al. 1994), and 60–90% in fish cell lines (Smith and Houlihan 1995). In the whole animal the fractional costs of protein synthesis range between 11 and 42% (Carter et al. 1993; Houlihan et al. 1988; Lyndon et al. 1992). A large variability in protein synthesis costs has been reported in fish, depending on species and tissue type. Moreover, synthesis may become more efficient (lower aerobic costs) at higher rates [reviewed by (Houlihan et al. 1995)]. Gills generally have a high protein turnover rate (in some fish species second only to liver and occasionally intestines) with a fractional contribution to whole animal protein synthesis between 2.5 and 18% per day [for review see (Lyndon and Houlihan 1998)].
Absolute rates and the fractional energy costs for transcription, translation and ion regulation reported here are among the lowest reported for tissues or cell types from different fish or mammals. In the light of low overall rates of energy turnover of Antarctic fish (Johnston et al. 1991), this indicates lower gill activity than in warm water fishes.
Impacts of hypercapnia on energy allocation
Total gill oxygen consumption remained constant under hypercapnia in gills of G. gibberifrons and N. coriiceps, as well as in the temperate Z. viviparus (see Table 2). However, we observed significant shifts in the branchial energy budget in the Antarctic notothenioids. Hypercapnic exposure of G. gibberifrons gills induced a significant two- to threefold increase in the energy demand of all examined processes. As oxygen consumption remained constant, the fraction of energy exploited by residual processes (e.g., proton leak) was reduced by about 50% (see Fig. 5). These effects were not as dramatic in N. coriiceps, where energy turnover allocated to translation and ion regulation was higher to begin with and increased to a lesser extent, but still significantly, 1.3–1.6 fold. At constant overall energy demand, residual energy turnover also decreased by about 50%. These results clearly show, that—even if the gills’ total energy demand is not affected by higher CO2 concentrations—rearrangements in energy budget occur to match the new requirements for ion and acid–base regulation. A higher ion transport activity might lead to a higher protein turnover rate for Na+/K+-ATPase and several other transporters and enzymes involved in acid–base regulation. Indeed, a higher energy demand for protein synthesis was measured in the gills of both species. An increased translational activity might be supported by a higher transcriptional activity, which became evident only in the gills of G. gibberifrons.
In line with these results, Na+/K+-ATPase enzyme activity, as well as transcription and translation rates increased under hypercapnia in gills of the common eelpout (Deigweiher et al. 2008). Elevated transcription and/or translation levels of a number of ion channels and transporter proteins under hypercapnia have also been shown in gills of rainbow trout (Ivanis et al. 2008; Perry et al. 2000, 2003; Shahsavarani and Perry 2006) and mummichog (Edwards et al. 2005).
Extracellular pH and bicarbonate levels of the perfusate used in our study reflected the blood status typically observed in marine fish after compensation of a hypercapnic acidosis (Larsen et al. 1997; Michaelidis et al. 2007; Toews et al. 1983) with elevated pH, PCO2 and bicarbonate levels (Heisler 1993). Under our experimental conditions, the observed shifts in energy allocation can thus be interpreted to reflect the branchial response to long-term PCO2 increments in the whole organism. Maintenance of high extracellular pH would explain why we did not see a decrease of cycloheximide sensitive respiration under hypercapnia as observed in isolated hepatocytes of Antarctic fish during hypercapnic acidosis (Langenbuch and Pörtner 2003). During non-compensated acidosis the liver cells underwent metabolic depression, thereby showing the same response pattern as previously demonstrated in an invertebrate model (Reipschläger and Pörtner 1996; Pörtner et al. 2000; Langenbuch and Pörtner 2002). In trout hepatocytes anoxia caused a 50% down regulation of protein synthesis. Interestingly, hepatocytes from anoxia-tolerant goldfish maintained protein synthesis rate under anoxia (Wieser and Krumschnabel 2001). However, the role of extracellular pH has not been addressed in both studies. Metabolic depression would indeed be a mechanism suitable to extend tolerance to environmental stressors like anoxia or hypercapnia. The present data indicate that such metabolic depression does not occur in branchial tissue under conditions of fully compensated extracellular acidosis.
