Abstract
To develop non-invasive techniques for monitoring steroid stress hormones in the feces of free-living animals, extensive knowledge of their metabolism and excretion is essential. Here, we conducted four studies to validate the use of an enzyme immunoassay for monitoring fecal cortisol metabolites in snowshoe hares (Lepus americanus). First, we injected 11 hares with radioactive cortisol and collected all voided urine and feces for 4 days. Radioactive metabolites were recovered predominantly in the urine (59%), with only 8% recovered in the feces. Peak radioactivity was detected an average of 3.5 and 5.7 h after injection in the urine and feces, respectively. Second, we investigated diurnal rhythms in fecal cortisol metabolites by measuring recovered radioactivity 2 days after the radioactive cortisol injection. The total amount of radioactivity recovered showed a strong diurnal rhythm, but the amount of radioactivity excreted per gram of feces did not, remaining constant. Third, we injected hares with dexamethasone to suppress fecal cortisol metabolites and 2 days later with adrenocorticotropic hormone to increase fecal cortisol metabolites. Dexamethasone decreased fecal cortisol metabolites concentrations by 61% and adrenocorticotropic hormone increased them by 1,000%, 8–12 h after injection. Fourth, we exposed hares to a simulated predator (dog). This increased the fecal cortisol metabolites concentrations by 175% compared with baseline concentrations 8–12 h after exposure. Thus, this enzyme immunoassay provides a robust foundation for non-invasive field studies of stress in hares.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adrenal cortex secretes glucocorticoids to aid in daily functions such as the regulation of energy storage and of diurnal rhythms, and in response to activities such as courtship, copulation, and hunting (Sapolsky et al. 2000). However, when presented with a stressor (defined as any environmental disturbance that disrupts homeostasis) animals respond by increasing their glucocorticoid secretion. This response is primarily mediated by the hypothalamic–pituitary–adrenal (HPA) axis (Owen et al. 2005).
Understanding stress hormones is key to the study of natural populations. This knowledge can address questions about how stressors affect the survival and reproductive success of free-living animals and broader ones pertaining to management strategies, relocation or reintroduction, habitat disturbance, and population dynamics (Boonstra and Singleton 1993; Wasser et al. 1997; Creel et al. 2002; Cyr and Romero 2007). Since a stress response leads to an increase of blood glucocorticoids, their concentrations have been used as an index of stress in a wide range of studies (e.g., Boonstra et al. 1998; Hopster et al. 2002; Häcklander et al. 2003; Romero and Reed 2005). However, capture, handling, and bleeding can cause a rapid increase in blood glucocorticoid concentrations (Haemisch et al. 1999; Romero and Romero 2002). An alternative, non-invasive method has been developed to monitor glucocorticoids through the use of their fecal metabolites (see review by Touma and Palme 2005; Palme et al. 2005).
Lagomorphs (hares, rabbits and pikas) have been the focus of a range of ecological research with studies on mating, dispersal, antipredator behaviors, population dynamics, among others. Since the ecology is so well known for many lagomorphs, they make excellent study species for investigating endocrinology of free-living animals. However, only two previously published studies (Teskey-Gerstl et al. 2000 on European hares; Monclús et al. 2006 on rabbits) have investigated non-invasive techniques for measuring stress hormones. Teskey-Gerstl et al. (2000) found that a group-specific enzyme immunoassay (EIA) for 11,17-dioxoandrostanes (11,17-DOA; 11-oxoetiocholanolone-EIA) established by Palme and Möstl (1997) was best suited for hares due to its high immunoreactivity. However, Teskey-Gerstl et al. (2000) did not use the rigorous validation method of dexamethasone (DEX) suppression and adrenocorticotropic hormone (ACTH) stimulation of adrenal cortisol and its subsequent appearance in the feces. Here, we extend their initial findings to assess the suitability of this EIA in the snowshoe hare, Lepus americanus. The primary glucocorticoid in snowshoe hares is also cortisol (Boonstra and Singleton 1993), and thus we will be monitoring fecal cortisol metabolites (FCM).
