Abstract
Pacific hagfish (Eptatretus stoutii) are marine scavengers and feed on decaying animal carrion by burrowing their bodies inside rotten carcasses where they are exposed to several threatening environmental stressors, including hypercapnia (high partial pressures of CO2). Hagfish possess a remarkable capacity to tolerate hypercapnia, and their ability to recover from acid–base disturbances is well known. To deal with the metabolic acidosis resulting from exposure to high CO2, hagfish can mount a rapid elevation of plasma HCO3− concentration (hypercarbia). Once PCO2 is restored, hagfish quickly excrete their HCO3− load, a process that likely involves the enzyme carbonic anhydrase (CA), which catalyzes HCO3− dehydration into CO2 at the hagfish gills. We aimed to characterize the role of branchial CA in CO2/HCO3− clearance from the plasma at the gills of E. stoutii, under control and high PCO2 (hypercapnic) exposure conditions. We assessed the relative contributions of plasma accessible versus intracellular (cytosolic) CA to gill HCO3− excretion by measuring in situ [14C]-HCO3− fluxes. To accomplish this, we employed a novel surgical technique of individual gill pouch arterial perfusion combined with perifusion of the gill afferent to efferent water ducts. [14C]-HCO3− efflux was measured at the gills of fish exposed to control, hypercapnic (48 h) and recovery from hypercapnia conditions (6 h), in the presence of two well-known pharmacological inhibitors of CA, the membrane impermeant C18 (targets membrane bound, plasma accessible CA) and membrane-permeant acetazolamide, which targets all forms of CA, including extracellular and intracellular cytosolic CAs. C18 did not affect HCO3− flux in control fish, whereas acetazolamide resulted in a significant reduction of 72%. In hypercapnic fish, HCO3− fluxes were much higher and perfusion with acetazolamide caused a reduction of HCO3− flux by 38%. The same pattern was observed for fish in recovery, where in all three experimental conditions, there was no significant inhibition of plasma-accessible CA. We also observed no change in CA enzyme activity (measured in vitro) in any of the experimental PCO2 conditions. In summary, our data suggests that there are additional pathways for HCO3− excretion at the gills of hagfish that are independent of plasma-accessible CA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the majority of fish species, carbon dioxide (CO2) is transported in the blood as bicarbonate (HCO3−), a process that is highly dependent on the conversion of CO2 to HCO3− catalyzed by the enzyme carbonic anhydrase (CA). The general teleost model for CO2 excretion starts at the production site (tissues) with CO2 entering the red blood cells (RBC) where it is immediately hydrated to HCO3− and H+ in the presence of CA. While H+ ions are buffered by hemoglobin (Hb), HCO3− ions are exchanged for Cl− by a band 3 anion Cl−/HCO3− exchanger (CBE) (Perry 1986; Tufts and Perry 1998). At the gas-exchanger organ, CA catalyzes the dehydration of HCO3− that enters the RBC from the plasma, and CO2 diffuses out through the respiratory epithelium. However, not all fishes conform to this teleost pattern of CO2 transport and excretion.
Hagfishes lack the presence of the band 3 CBE at the RBC membrane (Ellory et al. 1987; Tufts and Perry 1998), meaning that 90% of the CO2 produced from metabolism is found in the plasma as HCO3− (Tufts et al. 1998; Esbaugh et al. 2009; Gilmour and Perry 2009; Gilmour 2012). Furthermore, the RBC of hagfishes displays only a minor Haldane effect (Tufts et al. 1998) and a low measured RBC CA activity (Esbaugh et al. 2009; Dichiera et al. 2020). Therefore, the RBC of hagfishes appears to play a limited role in blood CO2 transport. Instead, these fishes possess several pools of plasma-accessible CA that, in addition to intracellular (cytosolic) CA in the gill epithelial cells, are thought to play a crucial role in CO2 excretion. Esbaugh et al. (2009) provided molecular and biochemical evidence of a three-compartment model for CO2 excretion at the hagfish gill, describing the presence of plasma-facing membrane-bound CA isozymes, CA-IV and CA-XV in the gill epithelial cells, in addition to CA-XV in RBCs, and even some levels of circulating CA in the plasma. These findings indicate that the efficiency of CO2 transport and the capacity to withstand blood acid–base perturbations are likely related to the presence of these pools of CAs localized at these three distinct compartments.
Pacific hagfish (Eptatretus stoutii) are jawless marine scavengers that routinely feed on decaying animal carrion by burrowing their way into carcasses where they can be exposed to several noxious environmental stressors (Martini 1998). Conditions inside the carcasses include low oxygen levels (hypoxia), high environmental ammonia, and high partial pressures of CO2 (PCO2: hypercapnia). Hagfishes possess a remarkable capacity to tolerate these environmental disturbances, being known as physiological champions amongst vertebrates (Cox et al. 2011; Baker et al. 2015; Clifford et al. 2015a, 2018). The ability to recover from acid–base disturbances resulting from manipulations of environmental and/or blood pH or increases in water PCO2 has been well documented (for review see: Clifford et al. 2015b).
Baker et al. (2015) exposed hagfish to hypercapnia levels (6% CO2) similar to what might be encountered in a recently deceased air-breathing vertebrate (e.g. whale carcass). They observed a severe blood acidosis (1.2 pH unit reduction) and complete recovery within 48 h, which was directly associated with a striking elevation in plasma HCO3− concentration to levels that had never been documented in water-breathing vertebrates (~ 75 mM HCO3−), paralleled by a nearly equimolar reduction in plasma Cl− levels (Baker et al. 2015). Recently, Clifford et al. (2018) exposed hagfish to similar hypercapnic conditions (4% CO2) for 48 h and observed a comparable compensatory increase in plasma HCO3− (~ 70 mM). When hagfish were removed from hypercapnia and placed in control normocapnic conditions (0.04% CO2) for recovery, plasma PCO2 was restored within 2 h, while HCO3− was still elevated for at least 6 h, resulting in a severe metabolic alkalosis. The rate of HCO3− clearance from the plasma within 8 h of recovery was remarkably high, posing the question as to what mechanism could be involved in achieving this feat. The authors tested the hypothesis that the kidney could be a viable route for HCO3− excretion through measurements of glomerular filtration rate and could not find support for a significant role of the kidney (Clifford et al. 2018). Then, they proposed that the rapid restoration of plasma HCO3− following hypercapnic exposure was linked to the presence of branchial CA, since a systemic injection of a potent CA inhibitor (acetazolamide) completely inhibited HCO3− excretion. However, the relative importance of the different gill compartments that contain CA (i.e., RBC, intracellular and membrane-bound plasma-accessible CA) was not investigated.