The present finding of a modified energy budget due to increased ion regulatory activity is in line with results obtained in salinity transfer studies, which may also have occurred under constant extracellular pH conditions. Interestingly, the fraction of ouabain sensitive respiration in isolated cutthroat trout gills increased from 25% in the freshwater control group to 37% in seawater acclimated fish, associated with reduced branchial oxygen consumption in seawater acclimated cutthroat trout (Morgan and Iwama 1999). Conversely, in flounder gill tissue ouabain sensitive oxygen consumption increased from 25% in the seawater control group to 28% after freshwater acclimation (Stagg and Shuttleworth 1982).
Conclusion and perspectives
The hypercapnia induced increments in the energy demand of ion exchange, protein and RNA synthesis and the concomitant 50% decrease of residual energy turnover in gills of both Antarctic species, G. gibberifrons and N. coriiceps, suggest a strong shift in tissue energy budgets, emphasizing the relevance of branchial acid–base regulation in the response to hypercapnia and during the resulting steady state conditions. This shift might indicate a large decrement in mitochondrial proton leakage, in line with its putative role in futile cycling of metabolism and rapid responsiveness to changes in energy turnover. This shift would be associated with enhanced efficiency in energy turnover. As comparative data are presently unavailable for hypercapnia effects on branchial energy budgets, it remains unclear, whether the present findings reflect a strong response of polar fishes to hypercapnia. Unchanged oxygen consumption rates in gills of Z. viviparus under hypercapnia confirm the same phenomenon in a temperate zone fish (under similar experimental conditions). The question also arises whether these phenomena will become involved under expected scenarios of ocean acidification, with an increase of CO2 concentrations from currently 380 ppm to about 1,000 ppm over the next 100 years (IPCC 2007). Effects become more likely under combined conditions of ocean warming and acidification when endogenous CO2 formation is elevated (Pörtner and Farrell 2008). However, high CO2 concentrations such as those applied in the present study are relevant in the light of proposed scenarios of CO2 disposal or storage (Caldeira et al. 2005), where high concentrations may be reached near injection or leakage sites (Caulfield et al. 1997). Future projects need to address the concentration dependence of observed effects as well as the role of long-term hypercapnic acclimation of the fish in branchial energy budget.
References
Bellamy D (1961) Movements of potassium, sodium and chloride in incubated gills from the silver eel. Comp Biochem Physiol 3:125–135
Boeuf G, Payan P (2001) How should salinity influence fish growth? Comp Biochem Physiol C Toxicol Pharmacol 130(4):411–423
Boutilier RG, Hemming TA, Iwama GK (1984) Physicochemical parameters for use in fish respirometry physiology. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press, New York, 10, pp 403–430
Bowgen AD, Fraser KP, Peck LS, Clarke A (2007) Energetic cost of synthesizing proteins in Antarctic limpet, Nacella concinna (Strebel, 1908), is not temperature dependent. Am J Physiol Regul Integr Comp Physiol 292(6):R2266–R2274
Burchett MS, Sayers PJ, North AW, White MG (1983) Some biological aspects of the nearshore fish populations at South Georgia. British Antarct Surv Bull 59:63–74
Buttgereit F, Brand MD (1995) A hierarchy of ATP-consuming processes in mammalian cells. Biochem J 312(Pt 1):163–167