Our study had four objectives. First, to determine the proportion of FCM excreted via the urine and feces, the time course of metabolite excretion, and the sex differences we injected administration of radioactively-labelled cortisol. Second, to investigate diurnal rhythms in snowshoe hares, we monitored FCM over 2 days. Third, to test whether changes in adrenocortical activity can be reliably monitored in FCM using this EIA, we carried out two tests: a DEX suppression test and an ACTH stimulation test. DEX acts as an artificial glucocorticoid agonist that mimics endogenous cortisol and reduces circulating cortisol concentrations via the negative feedback mechanism of the HPA axis (Axelrod and Reisine 1984). ACTH stimulation tests the responsiveness of the adrenals directly and acts to increase circulating blood cortisol concentrations (Miller and Tyrrell 1995). Previous work on snowshoe hares (Boonstra and Singleton 1993; Boonstra et al. 1998) has shown that this DEX-ACTH challenge causes major changes in plasma cortisol levels. Fourth, to assess whether a natural stressor resulted in an increase in FCM levels, we introduced a simulated predator (dog).
Materials and methods
Animals and housing
Snowshoe hares are the smallest of the hare species world wide. In the southwestern Yukon, adults weigh 1,200–1,800 g. They are found throughout Canada and the northern parts of the USA. They are most active at dusk and dawn when they do the majority of their feeding; however, due to the extremely long summer days in the far north hare’s active period ranges from 2000 to 0800 h during our study. In the boreal forest, hares have a 10-year population cycle that impacts the entire ecosystem. Hares can be subject to high predation risk, especially during the decline phase of their population cycle when approximately 95% die because of predation (Krebs et al. 1995).
Eleven adult (five males and six females, >12 months old) snowshoe hares were used for these experiments. Hares were live-trapped in the Shakwak Trench east of Kluane Lake, Yukon Territory (61°N, 138°W) using Tomahawk traps (Tomahawk Live Trap Co., Tomahawk Wisconsin, USA.) (see Krebs et al. 1986 for details). Hares were placed in individual pens 5–6 days prior to the start of the experiments to allow for habituation. Owing to space limitations, all animals were housed in the same room. Hares were exposed to ambient conditions of both external temperature and light.
Individual pens were 60 × 60 × 120-cm wire cages. Each cage was visually separated with a plywood wall, and partially covered on the top and front by a burlap cloth. The floor of each cage was made with 1.30-cm reinforced wire mesh that allowed both urine and feces to pass freely. Feces were then caught on a finer wire mesh placed on an angle below the cage. This allowed feces to roll to the collection tray and not be contaminated by urine. Voided urine passed through the finer mesh and was collected in a plastic catch basin below.
Animals were fed ad libitum with standard medicated rabbit chow (Unifeed, Okotoks, Alberta; Unifeed Ltd Cat. #19–2103, 18% protein, crude fat 2%, crude fiber 18%) supplemented daily with natural browse (small branches with leaves and bark from Salix sp.) and watered ad libitum.
Experimental design
The chronology of the entire experimental procedure, including acclimation time, time of initiation of each experiment, and exact times when feces were collected, can be found in Table 1. All injections were made between 1700 and 1900 h. Free-living hares experience intense predation pressure thus a trained dog, as a simulated predator, was chosen as a natural stressor.
For each hare, all feces or urine produced was collected at the end of the period and pooled; we analyzed an aliquot from each sample. Samples from all hares were analyzed separately. The weight of each fecal sample or volume of each urine sample was determined. Results in the figures are plotted at the time of collection.
Administration of radioactive cortisol
[1,2,6,7-3H] Cortisol (code#TRK407, batch#124) was obtained from Amersham Bioscience UK Ltd (Buckinghamshire, UK). It had a radiochemical purity of 98.9% as tested by high performance liquid chromatography (HPLC) on a Hypersil MOS column using a water:methanol gradient. Ten hares were injected with 1,110 kBq of tritiated cortisol in a 450 μl solution containing 6% toluene, 10% ethanol and 84% saline; one was injected with 610.5 kBq. All injections were into an ear vein when the hare was restrained in a burlap bag. The entire procedure took 5–15 min per hare.