The goal of our study was to characterize the relative roles of different branchial CAs in CO2/HCO3− excretion at the gills of Pacific hagfish (Eptatretus stoutii) under both control and high plasma HCO3− conditions. Using a pharmacological approach, we investigated the relative contributions of plasma accessible versus intracellular (cytosolic) CA to gill HCO3− excretion by measuring in situ HCO3− fluxes. Radiolabeled [14C]-HCO3− excretion was assessed through a novel surgical technique of individual gill pouch perfusion that combined the perfusion of both arterial and water supply (perifusion) through the cannulation of the dorsal aorta and the afferent and efferent gill water ducts (Clifford et al. 2022). In our working model, [14C]-HCO3− can move across the gills (from plasma to water) via the following pathways: (1) by being converted to CO2 by plasma-facing CA and diffusing across the gill epithelia (transcellularly); (2) entering gill ionocytes through a basolateral Cl−/HCO3− exchanger or an inward directed Na+/HCO3− co-transporter, subsequently being converted to CO2 by cytosolic CA and diffusing out and (3) similar to point (1) above, being converted to CO2 by plasma-facing CA, diffusing across the basolateral membrane, and being hydrated to HCO3− by cytosolic CA, and exchanged for Cl− via an apical Cl−/HCO3− exchanger. In our working models, the RBC is not considered as a valid pathway since in our in-situ preparations we used saline as a substitute for blood, without the addition of RBC. To tease apart the relative contributions of plasma accessible versus intracellular CA to the overall HCO3− offloading at the gills, we utilized two well-known pharmacological inhibitors of CA. The membrane-impermeant C18 (Supuran 2008) was used to assess the role of plasma accessible CA alone, while the membrane-permeant non-selective drug acetazolamide (Miller et al. 1950) was used to evaluate the contribution of total CA activity. The resulting intracellular (cytosolic) CA contribution was obtained from the difference between the two measures. As a membrane-impermeant drug, C18 can be used to test the validity of pathways (1) and (3) described above, while acetazolamide as a membrane permeant non-selective inhibitor, would have the potential to inhibit all three pathways. We also sought to determine whether the relative contributions of the different CAs at the gills are altered in response to hypercapnia, a condition that is known to result in compensatory elevations in plasma HCO3− (Baker et al. 2015; Clifford et al. 2018). Therefore, we tested hagfish kept in normocapnia (0.04% CO2), exposed to hypercapnia (5% CO2) for 48 h, and a third group that was allowed to recover from hypercapnia in normocapnia for 6 h. At the end of each exposure period, blood acid–base parameters and in situ [14C]-HCO3− flux across the isolated perfused gills were measured.
Material and methods
Animal collection and acclimation
Pacific hagfish (Eptatretus stoutii; average mass = 94.41 ± 3.72 g; total number animals used = 60) were caught using bottom-dwelling traps from Trevor Channel, near Bamfield Marine Sciences Centre (BMSC), British Columbia, Canada. Upon sorting on the boat, fish were immediately transferred to BMSC and held in 200-L darkened tanks with flow-through aerated sea water (12–13 ºC, 30 ppt) for 2–3 weeks. During acclimation, fish were fed frozen hake strips (Merluccius productus) weekly and were fasted for at least one week prior to any experimentation. All experiments were performed following the guidelines of the Canada Council for Animal Care, under joint approval of the animal care committees at BMSC (RS-19–12 and RS-20–14) and the University of Alberta (AUP00001126) and Department of Fisheries and Oceans Canada collection permits XR-185–2019 and XR-214–2020.
Hypercapnic exposure
Our experimental design consisted of three groups: normocapnia (control: 0.04% CO2), hypercapnia-exposed fish (5% CO2 for 48 h), and recovery from hypercapnia (6 h in control conditions). All fish were individually placed in 400-mL plastic containers with lids, which were modified with a fine mesh on each side to allow for free water flow while maintaining the fish inside. For the exposures, the fish containers were placed in darkened, 45-L plexiglass boxes, fitted with perimeter aeration, and served with flow-through seawater. Fish were allowed to settle in the experimental chambers for a minimum of 6 h or overnight before the start of the experiment. For hypercapnic exposure, the water flow to the boxes was reduced to a slow trickle, only what was necessary to avoid nitrogenous waste build-up, and regular aeration was replaced with a mixture of air (95%) and CO2 (5%). Gas flow delivery to the boxes from compressed CO2 cylinders (Praxair, Nanaimo, BC) was regulated by mass flow controllers (Alicat Scientific, Tucson, AZ, USA) connected to Flow Vision SC Software (v. 1.3.35). Preliminary tests showed that PCO2 equilibration within the boxes was achieved after 10–15 min of bubbling. A group of hagfish were exposed to hypercapnia (5% CO2) for 48 h and immediately anesthetized. In contrast, a recovery group was exposed to hypercapnia for the same duration and conditions as described above but allowed to recover in normocapnic water for 6 h prior to anesthesia and surgical procedures were conducted.
At the end of the experiment, fish were quickly transferred to an anesthetic solution (MS-222, Syndel Labs, Parksville, BC, Canada; 4 g/L neutralized to pH 7.8 with 5 M NaOH), and once anesthetized and weighed, a blood sample (~ 500 µL) was withdrawn from the posterior sinus using a heparinized syringe. The freshly-drawn blood was immediately transferred to a temperature-controlled water bath where pH was measured using an Orion ROSS micro pH electrode and meter (Fisher Scientific, Toronto, ON, Canada). After that, plasma was separated by centrifugation (12,000 g for 3 min), aliquoted, flash-frozen in liquid N2, and stored at – 80 ºC until analysis of total CO2 content were performed. Hagfish were then transferred to an operating table set up with a dissecting microscope for the surgical procedures.
In situ double cannulation of gill pouch
HCO3− fluxes were measured in individually isolated gill pouches from hagfish exposed to normocapnia, hypercapnia, or recovery. One gill pouch and its water ducts and blood supply were utilized for each hagfish. An incision was made along the anterior–posterior axis in the ventral region around the gill pouch openings. One gill pouch was then dissociated from its surrounding connective and fatty tissue but left intact in situ. To perifuse (water side of the gill epithelia) the gill pouch, the efferent and afferent water ducts of the 3rd–5th (usually the 4th pouch), from anterior (head) to posterior (tail) branchial pouch were cannulated with appropriately-sized PE-tubing (either PE90 or PE160; varying with animal size, BD, Intramedic, Franklin Lakes, NJ, USA) prefilled with artificial seawater (492.9 mM NaCl, 9.4 mM KCl, 10.2 mM CaCl2·2 H2O, 2.5 mM NaHCO3, 10 mM HEPES, 42.2 mM MgSO4·7 H2O, pH 7.9) and secured to the water ducts by a double knot using suture silk (braided silk, 5/0, Fine Science Tools, North Vancouver, BC, CA). Care was taken so that the tubing did not enter the pouch and damaged the internal ultrastructure of the gill. Blunt needles were used as bridges between the afferent water duct PE and peristaltic tubing, which were then connected to a peristaltic pump (Fisher Scientific) delivering artificial SW at a rate of 8 mL/h, being drawn-in from an air-equilibrated conical flask. The perifusate flow exited the gill pouch through the cannulated efferent water duct, and timed fractions were collected in pre-weighed 1.7 mL microcentrifuge tubes with a small (30 µL) amount of 1 M NaOH already in the vial to prevent loss of radioactivity as CO2. Perifusate volume was measured on a precision scale. Artificial SW was used to ensure consistency of ionic concentration and osmolality.