Caldeira K, Akai M, Brewer PG, Chen B, Haugan PM, Iwama T, Johnston P, Kheshgi H, Li Q, Ohsumi T, Pörtner HO, Sabine C, Shirayama Y, Thomson J (2005) Ocean storage. In: Metz B et al (eds) Carbon dioxide capture and storage: special report of the intergovernmental panel on climate change. Cambridge University Press, New York, pp 277–318
Carter G, Houlihan DF, Brechin J, McCarthy ID (1993) The relationships between intake and protein accretion, synthesis, and retention efficiency for individual grass carp, Ctenopharyngodon idella (Valenciennes). Can J Zool 71:392–400
Casaux RJ, Mazzotta AS, Barrera-Oro ER (1990) Seasonal aspects of the biology and diet of nearshore nototheniid fish at Potter Cove, South Shetland Islands, Antarctica. Polar Biol 11(1):63–72
Casaux R, Barrera-Oro E, Baroni A, Ramón A (2003) Ecology of inshore notothenioid fish from the Danco Coast, Antarctic Peninsula. Polar Biol 26:157–165
Casey TM, Pakay JL, Guppy M, Arthur PG (2002) Hypoxia causes downregulation of protein and RNA synthesis in noncontracting mammalian cardiomyocytes. Circ Res 90(7):777–783
Caulfield JA, Auerbach DI, Adams EE, Herzog HJ (1997) Near field impacts of reduced pH from ocean CO2 disposal. Energy Convers Manage 38:S343–S348
Claiborne JB, Evans DH (1992) Acid–base balance and ion transfers in the spiny dogfish (Squalus acanthias) during hypercapnia: a role for ammonia excretion. J Exp Zool 261:9–17
Claiborne JB, Heisler N (1983) Acid–base regulation and ion transfers in the carp (Cyprinus carpio) during and after exposure to environmental hypercapnia. J Exp Biol 108:25–43
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68(5):893–905
Decostere A, Henckaerts K, Ducatelle R, Haesebrouck F (2002) An alternative model to study the association of rainbow trout (Oncorhynchus mykiss L.) pathogens with the gill tissue. Lab Anim 36(4):396–402
Deigweiher K, Koschnick N, Pörtner HO, Lucassen M (2008) Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am J Physiol Regul Integr Comp Physiol 295(5):R1660–R1670
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. In: Gon O, Heemstra PC (eds) Fishes of the southern ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Di Prisco G (2000) Life style and biochemical adaptation in Antarctic fishes. J Mar Syst 27(1–3):253–265
Eastman JT (1993) Antarctic fish biology: evolution in a unique environment. Academic Press, San Diego
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28(2):93–107
Edwards SL, Wall BP, Morrison-Shetlar A, Sligh S, Weakley JC, Claiborne JB (2005) The effect of environmental hypercapnia and salinity on the expression of NHE-like isoforms in the gills of a euryhaline fish (Fundulus heteroclitus). J Exp Zoolog A Comp Exp Biol 303(6):464–475
Egginton S, Davison W (1998) Effects of environmental and experimental stress on Antarctic fish. In: Pörtner HO, Playle RC (eds) Cold Ocean Physiology. Society For Experimental Biology, Cambridge University Press, Cambridge, pp 299–326
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogenous waste. Physiol Rev 85(1):97–177
Gibbs A, Somero GN (1990) Na+-K+-adenosine triphosphatase activities in gills of marine teleost fishes: Changes with depth, size and locomotory activity level. Mar Biol 106(3):315–321
Gutt J (ed) (2008) The expedition ANTARKTIS-XXIII/8 of the research vessel “Polarstern” in 2006/2007: ANT-XXIII/8; 23 Nov 2006–30 Jan 2007 Cape Town-Punta Arenas. Ber Polarforsch/Rep Polar Res
Heisler N (1993) Acid–base-regulation. In: Evans DH (ed) The physiology of fishes. CRC Press Inc, Boca Raton, pp 343–377
Holeton GF (1970) Oxygen uptake and circulation by a hemoglobinless Antarctic fish (Chaenocephalus aceratus lonnberg) compared with three red-blooded Antartic fish. Comp Biochem Physiol 34(2):457–471