Diurnal rhythm
This experiment used data from the last 2 days of the radioinfusion experiment. Hares were not disturbed for 48 h prior to the start of this time course, and hares had recovered from the stress of handling and injection (see Fig. 1). We determined the amount of radioactivity excreted per gram of feces by measuring a 0.300 ± 0.05 g sample of homogenized ground feces for each sample time. We determined the total amount of radioactivity excreted at each time point by multiplying the amount of radioactivity per gram of feces by the weight of the entire fecal sample collected for that time point.
DEX suppression test
DEX was obtained from Sabex (Montreal, Canada). Seven hares were injected with both 0.4 mg/kg into the ear vein and 1.6 mg/kg into the thigh muscle. Four hares were used as controls and were injected with similar volumes of physiological saline. The entire procedure took 5–15 min per hare.
ACTH stimulation test
ACTH (Synacthen Depot) was obtained from, CIBA (Ontario, Canada). Seven hares were injected with 80 μg/kg into the thigh muscle. Four hares were used as controls and were injected with similar volumes of physiological saline. The entire procedure took 5–15 min per hare.
Natural stressor challenge
Hares were visually exposed to a medium sized, trained dog. The dog was allowed to smell the perimeter of each individual hare holding pen for 10–30 s at 1800, 2400 h, and the following morning at 0800 h. The dog was in contact for a total of 5 min at each time. The dog whined and sniffed against each cage but did not bark or lunge at any of the hares. Since all hares were housed in the same room, they served as there own control comparing FCM concentrations during and after the stressor.
Sample handling and extraction
Urine and feces were frozen at −80°C immediately after collection. They were transported to the University of Toronto at −20°C where fecal samples were stored at −80°C until drying and extraction, and urine samples were stored at 4°C until analyzed.
Fecal samples were first freeze dried using a Lyophilizer (LabConco, Missouri, USA) for 14–18 h to control for fiber and water content (Wasser et al. 1993) and homogenized with a coffee grinder. We then extracted 0.300 ± 0.05 g of the ground feces with 5 ml of 80% methanol (v/v) for 30 min at 15,000 rpm on a multi-vortexer. After centrifugation (15 min at 2,500g) an aliquot of the supernatant was either analyzed immediately if radioactive or diluted (1:10) with assay buffer and frozen at −80°C until analysis with the EIA.
Determination of radioactivity
After extraction a 250-μl aliquot of the fecal supernatant was counted with 5 ml ACS scintillation fluid (Amersham, USA) and its radioactivity measured in a liquid scintillation counter (Packard Tri-Carb 2900TR, Boston, MA) with quench correction. A 100-μl aliquot of each urine sample was counted with 5 ml ACS scintillation fluid with quench correction. The excretion of radioactivity in both the feces and urine was calculated by adjusting for the total weight of feces in each sample and the total volume of urine in each sample.
Determination of immunoreactivity
The immunoreactivity of fecal samples was determined using the 11-oxoetiocholanolone-EIA developed by Palme and Möstl (1997). Teskey-Gerstl et al. (2000) found that this EIA detected the highest amount of immunoreactive metabolites in fecal samples of the European hare (Lepus europaeus) as compared with cortisol- or corticosterone-EIAs. The assay had a sensitivity range from 2–500 pg per well. The inter- and intra-assay coefficients of variation were 18.6 and 6.7%, respectively.
Statistical analysis
All data are expressed as means ± 1 SEM, unless otherwise stated. Repeated measures ANOVA and t tests were performed using the software package STATISTICA 6. The assumption of normality was tested with Shapiro–Wilks test and of homogeneity of variances with Levene’s test. If these assumptions were not met, the appropriate adjustment was made (log-transformation of data or Greenhouse–Geisser adjustment; Quinn and Keough 2003). Comparisons of the means were considered significant if P < 0.05. Sexes were pooled as there were no significant differences between males and females for any variable examined (repeated measures ANOVA comparing sex at each variable; P > 0.05).