For simultaneous perfusion (vascular side of the gill blood vessel), the ventral aorta at the neighbouring region of the gill was cannulated with PE50 tubing ~ 1 cm prior to the branching afferent artery for the selected gill pouch. The PE50 was pre-filled with hagfish saline (490.4 mM NaCl, 8 mM KCl, 5 mM CaCl2·2H2O, 11 mM MgCl2·6H2O, 5 mM NaHCO3, 10 mM HEPES, 4 mM MgSO4·7 H2O, pH 7.75), and secured in place with 5/0 suture silk. The ventral aorta was then ligated after the branching to the selected gill pouch, and any other afferent gill arteries branching out from the ventral aorta and leading to other pouches were also tied with individual knots so that the only open vessel branching from the cannulated ventral aorta was the afferent blood vessel to the single perifused gill pouch. The afferent blood vessel was then connected to a different peristaltic pump (2 mL/h) using blunt needles as bridges between PE and peristaltic tubing. Flow rates chosen for the dual perfusion and perifusion are based on the early work of Forster and Fenwick (1994) and the recent work on E. stoutii by Clifford et al (2022). Practice trials were performed where the gill pouch was perifused and perfused with dyed solutions, to ensure that there was no leakage from one compartment to the other. Additionally, the integrity of the preparation was monitored throughout the duration of the flux experiments, by inspection of the gill pouch for the appearance of leaks, loose knots and connections. If any of the cannulas became loose and pulled away from the preparation, the data from that respective animal was not used. The entire surgical procedure was performed under a dissecting microscope and lasted between 30 and 45 min.
A preliminary experimental series was performed in order to evaluate the stability of the novel hagfish in situ double cannulations and perfusion surgical technique. This series was performed in control (normocapnic) fish only. The procedure described above was followed, and hagfish gill pouches were perfused with artificial SW (water side) and control saline (5 mmol/L HCO3−) for up to 120 min. Once it was confirmed that the gill preparation remained viable after this time frame, the experiments testing two known inhibitors of CA were performed.
In situ [14C]-HCO3 − fluxes
Prior trials were conducted to demonstrate the efficacy of C18 and acetazolamide in inhibiting hagfish CAs in vitro. To that, CA activity in the gills and plasma of normocapnic hagfish were assayed through the electrometric delta pH method (detailed below). To determine the role of CA in HCO3− excretion at the gills, individual hagfish gill pouches were perfused with CA inhibitor drugs, the membrane-impermeant C18 (0.2 mmol/L) or the membrane-permeant acetazolamide (0.2 mmol/L).
While the gill afferent water duct was always perifused with artificial seawater, the afferent blood vessel was perfused with hagfish saline at varying HCO3− concentrations that matched previously measured plasma HCO3− concentration in hagfish at normocapnic (5 mM HCO3−), hypercapnic (65 mM HCO3−) and during recovery (40 mM HCO3−) conditions (Clifford et al. 2018). The concentrations of HCO3− were adjusted to match the experimental conditions the fish had been subjected to prior to gill perfusion. PCO2 in the saline was always kept at a constant air-equilibrated level. Gill pouches were initially perfused with “cold” (radioactivity free) saline for 10 min and switched to saline spiked with radioactive [14C]-HCO3− (PerkinElmer, Waltham, MA, USA). The concentration of [14C]-HCO3− also varied, matching the varying concentrations of “cold” HCO3− in the saline. The ratio between hot and cold HCO3− (known as the specific activity: SA) was aimed to be maintained at ~ 30 cpm/nmol. However, SA varied between 15 and 50 cpm/nmol depending on the treatment. The gill was then perfused for 30 min with no-drug added (control period), followed by a 30 min perfusion with C18 and then 30 min perfusion with acetazolamide. The first 10 min of perifusate samples of each treatment were discarded, and the subsequent two 10-min samples were collected from each drug treatment for analysis of radioactivity. Perifusate seawater samples collected in the microcentrifuge tubes were considered to be indicative of the flux of HCO3− from the perfusate (arterial blood side) to the gill seawater side. At the end of the experiment, the perfused gill pouch was stripped of all tubing and suture silk, dissected, blotted dry with kimwipes, and weighed. Gill pouches were then dried at 65 ºC for 24–48 h and weighed again to obtain dry weight. An additional undisturbed gill pouch was collected immediately after the experiment, frozen in liquid N2, and stored at − 80 ºC for later analysis. Samples of the perfusion solutions (hagfish saline) were collected and processed for measurements of radioactivity and subsequent determination of the SA. Scintillation fluor (Optiphase, PerkinElmer, Waltham, MA, USA) was immediately mixed to the perifusate and perfusion solution samples in a 5:1 ratio (fluor: water), and samples were stored in the dark for 12 h to eliminate chemiluminescence prior to counting for beta emissions on a scintillation counter (LS6500; Beckman Coulter). The two 10-min perifusate replicates for each perfusion treatment (control, C18 and acetazolamide) were processed separately for radioactivity analyses and data was averaged.
The influence of endogenous plasma inhibitor on in situ [14C]-HCO3 − fluxes
To test for the possible presence of endogenous circulating inhibitors of CA in the hypercarbic hagfish plasma, we first exposed hagfish to 48 h of hypercapnia in conditions identical to those described earlier. After 48–54 h, fish were anesthetized and blood collected from the caudal sinus using heparinized syringes and needles. Plasma was obtained by centrifugation (12,000×g for 3 min), heparinized with 1000 IU/mL of sodium heparin in 500 mM NaCl, and kept on ice until use (usually within an hour). In addition, plasma from control (normocapnic) fish was also collected. Total CO2 content (TCO2) was measured in the samples of control and hypercapnia-exposed fish, and HCO3− concentration in the control plasma was adjusted to 70 mM to match the values measured in hypercapnia-exposed fish. These two pools of plasma were then spiked with appropriate amounts of radioactive [14C]-HCO3− to maintain approximately the same specific activity and used as the perfusion media for the measurements of in situ HCO3− fluxes (see above).
Individual gill pouches of control (normocapnic) hagfish were cannulated and perfused with heparinized plasma collected from normocapnic and hypercapnic fish in the following manner: 10 min with radioactivity-free plasma, followed by a 30 min perfusion with plasma collected from normocapnic fish. After 30 min, perfusion was switched to plasma collected from hypercapnia-exposed fish. The first 10 min of perfusate samples of each treatment were discarded and the subsequent two 10-min samples were collected for analysis of radioactivity. Afferent water ducts were perifused with artificial seawater prepared in the same manner as described above. The gill pouch was then excised and dried and perifusate samples were processed for measurements of [14C]-HCO3− flux as described above.
Analytical techniques and calculations
HCO3− specific activity (SA: cpm/nmol), which is the ratio between “hot” radiolabelled HCO3− and “cold” HCO3− in the perfusion solution was calculated as follows:
where total counts per mL (cpm/mL) were obtained as described previously and HCO3− concentration (µmol/mL) was obtained from measured TCO2 values and calculated as in Eq. 4. The total radioactivity in perfusate (cpmtotal) was obtained by multiplying the measured cpm/mL in the sample by the total perfusate volume (in mL).
HCO3− net fluxes (nmol HCO3−/mg/h) in the 10-min perifusate samples were calculated using the following calculation:
where cpmtotal (cpm/mL) was obtained as described above, time (min) is the duration of perfusate collection (10 min), W (mg) is the dry weight of the perfused gill pouch and SA is the specific activity as calculated in Eq. 1. Data from the two 10-min perifusate samples were then averaged for data analysis and statistical comparisons.
Plasma and saline total CO2 content (TCO2) were measured using a total CO2 analyzer (Corning 965 CO2 analyzer, Ciba Corning Diagnostic, Halstead, Essex, UK). The solubility coefficient of carbon dioxide (αCO2: mmol/L/Torr) and apparent pK of CO2 (pKapp) in hagfish plasma were calculated using equations from Heisler (1984), and described in full in Giacomin et al. (2018). With those two calculated parameters (αCO2 and pKapp), plasma PCO2 (Torr) was calculated from measured TCO2 and pH values using a modified Henderson-Hasselbalch equation:
Plasma [HCO3−] (mmol/L) was calculated as:
where TCO2 was measured as described above, αCO2 was calculated using equations from Heisler (1984), and PCO2 was calculated using Eq. 3.
Total carbonic anhydrase (CA, µmol H+/mg protein/min) activity was measured in gill or plasma samples from hagfish, using the electrometric delta pH assay (Henry 1991). Gill tissue (0.05–0.1 g) was homogenized with 8 volumes/g of tissue of ice-cold TRIS buffer (225 mM mannitol, 75 mM sucrose, 10 mM TRIS base, pH 7.4 adjusted with 10% H3PO4), using an automated bead homogenizer (Mini Bead Mill Homogenizer, VWR). The homogenate was centrifuged to remove cellular debris (7500×g for 20 min at 4 ºC) and the supernatant was separated and kept on ice until analysis. Plasma samples were assayed undiluted. The enzyme source (10 µL of supernatant or 50 µL of plasma) was added to a reaction vessel containing 5 mL of reaction buffer (TRIS buffer) and a stir bar, placed on ice over a magnetic stir plate, and allowed to equilibrate for 1 min. The reaction was initiated by adding 100 µL of CO2-saturated water, and the change in pH was observed over time using an Accumet AB15 pH meter (Fisher Scientific). The reaction rate was measured over the linear change in pH that occurred within the initial 1 min. The rate of uncatalyzed reaction (blank: no sample added) was also measured and subtracted from the catalyzed rate. The buffer capacity of the reaction buffer was measured and taken into account to convert the observed rate (pH units/min) to µmol H+/ min/mL. Gill and plasma enzyme activity were standardized by the amount of protein in the sample measured using the Bio-Rad (Bradford) protein assay using bovine serum albumin (BSA: Sigma-Aldrich) as a standard.
Statistical analyses
Data were tested for normality and homogeneity of variances (D’Agostino-Person and Bartlett’s tests, respectively) and, in case of failure, were transformed either using a log transformation or a square root transformation. Data were compared using either a one-way or repeated measures one-way analysis of variance (ANOVA) followed by post-hoc tests (Tukey’s and/or Bonferroni), depending on the data set analyzed. Mean values were considered significantly different when p < 0.05. All data are shown as means ± SEM (n = number of animals) with individual values present. All statistical analysis were performed using Graph Pad Prism v. 8.
Results
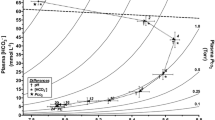
In control (normocapnia) conditions, hagfish blood pH was 7.69 ± 0.02 and 7.79 ± 0.01 in fish exposed to 48 h of hypercapnia (Fig. 1A), representing a fully compensated hypercapnic response. In fish that were recovered from hypercapnia for 6 h (return to normocapnic conditions), blood pH was 8.32 ± 0.02 (Fig. 1A). Hypercapnia-exposed fish showed a marked elevation (6.6 fold) in plasma HCO3−, in comparison to fish that remained in control conditions (Fig. 1B). Hagfish that were exposed to hypercapnia and allowed to recover for 6 h had a plasma HCO3− concentration of 68.3 ± 3.1 mmol/L (Fig. 1B). Blood PCO2 (Fig. 1C) was 5.7 ± 0.2 Torr in normocapnia, and 27.7 ± 1.2 Torr in hypercapnia-exposed fish (Fig. 1C), representing a 4.8-fold elevation. Blood PCO2 in recovery fish was not different than control fish (Fig. 1C). The graphical analysis of the blood acid–base status of hagfish exposed to hypercapnia is depicted in a pH/HCO3− (Davenport) diagram (Fig. 1D). Noteworthy, blood acid–base parameters were analyzed in different individuals, since we had three groups of fish exposed to the different PCO2 conditions.
Blood acid–base parameters in Eptatretus stoutii exposed to normocapnia (0.04% CO2: white bars), 48 h of hypercapnia (5% CO2: light grey bars), and 6 h of recovery from hypercapnia (0.04% CO2: dark grey bars). (A) blood pH, (B) plasma HCO3− (mmol/L), (C) blood PCO2 (Torr), (D) blood pH/HCO3− diagram, or Davenport diagram (Davenport, 1974). Each symbol in graphs A–C represents an individual data point. Bars sharing the same lowercase letters are not statistically different through a one-way ANOVA (p values for panels A–C are < 0.0001). Bars are means ± SEM (n numbers are specified at the base of each bar). Panel (D) graphically shows the changes in acid–base status of Eptatretus stoutii exposed to normocapnia (white triangles), 48 h of hypercapnia (grey squares), and 6 h of recovery from hypercapnia (black circles). The dashed straight line is the non-bicarbonate buffer line (NBB) determined by Wells et al. (1986). Grey lines are PCO2 isopleths (Torr). In this panel, some error bars are not visible due to the scale of pH and HCO3− axis (n numbers are the same as panels A–C)
The temporal stability of the novel hagfish in situ double cannulations and perfusion surgical technique was tested. HCO3− flux (calculated from the appearance of radiolabeled HCO3− in the perfusate) of 60–70 nmol HCO3−/mg/h was stable over time up to 110 min as there were no significant differences in fluxes measured every 10 min (Fig. 2).
HCO3− flux (nmol HCO3−/mg/h) in gills of Eptatretus stoutii perfused in situ under control conditions (no carbonic anhydrase CA inhibiting drug) over time. For this method control experiment, hagfish were maintained in normocapnic conditions (0.04% CO2). ns in the graph means that no statistically significant differences were detected through a repeated measures one-way ANOVA (p = 0.682). Horizontal lines are means ± SEM, which are represented as vertical lines above and below the mean (n = 6)
In vitro CA activity of gills and plasma of normocapnic hagfish was assayed in the presence of C18 or acetazolamide (at 0.2 mmol/L) (Fig. 3). Control gill CA activity was 682.25 ± 159.5 µmol H+/min/mg protein and C18 inhibited enzyme activity by 94.49% while acetazolamide resulted in a 97.9% inhibition (Fig. 3A). In plasma, control CA activity was 2.54 ± 1.3 µmol H+/min/mg protein and neither C18 nor acetazolamide had a significant inhibitory effect, despite a 77.8% reduction in the acetazolamide treatment (Fig. 3B).
Gill (A) and plasma (B) carbonic anhydrase (CA) enzyme activity (µmol H+/mg protein/min) of Eptatretus stoutii maintained in normocapnic conditions. In vitro enzyme activity (gill or plasma) was measured under control conditions (hatched bars), and in the presence of known CA inhibitors C18 at 0.2 mmol/L (grey bars) and acetazolamide (black bars) at 0.2 mmol/L. Bars sharing the same lowercase letters are not statistically different through a repeated measures one-way ANOVA. Bars are means ± SEM (n = 7 (A) and n = 5 (B))
HCO3− flux was evaluated in hagfish gill pouches perfused with control saline (without the addition of any CA inhibiting drugs), followed by saline spiked with the membrane-impermeant C18 and then saline spiked with membrane-permeable acetazolamide (Fig. 4). In fish kept in normocapnia, HCO3− flux under control conditions was 70.42 ± 14.48 nmol HCO3−/mg/h (Fig. 4A). There was no change in gill flux when perfused with C18 but a significant 72% reduction with the perfusion with acetazolamide (19.12 ± 4.05 nmol HCO3−/mg/h; Fig. 4A). In hypercapnia-exposed fish (Fig. 4B), control HCO3− flux rates were 747.9 ± 189.5 nmol HCO3−/mg/h. These rates were significantly inhibited by 38% with the perfusion with acetazolamide (458.02 ± 109.8 nmol HCO3−/mg/h; Fig. 4B). In hypercapnia-exposed fish, there was no significant differences in HCO3− flux between the perfusion with C18 and acetazolamide (Fig. 4B). In recovery from hypercapnia exposure, control HCO3− flux rates were 506.0 ± 62.3 nmol HCO3−/mg/h (Fig. 4C). Perfusion with C18 resulted in no significant inhibition of flux rates, while acetazolamide resulted in a significant 58% reduction (207.8 ± 44.63 nmol HCO3−/mg/h; Fig. 4C). There was a significant difference between C18 and acetazolamide, where fluxes were 46% lower in the latter treatment group (Fig. 4C).