Holeton GF (1974) Metabolic cold adaptation of polar fish: fact or artefact. Physiol Zool 47(3):137–152
Houlihan DF, Hall SJ, Gray C, Noble BS (1988) Growth rates and protein turnover in Atlantic cod Gadus morhua. Can J Fish Aquat Sci 45:951–964
Houlihan DF, Carter CG, McCarthy ID (1995) Protein turnover in animals. In: Walsh PJ, Wright PA (eds) Nitrogen metabolism and excretion. CRC Press, Boca Raton, pp 1–32
IPCC (2007) Climate change 2007: the physical science basis. In: Solomon S et al (eds) Contribution to Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK, pp 996
Ivanis G, Esbaugh AJ, Perry SF (2008) Branchial expression and localization of SLC9A2 and SLC9A3 sodium/hydrogen exchangers and their possible role in acid–base regulation in freshwater rainbow trout (Oncorhynchus mykiss). J Exp Biol 211(Pt 15):2467–2477
Jensen FB, Koldkjaer P, Bach A (2000) Anion uptake and acid–base and ionic effects during isolated and combined exposure to hypercapnia and nitrite in the freshwater crayfish, Astacus astacus. J Comp Physiol [B] 170(7):489–495
Johansen K, Pettersson K (1981) Gill O2 consumption in a teleost fish, Gadus morhua. Respir Physiol 44(3):277–284
Johnston IA, Clarke A, Ward P (1991) Temperature and metabolic rate in sedentary fish from the Antarctic, North Sea and Indo-West Pacific Ocean. Mar Biol 109(2):191–195
Kirk JM (1960) The mode of action of actinomycin D. Biochim Biophys Acta 42:167–169
Krumschnabel G, Malle S, Schwarzbaum PJ, Wieser W (1994) Glycolytic function in goldfish hepatocytes at different temperatures: relevance for Na+ pump activity and protein synthesis. J Exp Biol 192:285–290
Kunzmann A (1990) Gill morphometrics of two Antarctic fish species Pleuragramma antarcticum and Notothenia gibberifrons. Polar Biol 11(1):9–18
Kunzmann A (1991) Blood physiology and ecological consequences in Weddell Sea fishes. Ber Polarforsch/Rep Polar Res 91:1–79
Langenbuch M, Pörtner HO (2002) Changes in metabolic rate and N excretion in the marine invertebrate Sipunculus nudus under conditions of environmental hypercapnia: identifying effective acid–base variables. J Exp Biol 205(Pt 8):1153–1160
Langenbuch M, Pörtner HO (2003) Energy budget of hepatocytes from Antarctic fish (Pachycara brachycephalum and Lepidonotothen kempi) as a function of ambient CO2: pH-dependent limitations of cellular protein biosynthesis? J Exp Biol 206(Pt 22):3895–3903
Larsen BK, Pörtner HO, Jensen FB (1997) Extra- and intracellular acid–base balance and ionic regulation in cod (Gadus morhua) during combined and isolated exposures to hypercapnia and copper. Mar Biol 128:337–346
Lin H, Pfeiffer D, Vogl A, Pan J, Randall D (1994) Immunolocalization of H+-ATPase in the gill epithelia of Rainbow Trout. J Exp Biol 195(1):169–183
Lyndon AR (1994) A method for measuring oxygen consumption in isolated perfused gills. J Fish Biol 44(4):707–715
Lyndon AR, Houlihan DF (1998) Gill protein turnover: costs of adaptation. Comp Biochem Physiol A Mol Integr Physiol 119(1):27–34
Lyndon AR, Houlihan DF, Hall SJ (1992) The effect of short-term fasting and a single meal on protein synthesis and oxygen consumption in cod, Gadus morhua. J Comp Physiol [B] 162(3):209–215
Mark FC, Hirse T, Pörtner HO (2005) Thermal sensitivity of cellular energy budgets in some Antarctic fish hepatocytes. Polar Biol 28:805–814
Michaelidis B, Spring A, Pörtner HO (2007) Effects of long-term acclimation to environmental hypercapnia on extracellular acid–base status and metabolic capacity in Mediterranean fish Sparus aurata. Mar Biol 150:1417–1429