Results
Route and time course of steroid excretion
A total of 25 serial samples were collected after the injection of the isotope. The mean total recovery of administered radioactivity was 66.7%, with 58.30 ± 2.34% from the urine and 8.40 ± 0.55% from the feces. The pattern of radioactive excretion in both urine and feces varied significantly over time (urine: F 24,216 = 56.49, P < 0.0001; feces: F 23,230 = 26.06, P < 0.0001, Fig. 1). Radioactivity in urine samples peaked at 2000 h, 3.47 h ± 0.18 after injection, and declined rapidly thereafter. The radioactivity in the peak sample was significantly higher than in the samples collected immediately before (t 10 = –6.78, P < 0.0001) or after (t 10 = 2.91, P < 0.025) the peak. Radioactivity in fecal samples peaked at 2400, 5.73 ± 0.27 h after injection, and declined rapidly thereafter. The radioactivity in the peak sample was significantly higher than in the samples collected immediately before (t 10 = − 3.60, P < 0.005) or after (t 10 = 3.21, P < 0.01) the peak. In both the urine and the feces, background levels were reached within 2 days.
Diurnal rhythm
Snowshoe hares exhibited a diurnal rhythm in the total amount of radioactivity excreted in the feces after injection with radioactive cortisol. However, this pattern was not reflected in the amount of radioactivity per gram of feces, but rather was due to the amount of feces defecated (Fig. 2). The total amount of radioactivity excreted varied significantly over 48 h (F 10,100 = 5.81, P < 0.0001), with a clear pattern of increase and decline, with peak levels occurring between 2400 and 0400 h and minimum levels occurring at 1600 h. The amount of radioactivity excreted per gram of feces remained constant over the 48-h period. The slight decrease in radioactivity over time is due to the hares having less to excrete (the nature of a radioactive experiment). For all subsequent studies we compare the amount of FCM per gram of feces (i.e., FCM concentration).
DEX suppression test
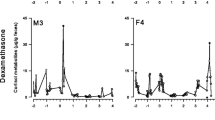
DEX suppressed FCM concentrations by 61% from 1,111 ng/g ± 328 (control) to 487 ± 85 (DEX) at 10 h post-injection (i.e., at 0400 h, t 9 = −2.61, P < 0.05; Fig. 3). FCM concentrations did not increase to baseline levels until 28 h after the injection.
ACTH stimulation test
ACTH injections increased FCM concentrations by 1,000%, from 1,054 ng/g ± 151 (control) to 1,1130 ± 3,317 (ACTH) at 10 h post-injection (i.e., at 0400 h, t 9 = 6.09, P < 0.0005, data log transformed; Fig. 4). FCM concentrations did not decline to baseline levels until 24 h after the injection.
Natural stressor challenge
In the simulated predator experiment, FCM concentrations varied significantly over time (F 12,120 = 5.72; P < 0.005; Fig. 5). To assess if the exposure to the dog stressed the hares, we compared FCM levels 10-h post-exposure (given that this was the lag time for both the DEX and ACTH challenges above) to FCM levels 24 h later. At 10-h post-exposure to the initial dog exposure (i.e., at 0400 h), FCM concentrations were 175% higher compared with FCM concentrations at 0400 h the next day (t 10 = 3.43, P < 0.01). To assess the stress response of hares to repeated dog challenges we exposed them a second (at 2400 h) and third (at 0800 h) time. Due to the timing of these challenges we could only compare FCM levels 8-h post-exposure to those 24 h later. At 8-h post-exposure to the second and third dog exposure, FCM concentrations were 151% (t 10 = 2.26, P = 0.054), and 173% (t 10 = 2.76, P < 0.05) higher than those 24 h later. FCM concentrations did not decline to baseline values until 24 h after the final exposure to the dog.
Discussion
This study validates a non-invasive method for assessing adrenocortical activity in hares. We show that the 11-oxoetiocholanolone-EIA can be used to reliably monitor changes in cortisol concentrations via measures of FCM in snowshoe hares. Specifically, we found that: (1) the mean excretion of radioactivity peaked in the urine and feces at 3.47 h ± 0.18 and 5.73 h ± 0.27, respectively; (2) there was a clear diurnal rhythm in the total amount of radioactivity excreted; (3) changes in adrenal functioning via DEX suppression or ACTH stimulation were detected in FCM concentrations; and (4) a dog stressor caused FCM concentrations to increase markedly.
Route and time course of steroid excretion
We recovered approximately 66% of the injected 3H-cortisol, with 8% being recovered in the feces. The loss of isotope is most likely due to loss in the urine collection. Copious amounts of urine were voided and thus isotope could have been absorbed into the plastic collection sheet, or dust that settled onto the sheet. As fecal pellets are large and conspicuous, we do not believe that there was isotope loss from feces and that fecal recovery reflects true FCM levels. Furthermore, this value is similar to that found by Teskey-Gerstl et al. (2000) in the European hare, L. europaeus. This limited recovery does not affect the measurement itself, as the group-specific EIAs are highly sensitive assays with an extremely low detection limit within the picogram range (Möstl et al. 2005).
The time delay between the 3H-cortisol injection and the peak appearance was 3–4 h in the urine and was 5–6 h in the feces (Fig. 1). Since we injected hares at 1800 h (the approximate start of the active phase of hares), this delay indicates the rate of cortisol metabolism and excretion during the active phase of snowshoe hares. In European hares, Teskey-Gerstl et al. (2000) found the peak appearance of radiometabolites in the feces occurred at 1,000, 23 h after the injection. However, they injected hares during the inactive phase at 1100 h (their active phase is from 1900 to 0700 h). Touma et al. (2003) suggested that an animal’s activity pattern and gut passage time plays an important role in the excretion of metabolites. Touma et al. (2003) found that the peak delay was 2.5 times later in mice injected at the end of their active phase compared with those injected at the beginning. Thus, if we had injected hares at the end of their active phase (i.e., 0700–0800 h), we would predict a 12–15-h delay. This delay is still not as long as Teskey-Gerstl et al. (2000) found, and may be due to species specific differences in active compared with inactive phase metabolism and excretion. This difference may also be due to the difference in diet and thus metabolism and excretion of the wild-caught hares used here compared with the captive-raised hares used by Teskey-Gerstl et al. (2000).
Diurnal rhythm
The total amount of radioactivity excreted showed a clear diurnal rhythm, but the amount of radioactivity per gram of feces did not (Fig. 2). Wasser et al. (1994) also found that the amount of steroid metabolites differed between the total amount excreted versus the amount excreted per gram of feces. Diurnal patterns in FCM (measured as per gram of feces) have been shown for Columbian ground squirrels (Bosson et al. 2008), mice (Touma et al. 2003), and rats (Bamberg et al. 2001). In snowshoe hares, peak levels occurred between 2400 and 0400 h, approximately 6–10 h after the beginning of their active period (Fig. 2). However, the diurnal rhythm seen here was not due to fluctuating concentrations of radioactivity excreted in the feces, as reflected by the per gram analysis of radioactivity (Fig. 2). Rather the diurnal rhythm was a function of the total amount of feces defecated (Fig. 2 inset). Touma et al. (2003) suggested that an animal’s activity rhythm and gut passage time play an important role in the diurnal rhythm of FCM. As the rate of excretion increases, the rate of bile secretion and thus metabolite secretion also increases (Randall et al. 2000). Therefore, times of peak defecation should contain peak amounts of FCM; however, if the total amount of FCM increases in proportion to the increase in total feces voided, FCM concentrations may not reflect the diurnal rhythm of the total FCM. This may be important in animals that defecate copious amounts, such as snowshoe hares, as it would dilute FCM concentrations and mute the diurnal rhythm. In animals that defecate large amounts, only a small portion of the total feces is collected during fecal analysis, and an even smaller portion is used. Thus a diurnal rhythm may not be detected or concentrations of FCM may be corrected for diurnal rhythms unnecessarily.
Though Wasser et al. (1994) showed that the overall rate of fecal steroid metabolite excretion differed from the concentration of fecal steroid metabolite excretion, they did not assess diurnal changes. Our study is the first to show this difference with fecal corticosteroid metabolites and the diurnal pattern. Thus, comparisons of FCM collected at different times of the day will only be valid if researchers have intimate knowledge of the diurnal rhythm.