HCO3− flux (nmol HCO3−/mg/h) in gills of Eptatretus stoutii perfused in situ in control conditions and in response to carbonic anhydrase (CA) inhibiting drugs C18 at 0.2 mmol/L (light grey bars) and acetazolamide at 0.2 mmol/L (dark grey bars). Symbols represent individual data points. Fish were exposed to (A) normocapnia, (B) 48 h of hypercapnia, and (C) 6 h of recovery from hypercapnia. Bars sharing the same lowercase letters are not statistically different through a repeated measures one-way ANOVA (p values for (A) is 0.008, (B) is 0.011 and (C) is < 0.0001). Bars are means ± SEM (n = 9 (A); 7 (B); and 10 (C))
HCO3− flux in hagfish gills perfused with plasma collected from control and hypercapnia-exposed fish is shown in Fig. 5. Perfusion with control plasma resulted in HCO3− flux of 654.37 ± 142.3 nmol HCO3−/mg/h (Fig. 5). There was a slight but significant increase in HCO3− flux when plasma collected from a hypercapnia-exposed fish was used as the perfusion solution (879.4 ± 112.0 nmol HCO3−/mg/h; Fig. 5).
HCO3− flux (nmol HCO3−/mg/h) in gills of normocapnic Eptatretus stoutii perfused in situ using plasma collected from fish kept in control (normocapnic) conditions (white bar) and plasma collected from fish that were exposed to 48 h of hypercapnia (dotted bar), as the perfusion solution. No CA inhibitors were used in this series. HCO3− concentration in the control fish plasma was matched to the measured concentration of the hypercapnia-exposed fish at 70 mmol/L. Bars are statistically different through a two-tailed, paired Student’s t-test (p = 0.0218). Symbols represent individual data points. Bars are means ± SEM (n = 7)
Total carbonic anhydrase enzyme activity in gills of normocapnic fish was 1450 ± 301.5 µmol H+/min/mg protein which was not significantly different from CA activity in fish exposed to hypercapnia (916.56 ± 98.78 µmol H+/min/mg protein), or fish that were recovered from hypercapnia exposure (1210.70 ± 131.3 µmol H+/min/mg protein; Fig. 6).
Total gill carbonic anhydrase (CA) enzyme activity (µmol H+/min/mg protein) of Eptatretus stoutii exposed to normocapnia (white bar), 48 h of hypercapnia (light grey bar), and 6 h of recovery from hypercapnia (dark grey bar). Assay was performed without the presence of CA inhibitors. ns in the graph means that no statistically significant differences were detected through a one-way ANOVA (p = 0.0748). Symbols represent individual data points. Bars are means ± SEM (n numbers are specified in each bar)
Discussion
Overview
The regulation of acid–base balance is amongst the most physiologically important processes in animals (Heisler, 1984). Pacific hagfish have a remarkable capacity to tolerate environmental changes in PCO2 and this ability is tightly linked to the capacity to mount an extensive accumulation of HCO3− to buffer the blood (Baker et al. 2015). Our goal was to characterize the relative contributions of the different CAs (plasma accessible and cytosolic) to the HCO3− offloading that occurs at the gills of hagfish. We employed a newly developed technique for in situ dual cannulation and perfusion of the individual gill pouch (Clifford et al. 2022), where both the afferent and efferent water ducts were cannulated, as well as the afferent blood vessel leading to the specific gill pouch being perfused. This technique allowed us to monitor the transport of radiolabeled [14C]-HCO3− from the blood side (saline perfusion) to the water side (perifusion). To evaluate the temporal stability of the surgical preparation, an experimental series was performed where HCO3− flux (measured as the appearance of radiolabeled [14C]-HCO3− in the perifusate) was assessed every 10 min. The rates are stable for up to 120 min (Fig. 2), attesting to the stability of the surgical preparation for an amount of time that is beyond the duration of a typical experiment, where gills would be perfused for approximately 90 min (see Methods). Overall, our HCO3− flux data cannot be entirely explained by the current proposed model of CO2 excretion at the gills of hagfishes, suggesting that plasma-accessible CA does not account for the all the basolateral transport of CO2/HCO3− from the plasma to the intracellular compartment, and subsequently to the water.
Role of different CAs in HCO3 − excretion under control conditions
The existing model for CO2 excretion across the gill in hagfishes suggests that the presence of a membrane-bound, plasma-accessible branchial CA is fundamental for the rapid dehydration of plasma HCO3− to molecular CO2, which will then diffuse across into the gill epithelium (Esbaugh et al, 2009; Gilmour, 2012; Clifford et al. 2015b; Brauner et al. 2019). Inside the gill epithelial cell, CO2 can either diffuse straight to the environment down a partial pressure gradient or be hydrated back to HCO3− and H+ (through a cytosolic form of CA), and be excreted at the apical membrane through a CBE in exchange for Cl− ions, and the H+ can be excreted in exchange for Na+ ions, through a Na+/H+ exchanger (NHE) (Tresguerres et al. 2007; Clifford et al. 2022). Thus, CO2 excretion at the gills would rely to a great extent on dehydration of HCO3− in the plasma catalyzed by branchial extracellular CA activity (Esbaugh et al. 2009). Based on this model, we predicted that HCO3− flux would be substantially inhibited when gill pouches were perfused in the presence of C18, a known membrane-impermeant inhibitor of CA (Scozzafava et al. 2000; Supuran 2008; Rummer et al. 2013). The presence of C18 has led to no change in normocapnic hagfish in situ HCO3− fluxes (Fig. 4A). We demonstrated that the concentration of C18 (0.2 mmol/L) used in the current study is sufficient to cause 94.49% inhibition of total CA activity in hagfish gill homogenates (measured in vitro) (Fig. 3A). The C18 concentration employed in our in situ perfusion experiments (0.2 mmol/L) is above threshold levels known to inhibit CA in a variety of different human cell lines (Mussi et al 2022) and also in in vivo rainbow trout experiments (Harter et al. 2019).
When gills were perfused with acetazolamide (0.2 mmol/L), a 72% inhibition in the rate of HCO3− flux was observed, suggesting that, under control conditions, intracellular cytosolic CA could be responsible for greater than half of the total HCO3− transport at the gills. These results point to an additional pathway of HCO3− transport that differs from the current understanding of how CO2/HCO3− are excreted at the gills of hagfishes (Brauner et al. 2019). Under control conditions, cytosolic CA could be operating by hydrating CO2 and supplying HCO3− ions exchanged for Cl− at the apical membrane through a transmembrane CBE (Clifford et al. 2015b). The Cl−/HCO3− exchange mechanism is an integral part of the response to perturbations in blood pH in Pacific hagfish (Baker et al. 2015; Clifford et al. 2018).
