Abstract
Annual patterns of fecal corticoid excretion were analyzed in the threatened Red-tailed parrot (Amazona brasiliensis) in captivity. Corticoid concentration over the 15 months of the study (mean ± standard error, 12.6 ± 0.32 ng g−1, n = 585) was lowest around May (the southern Fall), and greatest around September (late winter), just prior to their normal breeding period. Corticoid excretion follows a seasonal pattern best explained by reproductive cycles rather than climate, although climate may be involved in the timing of corticoid excretion. Fecal corticoids also show promise as a tool to measure stress levels. We demonstrate that fecal corticoid measurement is a simple, yet efficient method for monitoring adrenocortical activity in captive, and perhaps wild, parrots. Monitoring adrenocortical activity can inform researchers about imposed stress in captivity, whether pair-bonds are forming in captive birds, and of the timing of breeding both in captivity and in nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parrots (Psittacidae) are among the most threatened birds in the world, especially in the Neotropics. With more endangered species than any other bird family, ∼30% of neotropical parrots were considered threatened by the early 1990s (Collar et al. 1994). The Red-tailed parrot (Amazona brasiliensis) is threatened and endemic in southeastern Brazil. Extensive poaching (for the national and international bird trade) and habitat loss are its most serious threats (Wright et al. 2001). Despite heavy trapping pressure in the early 1990s, the population is now believed to be relatively stable, and has consequently been downlisted from endangered (Collar et al. 1994) to vulnerable by the IUCN (Bird Life International 2004). Understanding the threatened status of any species requires knowledge of population dynamics, especially breeding cycles.
Reproduction in birds carries predictable and unpredictable physical, energetic and social demands that stimulate behavioral and physiological responses that are mediated mostly through the hypothalamic–pituitary–adrenal (HPA) axis. Thus, monitoring HPA activity has great potential for understanding reproductive cycles and stressful periods. In birds, physiological responses to environmental challenges involve the secretion of glucocorticoids, primarily corticosterone (Oglesbee et al. 1997; Bentley 1998; Frigerio et al. 2004). For example, plasma corticosterone levels are associated with behavior in tits and owls (Silverin 1997; Belthoff and Dufty 1998) and with social interactions (e.g., courtship and parental care, Hirschenhauer et al. 2000; Breuner and Orchinik 2000). Birds in captivity may face unique challenges that force behavioral interactions, such as limited space, poor diet and greater proximity of individuals. Hence, stressful conditions peculiar to captivity may arise and perhaps may explain the low breeding success of many species in captivity. Since the Red-tailed parrot has few reports of successful breeding in captivity (Low 2006) understanding the factors that influence allostasis through monitoring HPA activity may be a very useful tool. However, monitoring such activity must be non-invasive so as to not introduce other stressful factors that may cause their own reactions, further reducing chances of breeding in captivity.
Fecal steroid analysis may offer a useful non-invasive method. Since avian steroids are metabolized by the liver and excreted as conjugates via bile into the gut or by the kidneys, non-invasive monitoring of adrenal function is possible by extracting steroid metabolites from feces (Wasser et al. 1997; Touma and Palme 2005). This approach is particularly useful for monitoring stress, since feces can be easily collected without disturbing the birds. Monitoring HPA can potentially measure adrenal response to a wide array of stressors (Morais et al. 1997; Touma and Palme 2005). The influence of acute and chronic stress, seasonality, reproductive status, age, migration and social organization on adrenal gland activity have all been monitored by fecal corticosteroids analysis in a variety of bird species (Wasser et al. 1997, 2000; Kortrschal et al. 1998, 2000; Hirschenhauser et al. 2000, 2005; Carere et al. 2003; Dehnhard et al. 2003; Millspaugh and Washburn 2004; Scheiber et al. 2005; Touma and Palme 2005).
In this study we examined fecal corticosteroids in a captive group of Red-tailed parrots to test that fecal corticoids may be used to indicate HPA axis activity in response to climate, reproduction and housing conditions. Specifically, we tested whether fecal corticosteroid variability indicates a response to seasonality or reproduction in captive parrots.
Methods
Study species and captivity
The Red-tailed parrot (or Red-tailed Amazon) occurs in the narrow coastal region of the states of São Paulo, Paraná and Santa Catarina (Scherer-Neto 1989; Cavalheiro 1999; Sipinski 2003). The breeding season begins in late August, with usually one nest per year. Sexual maturity and permanent pair-bonds occur at 3–4 years of age. Parental care is shared by both members of the pair (Scherer-Neto 1989; Rupley 1997; Cavalheiro 1999; Sipinski 2003).
Thirteen captive-reared adult parrots (7 males, 6 females, 7–8 years of age) were studied during 2000–2003. Parrots were kept at the Curitiba Zoological Park (25°S, 49°W) after recovery from illegal captivity in the southern Brazilian state of Paraná. In the zoo, all parrots were housed together away from public exhibits. At 3 months prior to this study they were separated into pairs (based on behavior) and each pair was placed in its own enclosure. Enclosures (4.10 × 2.5 × 2.6 m) allowed flight and were mostly open-air, including a covered area for shelter from rain and wind, and a breeding room (1.2 × 2.5 × 2.6 m) containing a nest box. One solitary male was housed separately (5.3 × 2.5 × 2.6 m). Adjacent enclosures permitted birds to see, hear and interact only with other parrots in the study. Daily care included feeding and cleaning. Diet comprised fruit, nuts and grain, supplemented with commercial dry dog food. Water was provided ad libitum. Parrot health was monitored regularly by the zoo veterinarian.
Fecal sampling
Feces were collected weekly twice from October 2003 to December 2004. During warmer weather, pairs were separated the evening prior to fecal sampling to determine the source of the feces, and feces were collected in the morning. Females were held in the breeding room with the nest box and males remained in the open-air part of the enclosure. The door to the breeding room was opened after fecal collection to allow the male and female free passage to all parts of the enclosures. In colder weather (4 June–17 August) birds (3 pairs and 1 single male) were held in separate cages overnight in the breeding room to separate and collect fecal samples. Samples were stored at −20°C in plastic bags until extraction and analysis. We assume that this procedure caused little or no reaction in the birds, since all were hand-reared, and they showed no adverse behaviors to the procedure.
Corticosteroid extraction and assay
Steroids were extracted following Schwarzenberger et al. (1991) and Javorouski (2003), with modifications. Whole feces homogenates were extracted (it was impossible to separate feces and uric acid components). An aliquot of ∼0.5 g of wet sample was transferred to a tube of 5.0 mL 90% ethanol in phosphate-buffered saline (PBS). After overnight inverted-mixing, tubes were centrifuged (1,500g/15 min) and extract diluted in PBS (1:1) and frozen (−20°C) until analysis. Fecal corticosteroid concentrations were measured in duplicate 100 μL aliquots of fecal extracts using a commercial double antibody 125I-corticosterone radioimmunoassay (Corticosterone ICN Biomedicals, USA). The antiserum description included the following cross-reactivities: 100% corticosterone, 0.34% desoxycorticosterone, 0.10% testosterone; 0.05% cortisol, 0.03% aldosterone, 0.02% progesterone, 0.01% androstenedione. The assay was validated for other bird species (Wasser et al. 2000) and for the Red-tailed parrot by demonstrating parallelism between dilutions (1:2; 1:4; 1:10; 1:20; 1:40; 1:80) of a pooled sample of fecal extracts (n = 120) from all individuals and the standard curve (r 2 > 0.97 for both regression lines, slope −0.18 for standards and −0.15 for fecal extracts) and by the recovery of exogenous corticosterone (12.5–500 ng mL−1) added to the extract pools (y = 0.94x − 0.38, r 2 = 0.99, P < 0.05). Testing the physiological relevance of fecal glucocorticoids in the studied birds was not possible due to limitations of working with few individuals of a threatened species. However, it was tested in adult Amazona aestiva (3 males, 3 females). Fecal corticoids increased (P < 0.01) from the mean value of 5.8 ± 0.3 ng g−1 3 days before to 9.7 ± 1.4 ng g−1 on the day after a physical restraining for blood collection. A total of 11 assays were used for validation and fecal extract analysis. Assay sensitivity, based on 89% maximum binding, was 12.5 ng mL−1. The mean inter-assay coefficient of variation for high and low controls (provided by the company) and for a fecal extract control was 6.1% and the intra-assay coefficient of variation was 3.2%. Fecal corticosteroid concentrations were corrected for the dilution factor of the assay standards (1:200) and are expressed as nanogram per gram−1 of wet feces.
Environment
Average daily temperature, relative humidity and rainfall were obtained from the Meteorological System of Paraná (SIMEPAR, http://www.simepar.br). Photoperiod was calculated for this latitude as the interval between sunrise and sunset. Partial correlation coefficients were used to test for climate and corticoid associations.
Data analysis
Fecal corticoid concentration was compared among sexes and pairs over time by repeated measures analysis of variance. Analysis of Variance was used to examine seasonal variation in overall fecal corticoids. Fecal corticoid concentrations were natural log transformed to fit the assumptions of the analyses (normality of residuals and equality of variances). Seasonality was also tested as an influence on corticoids by correlation with the environmental variables. We followed Marques et al. (2004) to examine climate by including lag-times of 30 and 60 days in the analysis and monthly averages of daylength, temperature and relative humidity. All tests used a significance level of 5%.
Results
Fecal corticoids
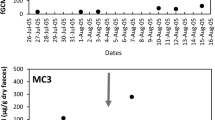
Fecal corticoid excretion varied over time (F 14, 112 = 3.67, P < 0.05). Also, pairs tended towards similar trends over time (Repeated Measures ANOVA, P > 0.1 for sex-pair, Fig. 3). May had the lowest corticoid levels (6.7 ± 0.4 ng g−1) and August and September the highest levels (12.4 ± 0.6 and 13.2 ± 0.6 ng g−1, respectively). Among seasons, winter had clearly the highest (mean ± 95% confidence interval, 10.7 ± 0.52 ng g−1; Tukey HSD, P < 0.05) concentration, and Fall the lowest (7.6 ± 0.51, Tukey HSD P < 0.05), while spring and summer were similar and intermediate (8.8 ± 0.44; Fig. 2).
Corticoid levels were correlated with photoperiod after a lag of 2 months (Fig. 1; partial r = 0.8, n = 13, P < 0.05). Since photoperiod and temperature are so highly correlated, the effects of temperature and photoperiod cannot be separated (Marques et al. 2004). Humidity apparently has no additional effect on corticoid levels (Fig. 2).
Monthly averages for fecal corticoid concentrations, daily temperature, and photoperiod during the 15 months of this study. Corticoids were most strongly correlated with photoperiod after a lag time of 2 months, but photoperiod and temperature are strongly correlated as well, so the relative importance of one over the other cannot be determined (see text)
Mean (±95% confidence interval) overall corticoid concentrations compared between seasons. Winter had clearly the highest concentration, and Fall the lowest, while spring and summer were similar. The ANOVA was based on log-transformed concentrations, which were then back-transformed for illustration purposes
Behavior
Corticoid excretion in females and males of pair number 1 (n = 14), 2 (n = 15) and 5 (n = 15) pairs (circles) were correlated over time (all r > 0.58, P < 0.05, Fig. 3), suggesting within-pair breeding synchrony. Corticoid excretion in the unpaired male was also correlated with that of two females (3, r = 0.58 and 5, r = 0.95, both P < 0.05). Since pairs varied somewhat, combining pairs for an overall analysis masks the trends shown by each pair.
Deviations from average corticoid excretion for individuals, grouped by pair and sex over the 15 months of this study (solid vertical lines are winter and summer solstices, dashed lines are spring and autumnal equinoxes). Corticoid excretion in females (open symbols) and males (filled symbols) of pairs number 1, 2 and 5 (circles) were correlated over time (all r > 0.58, P < 0.05), which suggests breeding synchrony. The female in pair number 2 laid eggs in November 2004. The female in pair number 4 plucked her own feathers, and her corticoid levels were most strongly correlated with those of the unpaired male, who is shown along side her as a hatched-square. Pairs identified by triangles were not synchronous (uncorrelated), although pairs 3 and 4 (despite the stronger correlation of the female with the unpaired male) displayed apparent courting behaviors. While the exact pattern over time varied among pairs, the magnitude (difference from each individual mean) of excretion was similar for all pairs
During this study, only one female laid eggs that were infertile and laid outside the nest box (female of pair number 2, Fig. 3). All individuals molted in early summer (3–12 December 2003, again 4–17 December 2004). One female (pair number 4; kept in cages) plucked her own feathers during winter (a common behavior for parrots in captivity, Bauck 1997; Rupley 1997), besides which all individuals seemed to exhibit normal behaviors of captive birds. There was no indication in any analysis overall or by individual of an influence due to routine care-taking and husbandry practices.
Discussion
Red-tailed parrot fecal corticoid excretion patterns follow the predicted hormonal cycle and demonstrate that this non-invasive method can be useful for monitoring hormonal cycles, including reproduction, in captive birds. Corticoid levels rose after May, when courting behaviors may begin (Sipinski 2003, Figs. 1, 3). Corticoid levels began to decline after September, when this species usually has occupied nest-sites and possibly begins egg-laying (Sipinski 2003). Although these birds did not breed, we believe that these results are promising for monitoring parrots. This breeding cycle explains the correlation between photoperiod and corticoid levels because they are both annual cycles (e.g., Marques et al. 2004).
In this first use of fecal corticoids to understand annual cycles in the family Psittacidae, we find the methods for extraction and assay of the corticosterone metabolites to be sufficiently simple, cost-effective and valuable for monitoring adrenal activity in the Red-tailed, and probably other parrots. We show that fecal corticoid excretion is seasonal in which hormonal changes precede reproduction. We suggest that temperature or photoperiod (or both) may trigger the hormonal changes associated with reproduction, and that completion of reproduction may trigger molting and return to stasis. Thus, correlations with climate should not be very strong or perhaps should be thought of as initiators but not terminators of hormonal activity. Further study will be required to determine the exact nature of the influence of climate on hormonal activity, or whether photoperiod alone is sufficient to initiate reproductive activity.
These subtle climate issues are not vital, however, for monitoring annual cycles in parrots. Fecal corticoids increased in the spring, when this species of parrot begins its breeding season (Juniper and Parr 1998; Sipinski 2003). Excretion reached a peak during the interval when parrots initiate nests and declined when nests should have been in use (Figs. 1, 3). Also, the slight rise in November may be in anticipation of molt, suggesting a possible extra metabolic cost of molting (Blem 2000). Prior to these changes and at the beginning of the experiment, a time interval of variable (especially for pairs 1, 4 and 6 in Fig. 3) excretion (October–May) may indicate acclimation of some birds to new surroundings.
The very strong correlation between the unmated male and female number 5 (r = 0.95), while unexplained, may indicate pair formation that was not obvious to the animal handlers. If so, a simple test could improve our identification of pair formation for captive birds. Continual monitoring and comparing correlation coefficients between different possible mating combinations could suggest pair formation in birds that are not in the same cage. This could then be tested by placing the birds together and analyzing behavior. These behaviors and correlations offer additional tools for understanding mating behaviors.
Here, we suggest biological and behavioral implications of fecal corticoid excretion in parrots. We recognize that detailed analysis, beyond the scope of this paper, will be required to test these possibilities, yet current understanding of the physiology of reproduction supports our views. For example, a reduction in basal activity of the HPA axis of the Red-tailed parrot may occur during the non-breeding season, as observed in greylag geese (Kotrschal et al. 1998). Thus, HPA axis activity may suggest two patterns—homeostasis when not breeding, and reproductive during the remainder of the year. The reproductive period perhaps would be preceded by a gradual rise associated with preparation for breeding. This “preparation” would be associated with the various behaviors that precede actual egg-laying. Pair-bonding increases, males feed females, nest-sites must be located and modified; all of these behaviors may be associated with increasing hormone levels. Thus, we expect fecal corticoids to change prior to the actual onset of egg-laying. Once egg-laying occurs, metabolic and behavioral demands change, and so a concomitant decline in fecal corticoids is expected. We will require detailed studies of nesting behaviors to confirm these predictions.
Similar patterns have been found in other species where hormones have been studied. In geese, increased hormonal reactivity is linked to metabolic and behavioral needs of reproduction, and confrontation with unknown individuals caused increased fecal corticoids only in the spring, during the breeding season (Kotrschal et al. 2000). Also in geese, similar to patterns observed here, fecal corticoid excretion is associated with breeding, with higher levels of corticoids in males during mating and nesting, followed by a decline during incubation of eggs (Hirschenhauser et al. 2000). Finally, pair bond formation improves birds “well-being” (von Holst 1998). In these parrots, while no reproduction occurred, all individuals molted. Molt, in December, was synchronous at the end of the breeding season and was anticipated by increased excretion of fecal corticoids in most individuals in November (Fig. 1, 3). The specific hormonal and metabolic changes associated with this event in parrots are still unknown.
In summary, fecal corticoids can provide a useful, non-invasive method for monitoring threatened and endangered birds in captivity and, perhaps with modification, in nature. The energetically demanding reproductive and molt cycles are especially important for monitoring, since they are closely tied to population dynamics. Thus, we recommend that further study, including collection of fecal samples from wild birds, be carried out to further examine this potentially useful tool for non-invasive monitoring of annual cycles in birds.
References
Bauck L (1997) Avian dermatology. In: Altman RB, Clubb SL, Dorrestein GM, Quesenberry K (eds) Avian medicine and surgery. W.B.Saunders, Philadelphia, pp 548–562
Beltholff JR, Dufty Jr AM (1998) Corticosterone, body condition and locomotor activity: a model for natal dispersal in birds. Anim Behav 55:405–415
Bentley PJ (1998) Comparative vertebrate endocrinology. Cambridge University Press, Cambridge, 526 p
BirdLife International 2004. Amazona brasiliensis. In: IUCN 2006. 2006 IUCN red list of threatened species. http://www.iucnredlist.org/. Downloaded 23 June 2006
Blem CR (2000) Energy balance. In: Whittow G (ed) Sturkie´s avian physiology, 5. Academic Press, New York, 685 p
Breuner CW, Orchinik M (2000) Downstream from corticosterone: seasonality of binding globulins, receptors and behavior in the Avian stress response. In: Dawson A, Chaturvedi CM (eds) Avian endocrinology. Narosa Publishing House, New Delhi, pp 1–11
Carere C, Groothuis TGG, Möstl E, Daan S, Koolhaas JM (2003) Fecal corticoids in a territorial bird selected for different personalities: daily rhythm and the response o social stress. Horm Behav 43:540–548
Cavalheiro ML (1999) Qualidade do ambiente e características fisiológicas do papagaio-de-cara-roxa (Amazona brasiliensis) na Ilha Comprida—São Paulo. (Quality of the environment and physiological characteristics of free-living Red-tailed parrots (Amazona brasiliensis) in Ilha Comprida—Sao Paulo). Master Sciences Dissertation, Federal University of Paraná, Curitiba-PR, p 105
Collar NJ, Crosby MJ, Stattersfield AJ (1994) Birds to watch. 2. The world list of threatened birds. Birdlife International, Washington
Dehnhard M, Schreer A, Krone O, Jewgenow K, Krause M, Grossmann R (2003) Measurement of plasma corticosterone and faecal glucocorticoid metabolites in the chicken (Gallus domesticus), the great cormorant (Phalacrocorax carbo), and the goshawk (Accipter gentilis). Gen Comp Endocrinol 131:345–352
Frigerio D, Dittami J, Möstl E, Kotrschal K (2004) Excreted corticosterone metabolites co-vary with ambient temperature and air pressure in male greylag geese. Gen Comp Endocrinol 137:29–36
Hirschenhauser K, Möstl E, Wallner B, Dittami J, Kotrschal K (2000) Endocrine and behavioural responses of male greylag geese (Anser anser) to pairbond challenges during the reproductive season. Ethology 106:63–77
Hirschenhauser K, Möstl E, Kotrschal K (2005) Synthesis of measuring steroid metabolites in goose feces. Ann N Y Acad Sci 1046:1–16
Javorouski ML (2003) Comparação da resposta adrenocortical em fêmeas de felídeos submetidas a anesthesia, laparoscopia e manipulação genital. (Comparative adrenocortical response in felid females submitted to anesthesia, laparoscopy and genital manipulation). Research Thesis for M. Sc. degree, Federal University of Paraná, Curitiba-PR, Brazil, 98 p
Juniper T, Parr M (1998) Parrots: a guide to parrots of the world. Yale University Press, New Haven, 584 p
Kotrschal K, Hirschenhauser K, Möstl E (1998) The relationship between social stress and dominance is seasonal in greylag geese. Anim Behav 55:171–176
Kotrschal K, Dittami J, Hirschenhauser K, Möstl E, Peczely P (2000) Effects of physiological and social challenges in different seasons on faecal testosterone and corticosterone in male domestic geese (Anser anser). Acta Ethol 2:115–122
Low R (2006) http://www.theparrotsocietyuk.org/Brasiliensis in Brazil conservation article.htm Downloaded 23 June
Marques MCM, Roper JJ, Salvalaggio APB (2004) Phenological patterns among plant life-forms in a subtropical forest in southern Brazil. Plant Ecol 173:203–213
Millspaugh JJ, Washburn BE (2004) Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138:189–199
Morais RN, Mucciolo RG, Gomes MLF, Lacerda O, Moraes W, Moreira N, Swanson WF, Brown JL (1997) Adrenal activity assessed by fecal corticoids and male reproductive traits in three South American felid species. Proc Am Ass Zoo Vet 220–223
Oglesbee BL, Orosz S, Dorrestein G (1997) The endocrine system. In: Altman RB, Clubb SL, Dorrestein GM, Quesenberry K (eds) Avian medicine and surgery. W.B. Saunders, Philadelphia, pp 475–488
Rupley AE (1997) Manual of avian practice. 1st edn. Saunders, Philadelphia, 556 p
Scheiber IB, Krajl S, Kotrschal K (2005) Sampling effort/frequency necessary to infer individual acute stress responses from fecal analysis in greylag geese (Anser anser). Ann N Y Acad Sci 1046:154–167
Scherer-Neto P (1989) Coontribuição à biologia do papagaio-de-cara-roxa (Amazona brasiliensis) [Contribution to the study of the biology of the Red-tailed parrot (Amazona brasiliensis)]. Research Thesis for M. Sc. degree, Federal University of Paraná, Curitiba-PR, 170 p
Schwarzenberger F, Möstl E, Bamberg E, Pammer J, Schmehlik O (1991) Concentration of progestagens and oestrogens in the faeces of pregnant Lipizzan, Trotter and Troughbred mares. J Reprod Fert Suppl 44:489–499
Silverin B (1997) The stress response and autumn dispersal behaviour in willow tits. Anim Behav 53:451–459
Sipinski EAB (2003) O papagaio-de-cara-roxa (Amazona brasiliensis) na Ilha Rasa,Pr—Aspectos ecológicos e reprodutivos e relação com o ambiente. [The Red-tailed parrot (Amazon brasiliensis) at the Ilha Rasa, Paraná- Ecological and reproductive aspects and its relationship with the environment]. Research Thesis for M. Sc. degree, Federal University of Paraná, Curitiba, Brazil, pp 74
Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann N Y Acad Sci 1046:54–68
von Holst D (1998) The concept of stress and its relevance for animal behavior. In: Moller AP (ed) Advances in the study of behavior, vol. 27. Academic Press, New York, pp 1–131
Wasser SK, Bevis K, King G, Hanson E (1997) Noninvasive physiological measures of disturbance in the northern spotted owl. Conserv Biol 11:1019–1022
Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Ugh JJ, Larbechert U, Millspason S, Monfort S (2000) A genereralized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120:260–275
Wright TF, Toft CA, Enkerlin-Hoeflich E, Gonzalez-Elizondo J, Albornoz M, Rodrigues-Ferraro A, Rojas-Suarez F, Sanz V, Trujillo A, