Abstract
Fecal steroid analysis is a powerful noninvasive tool for behavioral endocrinology, but enzyme immunoassays (EIAs) require experimental validation before they are applied. We conducted a physiological validation of an in-house EIA measuring fecal cortisol metabolites (FCMs) by performing an adrenocorticotrophic hormone (ACTH) challenge, dexamethasone suppression, and control saline solution injection experiment with six male and five female bearded capuchins (Sapajus libidinosus). We also took advantage of a presumably stressful incident to perform a biological validation for females. In addition, we conducted high-performance liquid chromatography (HPLC) immunograms to characterize the FCMs measured in both sexes of bearded capuchin, and in a closely related species (S. nigritus, black capuchin monkeys). Male and female S. libidinosus showed FCM peaks after ACTH injections, and females also showed FCM peaks in the biological validation. Three of four individuals (two males and one female) had an FCM peak shortly after injection of dexamethasone and both sexes then showed prolonged low FCM levels after dexamethasone injection. We observed no effects of saline solution injections. The time to peak FCM excretion after ACTH injection or a stressful incident varied 1.5–8.5 h. HPLC results revealed no differences in FCM profiles between sexes or species and suggest that the EIA can also be used in male and female S. nigritus. Our results validate an in-house EIA for both sexes of S. libidinosus but show large individual variation in FCM excretion, which highlights the need for carefully planned feces collection in endocrinologial research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cortisol is a hormone released by the adrenal glands in response to stressful situations (Möstl and Palme 2002), and therefore has been used as an indicator of well-being in captive and wild animals (Boinski et al. 1999; Schapiro et al. 1993; Sheriff et al. 2011). Cortisol measures provide quantitative physiological data that allow evaluation of the validity and/or efficiency of management practices. Researchers usually measure cortisol or its metabolites in matrices such as urine, feces, hair, and saliva (Sheriff et al. 2011) for which collection is noninvasive, inflicting little or no stress on the animals. Those matrices (except saliva) provide indirect measures of cortisol levels, because hormone metabolites are measured and not the hormone itself (Palme et al. 2005).
Enzyme immunoassays (EIAs) are frequently used to quantify hormone metabolites in fecal matrices. They depend on the cross-reactivity of the metabolites with the antibodies used in the assays (Möstl et al. 2005). Concentrations of fecal cortisol metabolites (FCMs) may vary between the sexes and across species (Möstl et al. 2005; Palme et al. 2005). Thus, it is essential to validate assays for FCMs for both sexes of a species (Dantzer et al. 2014; Möstl and Palme 2002).
There are two main types of validation experiments, physiological and biological validations (Touma and Palme 2005). Physiological validation is the most common and is ideally based on comparing measurements of FCMs among three experimental groups: one group that receives synthetic adrenocorticotropic hormone (ACTH), which should yield a peak in FCM levels; a second group that receives dexamethasone (a synthetic glucocorticoid) that should cause a reduction in FCM levels due to negative feedback on the hypothalamic–pituitary–adrenal (HPA) axis, inhibiting glucocorticoid release into the bloodstream (Touma and Palme 2005); and a third group that receives a saline solution as a control, where FCM levels should only reflect the effects of the experimental procedures (capture/restraint/injection) on the HPA axis.
Biological validation is used primarily in cases in which it is not ethical to conduct experiments, such as with species threatened with extinction. It consists of measuring FCMs before and after a relevant stressful event such as restraint, transportation, or physical examination (Touma and Palme 2005), which should yield a peak in FCM levels. This kind of validation shows that the technique can detect biologically meaningful changes in adrenocortical activity. However, biological validation does not allow the same controls as the physiological validation and negative results are not conclusive, given that the event used may not have been stressful enough to elicit FCM peaks (Fanson et al. 2017).
Capuchins (Sapajus) have been widely used in biomedical research (Mittermeier et al. 1994) and cognitive studies (Fragaszy and Adams-Curtis 1998). Capuchins are also common zoo exhibits (Fragaszy and Adams-Curtis 1998) and, therefore, require welfare monitoring. Of special interest is the bearded capuchin (Sapajus libidinosus), which has been studied in the context of tool use (Canale et al. 2009; Emidio and Ferreira 2012; Visalberghi et al. 2009), morphology (Wright et al. 2009), socioecology (Izar et al. 2012; Spagnoletti et al. 2012; Verderane et al. 2013), sexual behavior (Falótico and Ottoni 2013), endocrinology (Mendonça-Furtado et al. 2014), and interactions with humans (Spagnoletti et al. 2017). Only one study reporting a physiological validation is available for capuchins (colony hybrids, Sapajus sp.: Wheeler et al. 2013). That study tested four EIAs after an ACTH injection and found that a corticosterone EIA was the most sensitive to changes in glucocorticoid production. However, this study had a limited sample size (one male and one female) and cannot necessarily be generalized to other species owing to the possibility of differences in glucocorticoid metabolism in closely related species (Heistermann et al. 2006; Palme et al. 2005).
We conducted a physiological validation of an EIA for female and male bearded capuchins (Sapajus libidinosus), using three treatments: 1) ACTH injection, 2) dexamethasone injection, and 3) saline solution injection. We predicted that FCM levels in subjects would increase after ACTH administration and decrease after dexamethasone administration. The saline injection allowed us to control for FCM changes owing to stressful effects of handling the subjects. We also tested for intraspecific (between sexes) and interspecific differences (between S. libidinosus and the closely related species S. nigritus) in FCM profiles.
Methods
Subjects and Housing Conditions
We studied six male and five female captive adult bearded capuchins (Sapajus libidinosus) at the Centro de Triagem de Animais Silvestres (CETAS) in the city of Cabedelo/Paraíba, Brazil.
All subjects were wild born and arrived at CETAS after seizure or rescue by the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis, the Brazilian’s government agency responsible for wild animals, in various circumstances including possession by illegal breeders, subjects of animal traffic, captured invading homes, or illegal possession as pets (Pagano et al. 2009). O. Mendonça-Furtado identified the species based on body color pattern, hair distribution (Oliveira and Langguth 2006), body proportions (Wright et al. 2015), and the occurrence of capuchin species in Northeast Brazil (the region where the individuals were seized). We assumed that capuchins were not hybrids because they were wild born.
We used capuchins that the veterinarian staff of CETAS considered able to survive in the wild, and that were to be reintroduced soon after the study. We used only subjects that staff considered healthy when we conducted the experiment. All bearded capuchins kept in CETAS at the time of the experiment were housed collectively in a 6 × 2 × 3 m enclosure with indoor and outdoor areas and received monkey chow in the morning and fruits, roots, and vegetables in the afternoon. Water was available ad libitum.
Four days before the beginning of sample collection, we housed the 11 subjects individually in 1.5 × 1.0 × 0.5 m cages. FCM data suggest that this was enough time for subjects to habituate physiologically to this new housing condition. Provisioning of food and water was the same as in the group enclosure. To facilitate feces collection, we placed cages outdoors on a canvas from 07:00 to 17:30 h, during the days of the experiment (males: from October 19 to October 28, 2009; females: from February 22 to March 2, 2011). Between 17:30 and 07:00 h we placed cages in an indoor aisle near the subjects’ regular enclosure. We designed this procedure to prevent escapes or predator attacks during the hours when the observer was not allowed to stay in the CETAS facilities.
ACTH and Dexamethasone Administration
We divided capuchins randomly into three groups for treatment (Table I). We injected the ACTH group with Synacthen Dépôt (1 mg/ml, Novartis Pharma S.A.) injections. We injected the dexamethasone group with Decadron (2 mg/ml, Aché Laboratórios Farmacêuticos S.A.). We injected the saline solution group with a sterile isotonic saline solution. We gave all injections intramuscularly.
We first conducted the experiment with males and then later with females, when more females were available at CETAS. We administered the same amount of ACTH (100 μg/kg body weight) and dexamethasone (2.5 mg/kg body weight) to males as in a previous experiment with captive capuchins (Sapajus spp., previously Cebus apella; Torres-Farfan et al. 2008), and the saline solution injection had a fixed volume of 2 ml. As we had access to results of the experiment with males before starting the experiment with females, we increased ACTH dosage (200 μg/kg) for the females, expecting to produce a more pronounced FCM peak. Because females weigh less than males (Fragaszy et al. 2016), the injection volume was smaller for females than for males, so we set the volume of saline injection at the mean volume of the drugs injected in females from the ACTH and dexamethasone groups.
The experiment lasted 9 days. Immediately after the habituation period we started a 4-day feces collection period (more details in “Feces Collection and Steroid Analysis”) to measure baseline FCM concentrations. On the morning (ca. 09:00 h) of the fifth day, we restrained capuchins with a net, weighed them, and administered the appropriate treatment (Table I). We continued collecting feces from the fifth to the ninth day to observe changes in FCM related to the treatment received.
Biological Validation (“Bite Incident”)
A potential biological validation occurred on the last day of the females’ physiological validation experiment (fourth day after the injection; Fig. 1). The researcher was bitten on the thumb by a blond capuchin (Sapajus flavius) held in an enclosure located ca. 20 m away from the experimental subjects. When the researcher reacted to the bite, all the monkeys in the enclosure (about 10) vocalized intensely, similar to the way that they would react to a fight within the group. Although our experimental subjects could not see the S. flavius individuals that vocalized, they could hear the vocalizations, and might have been aroused by them.
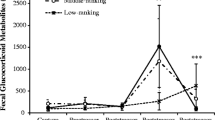
Individual fecal cortisol metabolite profiles from a male and a female bearded capuchin (Sapajus libidinosus) from groups injected with ACTH, dexamethasone, and saline solution. The experiment was performed at the Centro de Triagem de Animais Silvestres, in the city of Cabedelo/Paraíba, Brazil, from October 19 to October 28, 2009 (males) and from February 22 to March 2, 2011 (females). Gray dots represent outliers and black dots represent wild outliers. Asterisk indicates when a S. flavius bit the researcher, potentially arousing female subjects.
Feces Collection and Steroid Analysis
We collected any feces voided by the subjects at 30 min intervals from 07:00 to 17:30 h. This interval provides a good compromise between the time needed to release new feces (allowing us to collect all feces voided during the day) and prevention of steroid metabolism post-defecation. We collected 948 samples during the experiment (491 from males and 457 from females). We placed samples in polypropylene tubes, identified them, and stored them immediately in a freezer at –20 °C. We transported feces in a cooler with reusable ice packs to the Laboratory for Hormonal Measurement at the Federal University of Rio Grande do Norte, Brazil, where we extracted fecal cortisol metabolites using methanol, following the procedure (0.5 g of feces plus 5 ml of 80% methanol) described in Palme et al. (2013). We transferred an aliquot of 100 μl of each extract to a new propylene tube and placed it in a water bath coupled to an airflow until dry. We transported dried extracts to the Vetmeduni Vienna, where we resuspended them in 100 μl of methanol (80%) for EIA analysis. We measured FCM using an in-house cortisol enzyme immunoassay (EIA), described in detail by Palme and Möstl (1997). Briefly, this EIA used a polyclonal antibody raised in rabbits against cortisol-3-CMO:BSA and a biotinylated cortisol (also coupled to cortisol-3-CMO) as label. Its cross-reactions are as follows (ordered by decreasing percentage): cortisol, 100%; cortisone, 9.6%; corticosterone, 6.2%; 5α-dihydrocortisol, 4.6%; 5α-tetrahydrocortisol, 0.8%; 5β-tetrahydrocortisol, 0.1%; several other 5β-reduced metabolites, <0.01%. Although cortisol itself is rarely present in feces (Bahr et al. 2000), this assay cross-reacts with some of its metabolites, specifically those exhibiting a 21-ol-20-one structure (Heistermann et al. 2006). Serial dilutions (1:2.5) of three peak concentration samples (from both sexes) were in parallel with the standard curve. We also tested an 11-oxoetiocholanolone EIA (measuring metabolites with a 5β-3α-ol-11-one structure; for details of this EIA see Möstl et al. 2002) on all samples from two males and two females from 1 day before ACTH injection to 1.5 days after it (Electronic Supplementary Material [ESM] Fig. S1). As the results of this assay correlated with those for the cortisol EIA, but the increases were less pronounced in females, we analyzed all remaining samples only with the cortisol EIA. Intra- and interassay coefficients of variation of pool samples were <12% and the sensitivity of the EIA was 0.5 ng/g. We assayed all samples in duplicate. We expressed the concentration of FCM as nanograms per gram of wet fecal matter.
HPLC Separation
We intended to perform the validation experiment (ACTH and dexamethasone challenges) for Sapajus libidinosus and S. nigritus. However, we could not find captive S. nigritus subjects in a condition that would allow experimentation. Instead, we used high-performance liquid chromatography (HPLC) of pooled samples for each species and sex to characterize the metabolites produced and to test for sex and species differences (Möstl et al. 2005; Wheeler et al. 2013).
We collected these samples from wild populations of Sapajus nigritus in Parque Estadual Carlos Botelho, São Paulo, Brazil and S. libidinosus from Fazenda Boa Vista, Piauí, Brazil (Izar et al. 2012). We formed each pool group from six randomly chosen sample extracts. Before pool formation, we extracted samples according to the protocol described in the preceding text. We used 0.5 ml of each sample extract to form 3.0-ml pools that we packed in polypropylene tubes and dried as described in “Feces Collection and Steroid Analysis” before transporting them to Vienna. There we resuspended them and subjected them to a Novapak C18 column (3:9 × 150 mm, Millipore Corporation, Milford, MA, USA) with a Mini-Guard column (C18) and a solvent system of methanol: water at a flow rate of 1 ml/min (Schatz and Palme 2001; Teskey-Gerstl et al. 2000). We separated steroids using a linear gradient of 50–75% methanol. The percentage of methanol remained constant at 50% for 5 min. We then increased it linearly up to 75% over the next 30 min. We collected three fractions per minute. We also determined the elution positions of cortisol and corticosterone in the HPLC system. We obtained all steroids from Steraloids, Wilton, NH, USA. We determined the immunoreactivity of the fractions in the cortisol EIA to gain information about fecal metabolites picked up by the EIA.
Data Interpretation and Analysis
To test our predictions we used the collected data to calculate several indexes (Table II). To identify peaks, we used an iterative removal of wild (Norusis 1998) and regular outliers using the box plot function in IBM SPSS statistics 23. We also inspected FCM plots for each female visually to evaluate if FCM levels increased in relation to the potential biological validation incident.
Ethical Note
This research complied with protocols approved by the Animal Research Ethics Committee of the Institute of Psychology of the University of São Paulo and with the current Brazilian laws on ethical standards. The study met the ethical guidelines of the International Primatology Society for conducting research with nonhuman primates. The authors declare that they have no conflict of interest.
Results
Physiological Validation
Visual inspection of the FCM plots (Fig. 1 and ESM Fig. S2) shows that almost all subjects had daily FCM peaks during the entire duration of the experiment. Those peaks were normally less pronounced than the peaks observed shortly after injections; i.e., they were regular outliers rather than wild outliers, and occurred systematically between ca. 13:00 and 16:00 h.
Both males from the ACTH group had wide FCM peak spans and peak time spans (M1 > M2), and their highest FCM values were ca. 4.5 h after the injection, with FCM concentrations at least 10 times higher than baseline (Fig. 1; Table III). In both individuals, FCM levels returned to baseline ca. 24 h after injection.
One male (M3) from the dexamethasone group had a FCM peak 11 times higher than his baseline, 6 h after the injection. The other subject (M4) from the dexamethasone group had a FCM peak 4 times higher than his baseline, 5.7 h after the injection. Twenty-four hours after the injections, both males from the dexamethasone group had very low FCM levels and levels remained low for ca. another 48 h (Fig. 1 and ESM Fig. S2). Their lowest FCM values (troughs) were around 50 h after the injection (Fig. 1; Table III).
Males from the saline solution group lacked FCM peaks on the day of injections (Fig. 1 and ESM Fig. S2). All males except M4 from the dexamethasone group showed FCM peaks detected by wild outliers, although M5’s FCM peak seemed to be detached from the experiment, happening 76.3 h after the injection (Fig. 1). For male subjects, the time to return to FCM baseline values after the peak varied from 22.0 to 24.7 h.
Females in the ACTH group showed large individual differences in all variables (Fig. 1; Table III). One female (F3) from the dexamethasone group had a peak 14 times larger than her baseline, 5.2 h after the injection, which was the only wild outlier in females. The other subject (F4) showed no peak within 24 h of the dexamethasone injection. Twenty-five hours after the injections, both females from the dexamethasone group had reached their minimum values (troughs), and their values remained low for ca. another 48 h (Fig. 1 and ESM Fig. S2). The single female from the saline solution group (F5) had no peak on the day of the injection (Fig. 1).
The time between the injections and the first fecal sample voided and the number of samples voided before the peak sample varied in males and females but we observed no effect of treatment on either measure.
Biological Validation
All females involved in the experiment had a peak FCM level on the last day of the experiment (fourth day after the injection) between 11:00 and 15:00 h (1.5–5.5 h after the bite incident; Fig. 1 and ESM Fig. S2).
HPLC
The HPLC immunograms revealed very similar profiles of immunoreactive FCM between species and sexes (Fig. 2). Our cortisol EIA detected two main peaks, the first eluting around the position of cortisone and the second eluting around the position of cortisol (Fig. 2).
Fecal cortisol metabolite profile obtained after reverse-phase HPLC for four pool samples from female (upper panel) and male (lower panel) Sapajus nigritus and S. libidinosus (collected in Parque Estadual Carlos Botelho, São Paulo, Brazil and Fazenda Boa Vista, Piauí, Brazil, respectively, from January/2009 to December 2010).
Discussion
Our results demonstrate the validity of the cortisol EIA method for measuring FCM in Sapajus libidinosus. FCMs peaked after injection with ACTH and were low after dexamethasone treatment. However, some results require further explanation. First, we found FCM peaks a few hours after the dexamethasone injection in three out of four subjects of this treatment. This result seems to contradict our prediction and has also been reported in other species and may be due to an immediate excitatory influence of dexamethasone on the HPA axis before it exerts its suppressive effects (Palme et al. 1999). Second, all individuals subject to saline treatment showed peaks around 24 h after the injections. These peaks are not related to the injection because gut transit time in capuchins is very fast (Wehncke et al. 2003). These subjects also showed peaks almost every day around a similar time. Those peaks probably reflect other unknown stressors inside the CETAS, e.g., conspecific vocalizations, staff noises, food provisioning, or the circadian rhythm of cortisol. Third, all female subjects presented FCM peaks 4 days after the injections. Those peaks happened shortly after a disruptive incident in a group of another capuchin species, out of sight of our subjects (the bite incident described in Methods). This incident seemed to have been a strong psychological stressor and suggests that the method we used to measure FCMs detects biologically significant alterations in plasma cortisol level (Touma and Palme 2005). Unfortunately, as we conducted experiments with males and females at different occasions we do not have similar data for the males.
Our subjects showed extended peaks in FCM after ACTH injection and troughs after the dexamethasone injection. We obtained this information because the subjects defecated at a high frequency, thus voiding more than one sample with values considered as peaks or troughs. The amplitude and duration of these peaks and troughs varied between individuals. We also determined the time until the major FCM peak appeared in the feces, to estimate the time lag between the release of cortisol into the bloodstream and detection in the feces as FCM. Knowledge of this time delay is important to correlate FCM levels with other types of data, e.g., behavioral or climate data. In subjects that received the ACTH injection, we observed major FCM peaks at ca. 4.5 h and ca. 5 h in males and ca. 3 h and ca. 8.5 h in females. In the biological validation, we detected peaks between ca. 1.5 h and ca. 5.5 h after the stressful incident. In a similar experiment with a male and two female captive Sapajus spp. (probably capuchin hybrids) in the Instituto Di Scienze e Tecnologie Della Cognizione Primate Center (Rome), the time required to detect FCM peaks after an ACTH injection was 3 h and 3.5 h for the male and 2 h and 4.5 h for the females (Wheeler et al. 2013). Together these results show that the time delay between cortisol release and FCM peak detection varies considerably among individuals.
The time our subjects took to return to FCM baseline values after the peak related to the injection (ACTH or dexamethasone, for subjects that presented peaks) was longer (>8 h) than that in the previous study (between 4.0 and 8.4 h; Wheeler et al. 2013). The time between injection and release of first fecal sample varied from 0.5 h to 4.5 h in our subjects. These first samples after the ACTH injection were not the FCM peaks, unlike in the study of Wheeler et al. Our ACTH- and dexamethasone-treated subjects voided one to six samples before the peak sample occurred.
We suggest that factors such as individual differences in defecation frequency and in the ways this frequency is affected by other issues, e.g., stress (Ramos and Mormède 1998), are responsible for this variation. Differences in the results of the two studies could also be due to the drugs used. Wheeler et al. (2013) used Synacthen, which releases ACTH faster into the blood stream than the Synacthen Dépôt we used. This explanation is supported by the shorter time required to detect the peaks (1.5–5.5 h) in our biological validation, which did not rely on a synthetic drug. Another potential reason for the difference observed between the two studies is a possible species difference between Sapajus hybrids and S. libidinosus in cortisol metabolism and/or defecation frequency. However, the small sample sizes in both studies preclude any robust interpretation about species differences in the timing of cortisol release and FCM peaks. The variation in the times of FCM peaks highlights the importance of validation for each species, even for closely related ones, a cautious interpretation of times to FCM excretion, and the need for studies with larger sample sizes to better understand the factors influencing FCM excretion in Sapajus.
We also have results that point to a daily cortisol rhythm, showing FCM peaks in most subjects almost every day between ca. 13:00 and ca. 16:00 h. As we observed these peaks 8–11 h after capuchins awoke and our data point to a much shorter time lag to FCM peaks in feces after cortisol release, we do not think these peaks are related to awakening. They are more likely related to routine procedures that occurred daily at ca. 09:00 h (enclosure cleaning, feeding, interaction with the staff, etc.) that aroused the subjects. The fact that those peaks are sometimes more intense, e.g., M1/F2: Day 1; M2/M5: Day 3, supports this explanation and may point to some more disturbing (but unknown) events during the morning routine on those days.
Only one of five females showed a FCM peak determined by a wild outlier. Although we injected twice as much ACTH into females than into males, females showed less pronounced peaks (regular outliers instead of wild outliers as observed in several males). This is an unexpected result, because females normally show higher stress-induced HPA axis activity (for a review see Spencer and Deak 2017). These findings highlight the need for further research to fully understand how these mechanisms work.
Sapajus nigritus and S. libidinosus produced very similar fecal cortisol metabolites (as determined by the cortisol EIA), strongly suggesting that the experimental protocol validated here for S. libidinosus is also valid for S. nigritus. Another important conclusion based on the HPLC profiles is that, as in other primates (brown spider monkeys and red howlers: Rimbach et al. 2013, but also tufted capuchins: Wheeler et al. 2013), males and females from both species metabolize cortisol in a similar way, allowing comparisons of FCM values between sexes. The HPLC profiles showed two main immunoreactive FCM peaks, the second eluting around the position of the cortisol standard. However, this does not necessarily mean that subjects excrete unmetabolized cortisol, because other cortisol metabolites (5α-di/tetrahydrocortisol) elute close to this position. The second main peak and further even more polar, smaller peaks were not present in other primate species investigated with the same EIA or were present only to a minor extent (Heistermann et al. 2006; Wheeler et al. 2013), again revealing species differences in cortisol metabolism and excretion. The HPLC immunograms also indicate that cortisone (or its 5α-reduced metabolites), which also cross-reacts in our cortisol EIA and elutes around the position of the first main peak, may be present in the feces of primates (see also HPLC immunograms presented by Heistermann et al. 2006 and Wheeler et al. 2013).
The cortisol EIA we used shows cross-reactivity with several glucocorticoid metabolites (having a 21-ol-20-one structure in common) and detected a clear signal after ACTH/dexamethasone injections. The observed increases in FCM values after ACTH injection were 6.4 to 16.4 times greater than baseline levels, whereas dexamethasone injection suppressed FCM values to 1–10% of the respective baseline. A study using a single male and female Sapajus found that the same cortisol EIA measured the highest quantities of FCM when compared with three other EIAs (Wheeler et al. 2013). We also used one of those EIAs (an 11-oxoetiocholanolone EIA) on a subset of ACTH treatment samples, and also found lower FCM levels, and a less pronounced increase, especially in females. Unfortunately, the corticosterone EIA used by Wheeler et al. (2013), which yielded the strongest increase, was not available for our study, suggesting that other assays might be even more sensitive for assessing adrenocortical activity. Importantly, whatever EIA is used for measuring FCM in a species, a successful physiological/biological validation is needed before application (Sheriff et al. 2011; Touma and Palme 2005).
In summary, our study successfully validates a cortisol EIA for male and female Sapajus libidinosus and enables application of this noninvasive method in future studies of behavior and endocrinology in the field. The timing of FCM peaks varied between individuals, highlighting the need for frequent feces collection after treatment in validation experiments (Palme 2012) and in applied socioendocrinological research. Furthermore, our study highlights the need for future studies to clarify cortisol metabolism in females. HPLC immunograms showed that the sexes have similar FCM profiles and that S. libidinosus and S. nigritus have similar profiles. The latter finding suggests that our method can also be used for S. nigritus.
References
Bahr, N., Palme, R., Möhle, U., Hodges, K., & Heistermann, M. (2000). Comparative aspects of the metabolism and excretion of cortisol in three individual nonhuman primates. General and Comparative Endocrinology, 117, 427–438.
Boinski, S. U., Swing, S. P., Gross, T. S., & Davis, J. K. (1999). Environmental enrichment of brown capuchins (Cebus apella): Behavioral and plasma and fecal cortisol measures of effectiveness. American Journal of Primatology, 68, 49–68.
Canale, G. R., Guidorizzi, C. E., Kierulff, M. C. M., & Gatto, C. A. F. R. (2009). First record of tool use by wild populations of the yellow-breasted capuchin monkey (Cebus xanthosternos) and new records for the bearded capuchin (Cebus libidinosus). American Journal of Primatology, 71, 366–372.
Dantzer, B., Fletcher, Q. E., Boonstra, R., & Sheriff, M. J. (2014). Measures of physiological stress: A transparent or opaque window into the status, management and conservation of species? Conservation Physiology, 2, 1–18.
Emidio, R. A., & Ferreira, R. G. (2012). Energetic payoff of tool use for capuchin monkeys in the caatinga: Variation by season and habitat type. American Journal of Primatology, 74, 332–343.
Falótico, T., & Ottoni, E. B. (2013). Stone throwing as a sexual display in wild female bearded capuchin monkeys, Sapajus libidinosus. PloS One, 8, e79535.
Fanson, K. V., Best, E. C., Bunce, A., Fanson, B. G., Hogan, L. A., et al (2017). One size does not fit all: Monitoring faecal glucocorticoid metabolites in marsupials. General and Comparative Endocrinology, 244, 146–156.
Fragaszy, D. M., & Adams-Curtis, L. E. (1998). Growth and reproduction in captive tufted capuchins (Cebus apella). American Journal of Primatology, 44, 197–203.
Fragaszy, D. M., Izar, P., Liu, Q., Eshchar, Y., Young, L. A., & Visalberghi, E. (2016). Body mass in wild bearded capuchins, (Sapajus libidinosus): Ontogeny and sexual dimorphism. American Journal of Primatology, 78, 473–484.
Heistermann, M., Palme, R., & Ganswindt, A. (2006). Comparison of different enzymeimmunoassays for assessment of adrenocortical activity in primates based on fecal analysis. American Journal of Primatology, 68, 257–273.
Izar, P., Verderane, M. P., Peternelli-dos-Santos, L., Mendonça-Furtado, O., Presotto, A., et al (2012). Flexible and conservative features of social systems in tufted capuchin monkeys: Comparing the socioecology of Sapajus libidinosus and Sapajus nigritus. American Journal of Primatology, 74, 315–331.
Mendonça-Furtado, O., Edaes, M., Palme, R., Rodrigues, A., Siqueira, J., & Izar, P. (2014). Does hierarchy stability influence testosterone and cortisol levels of bearded capuchin monkeys (Sapajus libidinosus) adult males? A comparison between two wild groups. Behavioural Processes, 109, 79–88.
Mittermeier, R. A., Konstant, W. R., & Mast, R. B. (1994). Use of neotropical and Malagasy primate species in biomedical research. American Journal of Primatology, 34, 73–80.
Möstl, E., & Palme, R. (2002). Hormones as indicators of stress. Domestic Animal Endocrinology, 23, 67–74.
Möstl, E., Maggs, J., Schrötter, G., Besenfelder, U., & Palme, R. (2002). Measurement of cortisol metabolites in faeces of ruminants. Veterinary Research Communications, 26, 127–139.
Möstl, E., Rettenbacher, S., & Palme, R. (2005). Measurement of corticosterone metabolites in birds’ droppings: An analytical approach. Annals of the New York Academy of Sciences, 1046, 17–34.
Norusis, M. (1998). Guide to data analysis: SPSS 8. Englewood Cliffs: Prentice Hall.
Oliveira, M. M., & Langguth, A. (2006). Rediscovery of Marcgrave’s capuchin monkey and designation of a neotype for Simia flavia Schreber, 1774 (primates, Cebidae). Boletim do Museu Nacional, 523, 1–16.
Pagano, I. S. A., Sousa, A. E. B. A., Wagner, P. G. C., & Ramos, R. T. C. (2009). Aves depositadas no Centro de Triagem de Animais Silvestres do IBAMA na Paraíba: Uma amostra do tráfico de aves silvestres no estado. Ornithologia, 3, 132–144.
Palme, R. (2012). Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Animal Welfare, 21, 331–337.
Palme, R., & Möstl, E. (1997). Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. International Journal of Mammalian Biology, 62(Suppl. 2), 192–197.
Palme, R., Robia, C., Meßmann, S., Hofer, J., & Möstl, E. (1999). Measurement of faecal cortisol metabolites in ruminants: A non-invasive parameter of adrenocortical function. Wiener Tierärztliche Monatsschrift, 86, 237–241.
Palme, R., Rettenbacher, S., Touma, C., El-Bahr, S. M., & Möstl, E. (2005). Stress hormones in mammals and birds: Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Annals of the New York Academy of Sciences, 1040, 162–171.
Palme, R., Touma, C., Arias, N., Dominchin, M. F., & Lepschy, M. (2013). Steroid extraction: Get the best out of faecal samples. Wien Tierärztliche Monatsschrift – Veterinary Medicine Austria, 100, 238–246.
Ramos, A., & Mormède, P. M. (1998). Stress and emotionality: A multidimensional and genetic approach. Neuroscience and Biobehavioral Reviews, 22, 33–57.
Rimbach, R., Heymann, E. W., Link, A., & Heistermann, M. (2013). Validation of an enzyme immunoassay for assessing adrenocortical activity and evaluation of factors that affect levels of fecal glucocorticoid metabolites in two new world primates. General and Comparative Endocrinology, 191, 13–23.
Schapiro, S. J., Bloomsmith, M. A., Kessel, A. L., & Shively, C. (1993). Effects of enrichment and housing on cortisol response in juvenile rhesus monkeys. Applied Animal Behaviour Science, 37, 251–263.
Schatz, S., & Palme, R. (2001). Measurement of faecal cortisol metabolites in cats and dogs: A non-invasive method for evaluating adrenocortical function. Veterinary Research Communications, 25, 271–287.
Sheriff, M. J., Dantzer, B., Delehanty, B., Palme, R., & Boonstra, R. (2011). Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia, 166, 869–887.
Spagnoletti, N., Visalberghi, E., Verderane, M. P., Ottoni, E. B., Izar, P., & Fragaszy, D. (2012). Stone tool use in wild bearded capuchin monkeys, Cebus libidinosus. Is it a strategy to overcome food scarcity? Animal Behaviour, 83, 1285–1294.
Spagnoletti, N., Cardoso, T. C. M., Fragaszy, D., & Izar, P. (2017). Coexistence between humans and capuchins (Sapajus libidinosus): Comparing observational data with farmers’ perceptions of crop losses. International Journal of Primatology, 38, 243–262.
Spencer, R. L., & Deak, T. (2017). A users guide to HPA axis research. Physiology and Behavior. 78, 43–65.
Teskey-Gerstl, A., Bamberg, E., Steineck, T., & Palme, R. (2000). Excretion of corticosteroids in urine and faeces of hares (Lepus europaeus). Journal of Comparative Physiology B, 170, 163–168.
Torres-Farfan, C., Valenzuela, F. J., Ebensperger, R., Méndez, N., Campino, C., et al (2008). Circadian cortisol secretion and circadian adrenal responses to ACTH are maintained in dexamethasone suppressed capuchin monkeys (Cebus apella). American Journal of Primatology, 70, 93–100.
Touma, C., & Palme, R. (2005). Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. Annals of the New York Academy of Sciences, 1046, 54–74.
Verderane, M. P., Izar, P., Visalberghi, E., & Fragaszy, D. M. (2013). Socioecology of wild bearded capuchin monkeys (Sapajus libidinosus): An analysis of social relationships among female primates that use tools in feeding. Behaviour, 150, 659–689.
Visalberghi, E., Addessi, E., Truppa, V., Spagnoletti, N., Ottoni, E., et al (2009). Selection of effective stone tools by wild bearded capuchin monkeys. Current Biology, 19, 213–217.
Wehncke, E. V., Hubbell, S. P., Foster, R. B., & Dalling, J. W. (2003). Seed dispersal patterns produced by white-faced monkeys: Implications for the dispersal limitation of neotropical tree species. Journal of Ecology, 91, 677–685.
Wheeler, B. C., Tiddi, B., Kalbitzer, U., Visalberghi, E., & Heistermann, M. (2013). Methodological considerations in the analysis of fecal glucocorticoid metabolites in tufted capuchins (Cebus apella). International Journal of Primatology, 34, 879–898.
Wright, B. W., Wright, K. A., Chalk, J., Verderane, M. P., Fragaszy, D., et al (2009). Fallback foraging as a way of life: Using dietary toughness to compare the fallback signal among capuchins and implications for interpreting morphological variation. American Journal of Physical Anthropology, 140, 687–699.
Wright, K. A., Wright, B. W., Ford, S. M., Fragaszy, D., Izar, P., et al (2015). The effects of ecology and evolutionary history on robust capuchin morphological diversity. Molecular Phylogenetics and Evolution, 82, 455–466.
Acknowledgments
The authors thank Victor Shiramizu, Edilma Chagas, and Eliane Morgado for help with samples in the LDH/UFRN; Dra. Maria Bernardete Cordeiro de Souza for opening her laboratory to us; CETAS employees: Dr. Paulo, Michele, Mércia, Ronny, Adair, Hércules, Aluilson, Zé, and Dinho for help throughout the experiment; Dra. Renata Ferreira for sample transportation; Edith Klobetz-Rassam for help with steroid analyses and Gisele Zago for logistic help throughout this research. We also thank Dr. Alex Hubbe, Dr. Brandon Wheeler, Dr. James Higham, Dr. Joanna Setchell, and an anonymous reviewer for helpful comments on previous drafts of this manuscript. This research was funded by FAPESP (O. Mendonça-Furtado: 08/52293-3 and P. Izar: 08/55684-3) and Capes (OMF: 13/1537). Permits to collect data were granted from ICMBio to P. Izar (21406).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Joanna M. Setchell
Electronic Supplementary Material
ESM 1
(DOCX 685 kb)
Rights and permissions
About this article
Cite this article
Mendonça-Furtado, O., Izar, P. & Palme, R. Validation of an Enzyme Immunoassay for Measuring Fecal Cortisol Metabolites of Bearded (Sapajus libidinosus) and Black (Sapajus nigritus) Capuchins. Int J Primatol 38, 1002–1016 (2017). https://doi.org/10.1007/s10764-017-9993-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-017-9993-6