Our results provide functional evidence to only part of the current proposed model of CO2 excretion (Esbaugh et al. 2009; Gilmour and Perry, 2010; Gilmour 2012), and that being the direct role of intracellular CA in the clearance of HCO3− ions from the plasma to the water, calling into question the extent of the role of plasma-accessible CA in the maintenance of acid–base balance in Pacific hagfish. It should be noted that our perfusion protocol did not contain red blood cells (RBCs) in the perfusion saline, therefore we cannot account for any contribution of this compartment. However, it has been previously found that hagfish RBCs do not possess a CBE in the RBC membrane (Ellory et al. 1987; Tufts et al. 1998) and RBCs display very low CA activity (Esbaugh et al. 2009). In order for plasma accessible CA to operate at optimal conditions, it requires an availability of protons. One potential caveat that should be noted is that the buffer capacity of the saline utilized in our perfused gill preparation was lower than that of hagfish plasma. The buffer capacity of E. stoutii plasma measured and reported in Esbaugh et al (2009) is 5 mM CO2 pH unit−1, while the buffer capacity of our different salines ranged from 0.34 to 0.92 mmol H+ pH unit−1. The lack of appreciable C18 inhibition of plasma accessible CA could partially be influenced by the lowered buffer capacity of the salines utilized in this study.
Role of different CAs in HCO3 − excretion under hypercapnia and recovery conditions
Hagfish are known for their ability to tolerate extreme elevations in water PCO2 (hypercapnia) and for responding to it with an elevation in plasma HCO3− concentrations to levels higher than 100 mM to compensate for the severe respiratory acidosis (Baker et al. 2015; Clifford et al. 2018). In our study, after exposure to 5% CO2 for 48 h, hagfish blood pH rose slightly to 7.79, accompanied by a large elevation in plasma PCO2 from pre-exposure levels. Plasma HCO3− concentration also increased by 6.6-fold and once the 5% CO2 stimulus was removed, plasma PCO2 returned to near-normal levels by diffusion of CO2 down a partial pressure gradient from the blood to the environment. As a result, a very large metabolic alkalosis was present in the plasma due to the elevated plasma HCO3− (76.58 mmol/L) relative to the low PCO2. Baker et al. (2015) observed that within the first few hours (0–24 h) of exposure to hypercapnia, plasma Cl− decreased while plasma HCO3− increased in an almost 1:1 ratio, being nearly equimolar. As soon as hagfish were placed in normocapnic water, the opposite happened, where plasma HCO3− was reduced concomitantly as plasma Cl− increased, again in near equimolar amounts (Clifford et al. 2018). Similar equimolar changes in HCO3− and Cl− in the plasma have been found for rainbow trout in recovery from a metabolic alkalosis caused by an infusion with NaHCO3− (Goss and Wood 1990). These findings were later confirmed by the detection of slc26a6 transcripts (solute carrier anion exchanger protein) in the tissues of rainbow trout (Boyle et al. 2015), linking the involvement of CBE in acid–base maintenance in freshwater and marine fishes (Deigweiher et al. 2008; Boyle et al. 2015).
Interestingly, we were expecting to observe a far greater decrease in plasma HCO3− concentration at 6 h of recovery from hypercapnia (Fig. 1B). While Clifford et al. (2018), using similar experimental conditions, observed a 62% decrease in plasma HCO3− at 6 h, we observed none. At present, we are at a loss as to explain why the fish in our study were not displaying a similar response. We can assume that intraspecific differences and “batch effects” of fish that were collected from the wild many years apart could have an influence in these differences, but at this point we can only speculate. Also, it would have been interesting to follow plasma HCO3− concentrations for longer than 6 h as to gain a better understanding of the time-dependency of the physiological adjustments of our fish to the hypercapnia-induced hypercarbia. Additionally, measurements of plasma Cl− following hypercapnia and in recovery could have been useful in informing us regarding the extent of the recovery response. We observed a 32% reduction in HCO3− flux at the individually perfused gill pouch from hypercapnia-exposed to recovery fish under control conditions (no drug added) (Fig. 4B, C). Based on our plasma HCO3− concentration being higher than we were predicting at 6 h of recovery, our HCO3− flux in recovery conditions could have been underestimated, since the fish were not displaying a reduction in plasma HCO3− at 6 h. Clifford et al. (2018) hypothesized that an apically located, Cl−/HCO3− exchanger could be the dominant mechanism to regulate and transport HCO3− from the ionocytes to the environment. Once in recovery and after the loss of favourable partial pressure gradients for respiratory CO2 loss to the environment, it is likely that hagfish would resort to other mechanisms for HCO3− excretion rather than passive loss. While Clifford et al. (2018) found that branchial carbonic anhydrase (CA) mediated HCO3− excretion is an essential mechanism for the reduction in plasma HCO3− in a hypercapnia compensated hagfish, accounting for about 2/3 of the total HCO3− offloading at the gills, the authors could not determine the relative contributions of intracellular versus plasma accessible CA.
Our findings indicate that plasma accessible CA plays only a minor role in the excretion of HCO3− at the gills, in both hypercapnia and recovery conditions. We found no significant effect of C18 on HCO3− flux at the gills (Fig. 4A, B, C). In hypercapnia-exposed fish, acetazolamide inhibited HCO3− flux by 51%, while in recovery from hypercapnia, HCO3− flux was inhibited by 59%. Moreover, these results are very similar to the inhibition seen in control conditions (Fig. 4A). Therefore, based on our data, it seems that the importance of CA in the acid–base regulation and, more specifically, in the recovery from metabolic alkalosis is no different than its role under control conditions. Even though there are no working models for compensation from a post-hypercapnic metabolic alkalosis in hagfish, it is known that CA can respond to changes in ambient CO2 levels. Studies in marine teleosts have shown that after exposure to elevated PCO2, mRNA levels for cytosolic CA were reduced (Esbaugh et al. 2012; Tseng et al. 2013). Cytosolic CAs are known to hydrate CO2 and provide H+ and HCO3− for transport processes at the apical membrane (Gilmour and Perry, 2009; Esbaugh et al. 2012; Gilmour, 2012). When aquatic animals are faced with a respiratory acidosis due to rising environmental PCO2, they can compensate by both excreting H+ apically using a Na+/H+ exchanger NHE (Tresguerres et al. 2006) and reabsorbing HCO3− into the plasma across the basolateral membrane using either apical and basolateral isoforms of Cl−/HCO3− exchangers (CBE) or Na+/HCO3− (NBC) co-transporters (Hyde and Perry, 1987; Parks et al. 2007). Similarly, during a metabolic alkalosis, an animal will preferentially excrete HCO3− apically via a CBE and reabsorb H+ into the plasma. Our results suggest that in hagfish, the mechanism for HCO3− efflux during severe metabolic alkalosis is governed primarily by the combined actions of an intracellularly located CA and both a basolateral and an apical CBE on the gill epithelia. Overall, there was little contribution to HCO3− excretion from any plasma accessible CA present in the gill vasculature. Future experiments using combinations of stilbene inhibitors of HCO3− transporters (e.g., DIDS targeting Na+/HCO3− cotransporter) (Claiborne et al. 1997; Boyle et al. 2015) or the use of PI-PLC to remove plasma-facing CA from the gill vasculature will help to clarify the role, if any, of plasma accessible CA in recovery from a severe metabolic alkalosis.