Adrenal suppression (DEX) and stimulation (ACTH)
Boonstra et al. (1998) showed that in the snowshoe hare both DEX and ACTH injections resulted in rapid changes in plasma cortisol concentrations. Using the 11-oxoetiocholanolone-EIA, we were able to detect changes in FCM concentrations 10 h after either the DEX or ACTH injection compared with saline injected controls (Figs. 3, 4). Since we collected samples every 4 h, the changes in FCM concentrations could occur as early as 8 h post-injection or as late as 12 h. In other small mammals, changes in adrenal functioning have also been detected in FCM concentrations within 24 h [e.g., 4–10 h in mice (Touma et al. 2003); 6–30 h in Belding’s ground squirrels (Mateo and Cavigelli 2005); 12 h in European rabbits (Monclús et al. 2006)]. This lag time between hormonal changes in the blood and their appearance as metabolites in the feces is likely due to the variation in gut passage time of the animal. This relationship between FCM excretion and gut passage time is well documented for a diverse array of mammalian and avian species (Wasser et al. 2000; Palme et al. 2005; Touma and Palme 2005).
Natural stressor challenge
The dog stressor significantly increased FCM concentrations 175% 10 h after the initial exposure compared with levels 24 h later (Fig. 5). Although the second and third stress events did not continue to increase FCM concentrations, levels were still higher than the following day. The reason the latter stressors did not continue to increase cortisol levels may be due to a short-term corticoid induced feedback inhibition of the HPA axis and incomplete recovery of the HPA axis responsiveness. Other studies of changes in HPA responsiveness as a result of previous stress exposure have resulted in habituation of the HPA axis to repeated stressors of the same type (Gądek-Michalska and Bugajski 2003; Jaferi et al. 2003). In the snowshoe hare, a species subject to intense predation pressure, this temporary HPA habituation to repeated short-term stressors might function to protect the animal from the negative effects of long-term exposure to high cortisol concentrations.
Although, other studies on lagomorphs have also found significantly elevated FCM concentrations after a biological stressor, homogenized pooled samples were used and short time-scale changes in FCM concentrations could not be detected (Teskey-Gerstl et al. 2000; Monclús et al. 2006). This is the first study on lagomorphs with such a high-resolution sampling regime that allowed us to track short time-scale changes in FCM concentrations. Clearly, the EIA is able to detect changes in adrenal functioning in the snowshoe hare.
Abbreviations
- FCM:
-
Fecal cortisol metabolites
- EIA:
-
Enzyme immunoassay
- DEX:
-
Dexamethasone
- ACTH:
-
Adrenocorticotropic hormone
- 11,17-DOA:
-
11,17-Dioxoandrostanes
- HPA:
-
Hypothalamic–pituitary–adrenal
References
Axelrod J, Reisine TD (1984) Stress hormones; their interaction and regulation. Science 224:452–459
Bamberg E, Palme R, Meingassner JG (2001) Excretion of corticosteroid metabolites in urine and faeces of rats. Lab Anim 35:307–314
Boonstra R, Singleton GR (1993) Population declines in the snowshoe hare and the role of stress. Gen Comp Endocrinol 91:126–143
Boonstra R, Hik D, Singleton GR, Tinnikov A (1998) The impact of predator-induced stress on the snowshoe hare cycle. Ecol Monogr 79:371–394
Bosson OC, Palme R, Boonstra R (2008) Assessment of the stress response in Columbian ground squirrels: laboratory and field validation of an enzyme immunoassay for fecal cortisol metabolites. Physiol Biochem Zool (in press)
Creel S, Fox JE, Hardy A, Sands J, Garrott B, Peterson RO (2002) Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv Biol 16:809–814
Cyr NE, Romero LM (2007) Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen Comp Endocrinol 151:82–89
Gądek-Michalska A, Bugajski J (2003) Repeated handling, restraint, or chronic crowding impair the hypothalamic–pituitary–adrenocortical response to acute restraint stress. J Physiol Pharmacol 54:449–459
Häcklander K, Möstl E, Arnold W (2003) Reproductive suppression in female Alpine marmots, Marmota marmota. Anim Behav 65:1133–1140
Haemisch A, Guerra G, Furkert J (1999) Adaptation of corticosterone—but not β-endorphin—secretion to repeated blood sampling in rats. Lab Anim 33:185–191