Mommsen TP (1984) Metabolism of the fish gill. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press (Harcourt Brace Jovanovich, Publishers), New York, 10, pp 203–238
Morgan JD, Iwama GK (1999) Energy cost of NaCl transport in isolated gills of cutthroat trout. Am J Physiol 277(3 Pt 2):R631–R639
Morgan CD, Mills KC, Lefkowitz DL, Lefkowitz SS (1991) An improved colorimetric assay for tumor necrosis factor using WEHI 164 cells cultured on novel microtiter plates. J Immunol Methods 145(1–2):259–262
Morris DJ, North AW (1984) Oxygen consumption of five species of fish from South Georgia. J Exp Mar Biol Ecol 78(1–2):75–86
Nobes CD, Brown GC, Olive PN, Brand MD (1990) Non-ohmic proton conductance of the mitochondrial inner membrane in hepatocytes. J Biol Chem 265(22):12903–12909
Obrig TG, Culp WJ, McKeehan WL, Hardesty B (1971) The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J Biol Chem 246(1):174–181
Perry SF (1982) The regulation of hypercapnic acidosis in two salmonids, the freshwater trout (Salmo gairdneri) and the seawater salmon (Onchorynchus kisutch). Mar Hehav Physiol 9:73–79
Perry SF, Walsh PJ (1989) Metabolism of isolated fish gill cells: contribution of epithelial chloride cells. J Exp Biol 144:507–520
Perry SF, Beyers ML, Johnson DA (2000) Cloning and molecular characterisation of the trout (Oncorhynchus mykiss) vacuolar H+-ATPase B subunit. J Exp Biol 203(Pt 3):459–470
Perry SF, Furimsky M, Bayaa M, Georgalis T, Shahsavarani A, Nickerson JG, Moon TW (2003) Integrated responses of Na+/HCO3 − cotransporters and V-type H+-ATPases in the fish gill and kidney during respiratory acidosis. Biochim Biophys Acta 1618(2):175–184
Pörtner HO (2006) Climate-dependent evolution of Antarctic ectotherms: an integrative analysis. Deep Sea Res II 53(8–10):1071–1104
Pörtner HO (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol Prog Ser 373:203–217
Pörtner HO, Farrell AP (2008) Ecology, physiology and climate change. Science 322(5902):690–692
Pörtner HO, Bock C, Reipschläger A (2000) Modulation of the cost of pHi regulation during metabolic depression: a 31P-NMR study in invertebrate (Sipunculus nudus) isolated muscle. J Exp Biol 203:2417–2428
Pörtner HO, Langenbuch M, Reipschläger A (2004) Biological impact of elevated CO2 concentrations: lessons from animal physiology and earth history? J Oceanogr 60:705–718
Reipschläger A, Pörtner HO (1996) Metabolic depression during environmental stress: the role of extracellular versus intracellular pH in Sipunculus nudus. J Exp Biol 199(Pt 8):1801–1807
Richards BD, Fromm PO (1969) Patterns of blood flow through filaments and lamellae of isolated-perfused rainbow trout (Salmo gairdneri) gills. Comp Biochem Physiol 29:1063–1070
Rolfe DF, Brand MD (1996) Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate. Am J Physiol 271(4 Pt 1):C1380–C1389
Rolfe DFS, Brand MD (1997) The physiological significance of mitochondrial proton leak in animal cells and tissues. Biosci Rep 17(1):9
Rolfe DF, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77(3):731–758
Schmid D, Burmester GR, Tripmacher R, Kuhnke A, Buttgereit F (2000) Bioenergetics of human peripheral blood mononuclear cell metabolism in quiescent, activated, and glucocorticoid-treated states. Biosci Rep 20(4):289–302
Seidelin M, Brauner CJ, Jensen FB, Madsen SS (2001) Vacuolar-type H+-ATPase and Na+, K+-ATPase expression in gills of Atlantic salmon (Salmo salar) during isolated and combined exposure to hyperoxia and hypercapnia in fresh water. Zoolog Sci 18(9):1199–1205
Shahsavarani A, Perry SF (2006) Hormonal and environmental regulation of epithelial calcium channel in gill of rainbow trout (Oncorhynchus mykiss). Am J Physiol Regul Integr Comp Physiol 291(5):R1490–R1498
Shuttleworth TJ (1972) A new isolated perfused gill preparation for the study of the mechanisms of ionic regulation in teleosts. Comp Biochem Physiol A Mol Integr Physiol 43(1):59–64
Smith RW, Houlihan DF (1995) Protein synthesis and oxygen consumption in fish cells. J Comp Physiol [B] 165(2):93–101
Smith MP, Dombkowski RA, Wincko JT, Olson KR (2006) Effect of pH on trout blood vessels and gill vascular resistance. J Exp Biol 209(Pt 13):2586–2594
Sobell HM (1985) Actinomycin and DNA transcription. Proc Natl Acad Sci USA 82(16):5328–5331
Somero GN, De Vries AL (1967) Temperature tolerance of some Antarctic fishes. Science 156(772):257–258
Somero GN, Fields PA, Hofmann GE, Weinstein RB, Kawall H (1998) Cold adaptation and stenothermy in Antarctic notothenioid fishes: what has been gained and what has been lost. In: Di Prisco G et al (eds) Fishes of Antarctica. A biological overview. Springer, Milan, pp 97–109
Stagg RM, Shuttleworth TJ (1982) Na+, K+ ATPase, quabain binding and quabain-sensitive oxygen consumption in gills from Platichthys flesus adapted to seawater and freshwater. J Comp Physiol 147:93–99
Sullivan G, Fryer J, Perry S (1995) Immunolocalization of proton pumps (H+-ATPase) in pavement cells of rainbow trout gill. J Exp Biol 198(Pt 12):2619–2629
Sullivan GV, Fryer JN, Perry SF (1996) Localization of mRNA for the proton pump (H+-ATPase) and Cl−/HCO3 − exchanger in the rainbow trout gill. Can J Zool 74(11):2095–2103
Takahashi M, Iwami T (1997) The summer diet of demersal fish at the South Shetland Islands. Antarct Sci 9(4):407–413
Toews DP, Holeton GF, Heisler N (1983) Regulation of the acid–base status during environmental hypercapnia in the marine teleost fish Conger conger. J Exp Biol 107(1):9–20
Wheeler KP, Whittam R (1962) Some properties of a kidney adenosine triphosphatase relevant to active cation transport. Biochem J 85:495–507
Whittam R (1962) The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J 84(1):110
Wieser W, Krumschnabel G (2001) Hierarchies of ATP-consuming processes: direct compared with indirect measurements, and comparative aspects. Biochem J 355(Pt 2):389–395
Zimmerman C (1997) On the ecology of Arctic and Antarctic fish: activity, sensory capabilities and behaviour. Ber Polarforsch/Rep Polar Res 231:1–137
Acknowledgments
The authors would like to thank Zora Zittier, Olaf Heilmeyer, Karl-Hermann Kock and his group and the Crew of Jubany Station and RV ‘Polarstern’ during cruise ANTXXIII/8 for their unfailing and excellent help in fish catching, maintenance and preparation. Furthermore, we want to thank Erich Dunker for constructing the sophisticated respiration chambers and Gijs de Rue for expert support with the technical drawings. This work is a contribution to the “European Project on Ocean Acidification” (EPOCA) which received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 211384. It is also a contribution to the PACES research program of the Alfred Wegener Institute and the BIOACD program funded by the Federal Ministry of Research, Germany. The study was supported by a student grant of the University of Bremen.
All animal experiments were conducted following German legislation. An approval of the work was issued by competent German authority (Freie Hansestadt Bremen, reference number 522-27-11/2-0; 28 November 2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deigweiher, K., Hirse, T., Bock, C. et al. Hypercapnia induced shifts in gill energy budgets of Antarctic notothenioids. J Comp Physiol B 180, 347–359 (2010). https://doi.org/10.1007/s00360-009-0413-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-009-0413-x