One of the interesting aspects of the large hypercapnia-induced hypercarbia is that the HCO3− is retained in the plasma despite a very large gradient for loss. The absence of a significant contribution from gill plasma accessible CA during this period would aid in the fish maintaining such a large blood-to-water gradient for HCO3− even when plasma HCO3− exceeds 70 mM (Baker et al. 2015; Clifford et al. 2018). Based on our findings, it seems like plasma-accessible CA had a minimal role in HCO3− excretion in all three experimental conditions. Interestingly, we also observed that hagfish branchial CA activity did not change between control and hypercapnia-exposed fish (Fig. 6).
The presence of circulating levels of CA inhibitors in the plasma is variable amongst fishes (Henry et al. 1997; McMillan et al. 2019; Brauner et al. 2019) and the functional significance of having circulating CA inhibitors and their distribution amidst fishes is still not fully resolved. However, in the case of the hypercarbic hagfish, it would work to preserve the high concentrations of HCO3− in the plasma. Therefore, we investigated if there were any endogenous inhibitors of CA activity in the plasma of hypercarbic hagfishes by measuring in situ HCO3− flux in control fish using plasma collected from hypercarbic hagfish (see methods) as the perfusion “saline”. We found that instead of an inhibitory effect, perfusion with hypercarbic plasma resulted in a small but significant stimulation of HCO3− flux (Fig. 5). Therefore, similar to elasmobranchs (Gilmour et al. 2002; Gilmour and Perry 2010; Mcmillan et al. 2019), the plasma of hagfish does not seem to possess an endogenous inhibitor of CA as previously demonstrated by Esbaugh et al. (2009), and hypercapnic exposure does not seem to stimulate the release of any other type of endogenous CA inhibitor. In fact, our results could indicate that hypercarbic hagfish may possess a certain degree of circulating levels of CA in the plasma. For our preliminary experimental series, where we tested the efficacy of C18 and acetazolamide as hagfish CA inhibitors, we also measured the enzyme activity in the plasma (Fig. 3B). Although our results are somewhat lower than the ones reported by Esbaugh et al (2009), we could still detect a small level of CA activity in the plasma of normocapnic hagfish. Studies performed in teleost fishes have shown that both infusion (Esbaugh et al. 2012) and injection (Tzaneva et al. 2011) of bovine CA can lower plasma PCO2 during hypercapnia exposure, as the injection of CA can alleviate the chemical constrains that limit CO2 diffusion during hypercapnia. Therefore, to the contrary of what we had initially predicted, hypercapnia-exposed hagfish could be releasing more CA into the plasma as a mean to increase CO2 excretion against a concentration gradient.
If plasma inhibition of CA does not seem to be a mechanism involved in the retention of HCO3 in hypercarbic hagfish, how can hagfish maintain such a large HCO3− gradient between plasma and water? The decrease in HCO3 permeability at the fish gill could be associated with a downregulation of the putative NBC transporter at the basolateral membrane of the gill epithelia (Tresguerres et al. 2007), or even a downregulation of the CBE at the apical membrane, or a combination of both. Alternatively, elevation of plasma HCO3− could use a primary active NHE that has been recently shown to occur on the apical membrane in hagfish gills (Clifford et al. 2022) in concert with a basolateral CBE and cytoplasmic CA to actively acquire HCO3− in the plasma space.
Conclusions and perspectives
Our results point to lesser contribution of plasma-accessible CA in clearing accumulated HCO3− in the plasma, while cytosolic intracellular CA seems to be mediating more than half of the total HCO3− efflux at the hagfish gill. These results are unique for hagfish and generates questioning into additional putative transport mechanisms that are available for hagfish to move CO2/HCO3− from the plasma to the intracellular compartment and further to the water. Some of the known transporters associated with acid–base and ion regulation in teleost fish have not yet been identified in hagfish. Recently, the whole genome of a closely related hagfish species (E. burgeri; Yamaguchi et al. 2021) has been sequenced, opening an entire avenue of potential new transporters to be characterized. Undoubtedly, CA is an important component for the acid–base balance of hagfish, and further research into its role in regulating plasma HCO3− is warranted.
Abbreviations
- CO2 :
-
Carbon dioxide
- HCO3 − :
-
Bicarbonate
- CA:
-
Enzyme carbonic anhydrase
- H+ :
-
Proton
- Hb:
-
Hemoglobin
- CBE:
-
Chloride/bicarbonate exchanger
- RBC:
-
Red blood cell
- PCO2 :
-
Partial pressure of CO2
- SA:
-
Specific activity
- C18:
-
Membrane-impermeant inhibitor of carbonic anhydrase
References
Baker DW, Sardella B, Rummer JL, Sackville M, Brauner CJ (2015) Hagfish: champions of CO2 tolerance question the origins of vertebrate gill function. Sci Rep 5:1–8
Boyle D, Clifford AM, Orr E, Chamot D, Goss GG (2015) Mechanisms of Cl- uptake in rainbow trout: cloning and expression of slc26a6, a prospective Cl-/HCO3- exchanger. Comp Biochem Physiol (a) 180:43–50
Brauner CJ, Shartau RB, Damsgaard C, Esbaugh AJ, Wilson RW, Grosell M (2019) Acid-base physiology and CO2 homeostasis: regulation and compensation in response to elevated environmental CO2. In: Grosell M, Munday PL, Farrell AP, Brauner CJ (eds.) Fish Physiology Vol 37, Elsevier Inc, pp. 69–132.
Claiborne JB, Perry E, Bellows S, Campbell J (1997) Mechanisms of acid-base excretion across the gills of a marine fish. J Exp Zool 279:509–520
Clifford AM, Goss GG, Wilkie MP (2015a) Adaptations of a deep sea scavenger: high ammonia tolerance and active NH4+ excretion by the Pacific hagfish (Eptatretus stoutii). Comp Biochem Physiol (a) 182:64–74
Clifford AM, Weinrauch AM, Goss GG (2018) Dropping the base: recovery from extreme hypercarbia in the CO2 tolerant Pacific hagfish (Eptatretus stoutii). J Comp Physiol B 188:421–435
Clifford AM, Goss GG, Roa JN, Tresguerres M (2015b) Acid/base and ionic regulation in hagfish. In: Edwards, S. L. and Goss, G. G. (eds.) Hagfish Biology, CRC Press pp. 277–292.
Clifford AM, Wilkie MP, Edwards SL, Tresguerres M, Goss GG (2022) Dining on the dead in the deep: active NH4+ excretion via Na+/H+ (NH4+) exchange in the highly ammonia tolerant Pacific hagfish, Eptatretus stoutii. Acta Physiologica, doi:https://doi.org/10.1111/apha.13845.
Cox GK, Sandblom E, Richards JG, Farrell AP (2011) Anoxic survival of the Pacific hagfish (Eptatretus stoutii). J Comp Physiol B 181:361–371
Davenport HW (1974) The ABC of acid-base chemistry: The elements of physiological blood-gas chemistry for medical students and physicians. 6ed. University of Chicago Press, Chicago, IL.