Hopster H, Bruckmaier RM, van der Werf JTN, Korte SM, Machuhova J, Korte-Bouws G, van Reenen CG (2002) Stress responses during milking; comparing conventional and automatic milking in primiparous dairy cows. J Dairy Sci 85:3206–3216
Jaferi A, Nowak N, Bhatnagar S (2003) Negative feedback functions in chronically stressed rats: role of the posterior paraventricular thalamus. Physiol Behav 78:365–373
Krebs CJ, Gilbert BS, Boutin S, Sinclair ARE, Smith JNM (1986) Population biology of snowshoe hares. I. Demography of food-supplemented populations in the southern Yukon, 1976–84. J Anim Ecol 55:963–982
Krebs CJ, Boutin S, Boonstra R, Sinclair ARE, Smith JNM, Dale MRT, Martin K, Turkington R (1995) Impact of food and predation on the snowshoe hare cycle. Science 269:1112–1115
Mateo JM, Cavigelli SA (2005) A validation of extraction methods for noninvasive sampling of glucocorticoids in free-living ground squirrels. Physiol Biochem Zool 78:1069–1084
Miller WL, Tyrrell JB (1995) The adrenal cortex. In: Felig P, Baxter JD, Frohman LA (eds) Endocrinology and metabolism, 3rd edn. McGraw-Hill, New York, pp 555–711
Monclús R, Rödel HG, Palme R, von Holst D, de Miguel J (2006) Non-invasive measurement of the physiological stress response of wild rabbits to the odour of a predator. Chemoecology 16:25–29
Möstl E, Rettenbacher S, Palme R (2005) Measurement of corticosterone metabolites in birds’ droppings: an analytical approach. Ann NY Acad Sci 1046:1–18
Owen D, Andrews MH, Matthews SG (2005) Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev 29:209–226
Palme R, Möstl E (1997) Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Int J Mammal Biol 62(Suppl II):192–197
Palme R, Rettenbacher S, Touma C, El-Bahr SM, Möstl E (2005) Stress hormones in mammals and birds. Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann NY Acad Sci 1040:1–10
Quinn GP, Keough MJ (2003) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Randall DJ, Burgren W, French K (2000) Eckert animal physiology. mechanisms and adaptations, 4th edn. W·H. Freeman and Company, New York
Romero LM, Reed JM (2005) Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp Biochem Physiol A 140:73–79
Romero LM, Romero RC (2002) Corticosterone responses in wild birds: the importance of rapid initial sampling. Condor 104:129–135
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress response? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Teskey-Gerstl A, Bamberg E, Steineck T, Palme R (2000) Excretion of corticosteroids in urine and faeces of hares (Lepus europaeus). J Comp Physiol B 170:163–168
Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann New York Acad Sci 1046:54–74
Touma C, Sachser N, Möstl E, Palme R (2003) Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278
Wasser SK, Thomas R, Nair PP, Guidry C, Southers J, Lucas J, Wildt DE, Monfort SL (1993) Effects of dietary fibre on fecal steroid measurements in baboons (Papio-cynocephalus-cynocephalus). J Reprod Fert 97:569–574
Wasser SK, Monfort SL, Southers J, Wildt DE (1994) Excretion rates and metabolites of oestradiol and progesterone in baboon (Papio cynocephalus) faeces. J Reprod Fert 101:213–220
Wasser SK, Bevis K, King G, Hanson E (1997) Noninvasive physiological measures of disturbance in the northern spotted owl. Conserv Biol 11:1019–1022
Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL (2000) A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120:260–275
Acknowledgments
We thank Andrew T. Sheriff for his contribution to this project. The Natural Sciences and Engineering Research Council of Canada, and the Department of Indian Affairs and Northern Development supported this research. We thank Andrew Williams and the Arctic Institute of North America, University of Calgary, for providing facilities at Kluane Lake. The University of British Columbia Animal Care Committee approved all procedures in accordance with the guidelines of the Canadian Council for Animal Care.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Sheriff, M.J., Bosson, C.O., Krebs, C.J. et al. A non-invasive technique for analyzing fecal cortisol metabolites in snowshoe hares (Lepus americanus). J Comp Physiol B 179, 305–313 (2009). https://doi.org/10.1007/s00360-008-0314-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-008-0314-4