Deigweiher K, Koschnick N, Pörtner HO, Lucassen M (2008) Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am J Physiol Regul Integr Comp Physiol 295:1660–1670
Dichiera AM, Mcmillan OJL, Clifford AM, Goss GG, Brauner CJ, Esbaugh AJ (2020) The importance of a single amino acid substitution in reduced red blood cell carbonic anhydrase function of early-diverging fish. J Comp Physiol B 190:287–296
Ellory JC, Wolowyk MW, Young JD (1987) Hagfish (Eptatretus stoutii) erythrocytes show minimal chloride transport activity. J Exp Biol 129:377–383
Esbaugh AJ, Gilmour KM, Perry SF (2009) Membrane-associated carbonic anhydrase in the respiratory system of the Pacific hagfish (Eptatretus stouti). Respir Physiol Neurobiol 166:107–116
Esbaugh AJ, Heuer R, Grosell M (2012) Impacts of ocean acidification on respiratory gas exchange and acid-base balance in a marine teleost, Opsanus beta. J. Comp Physiol B Biochem Syst Environ Physiol 182:921–934
Forster ME, Fenwick JC (1994) Stimulation of calcium efflux from the hagfish, Eptatretus cirrhatus, gill pouch by an extract of corpuscles of stannius from an eel (Anguilla dieffenbachii): Teleostei. Gen Comp Endocrinol 94(1):92–103
Gilmour KM (2012) New insights into the many functions of carbonic anhydrase in fish gills. Respir Physiol Neurobiol 184:223–230
Gilmour KM, Perry SF (2009) Carbonic anhydrase and acid-base regulation in fish. J Exp Biol 212:1647–1661
Gilmour KM, Perry SF (2010) Gas transfer in dogfish: a unique model of CO2 excretion. Comp Biochem Physiol (a) 155:476–485
Gilmour KM, Shah B, Szebedinszky C (2002) An investigation of carbonic anhydrase activity in the gills and blood plasma of brown bullhead (Ameiurus nebulosus), longnose skate (Raja rhina), and spotted ratfish (Hydrolagus colliei). J Comp Physiol B 172:77–86
Goss GG, Wood CM (1990) Na+ and Cl- uptake kinetics, diffusive effluxes and acidic equivalent fluxes across the gills of rainbow trout. II. Responses to bicarbonate infusion. J Exp Biol 152:549–571
Harter TS, Zanuzzo FS, Supuran CT, Gamperl AK, Brauner CJ (2019) Functional support for a novel mechanism that enhances tissue oxygen extraction in a teleost fish. Proc R Soc B 286:20190339
Heisler N (1984) Acid-base regulation in fishes. In: Hoar WS, Randall DJ (eds.) Fish physiology Vol. 10A (eds), Academic Press. pp. 315–401.
Henry RP, Gilmour KM, Wood CM, Perry SF (1997) Extracellular carbonic anhydrase activity and carbonic anhydrase inhibitors in the circulatory system of fish. Physiol Zool 70:650–659
Hyde BYDA, Perry SF (1987) Acid-base and ionic regulation in the American eel (Anguilla rostrata) during and after prolonged aerial exposure: branchial and renal adjustments. J Exp Biol 133:429–447
Martini FH (1998) The ecology of hagfishes. In: Jorgensen JM, Lomholt JP, Weber RE, Malte H (eds) The biology of hagfishes. Springer, Netherlands Dordrecht, pp 55–75
McDonald DG, Cavdek V, Calvert L, Milligan LC (1991) Acid-base regulation in the atlantic hagfish Myxine glutinosa. J Exp Biol 215:201–215
Mcmillan OJL, Dichiera AM, Wilson JM, Esbaugh AJ, Brauner CJ (2019) Blood and gill carbonic anhydrase in the context of a chondrichthyan model of CO2 excretion. Phys Biochem Zool 92:554–566
Miller WH, Dessert AM, Roblin RO (1950) Heterocyclic sulfonamides as carbonic anhydrase inhibitors. J Am Chem Soc 72:4893–4896
Mussi S, Rezzola S, Chiodelli P, Nocentini A, Supuran CT, Ronca R (2022) Antiproliferative effects of sulphonamide carbonic anhydrase inhibitors C18, SLC00111 and acetazolamide on bladder, gliobastoma and pancreatic cancer cell lines. J Enzyme Inhib Med Chem 37:280–286
Nikinmaa M, Berenbrink M, Brauner CJ (2019) Regulation of erythrocyte function: Multiple evolutionary solutions fo respiratory gas transport and its regulation in fish. Acta Physiol 227:e13299
Parks SK, Tresguerres M, Goss GG (2007) Blood and gill responses to HCl infusions in the Pacific hagfish (Eptatretus stoutii). Can J Zool 85:855–862
Perry SF (1986) Carbon dioxide excretion in fishes. Can J Zool 64:565–572
Rummer JL, Mckenzie DJ, Innocenti A, Supuran CT, Brauner CJ (2013) Root effect hemoglobin may have evolved to enhance general tissue oxygen delivery. Science 340:1327–1329
Scozzafava A, Briganti F, Ilies MA, Supuran CT (2000) Carbonic anhydrase inhibitors: synthesis of membrane-impermeant low molecular weight sulfonamides possessing in vivo selectivity for the membrane-bound versus cytosolic isozymes. J Med Chem 43:292–300
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7:168–181
Tresguerres M, Parks SK, Goss GG (2006) V-H+-ATPase, Na+/K+-ATPase and NHE2 immunoreactivity in the gill epithelium of the Pacific hagfish (Eptatretus stoutii). Comp Biochem Physiol A 145:312–321
Tresguerres M, Parks SK, Goss GG (2007) Recovery from blood alkalosis in the Pacific hagfish (Eptatretus stoutii): Involvement of gill V-H+ –ATPase and Na+/K+ –ATPase. Comp Biochem Physiol (a) 148:133–141
Tseng YC, Hu MY, Stumpp M, Lin LY, Melzner F, Hwang PP (2013) CO2-driven seawater acidification differentially affects development and molecular plasticity along life history of fish (Oryzias latipes). Comp Biochem Physiol (a) 165:119–1130
Tufts BL, Perry SF (1998) Carbon dioxide transport and excretion. In: Perry SF, Tufts BL (eds) Fish Physiology, vol 17. Academic Press, pp 229–281
Tufts BL, Vincent CJ, Currie S (1998) Different red blood cell characteristics in a primitive agnathan (M. glutinosa) and a more recent teleost (O. mykiss) influence their strategies for blood CO2 transport. Comp Biochem Physiol (a) 119:533–541
Tzaneva V, Gilmour KM, Perry SF (2011) Respiratory responses to hypoxia or hypercapnia in goldfish (Carassius auratus) experiencing gill remodelling. Respir Physiol Neurobiol 175:112–120
Wells RMG, Forster ME, Davidson W, Taylor HH, Davie PS, Satchell GH (1986) Blood oxygen transport in the free-swimming hagfish, Eptatretus cirrhatus. J Exp Biol 123:43–53
Yamaguchi K, Hara Y, Tatsumi K, Nishimura O, Jeramiah JS, Kadota M, Kuraku S (2020) Inference of a genome-wide protein-coding gene set of the inshore hagfish Eptatretus burgeri. bioRxiv. https://doi.org/10.1101/2020.07.24.218818
Acknowledgements
This study was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant G.G.G. (#203736). J.M.D. was supported by an NSERC Undergraduate Student Research Award (USRA). The authors wish to acknowledge two anonymous reviewers whose invaluable comments have greatly improved our manuscript. We would like to thank Drs. Eric Clelland and Tao Eastham (Bamfield Marine Sciences Centre research coordinators) and the BMSC research and animal care supporting staff for excellent and invaluable support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Pelster.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giacomin, M., Drummond, J.M., Supuran, C.T. et al. The roles of plasma accessible and cytosolic carbonic anhydrases in bicarbonate (HCO3−) excretion in Pacific hagfish (Eptatretus stoutii). J Comp Physiol B 192, 713–725 (2022). https://doi.org/10.1007/s00360-022-01459-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-022-01459-0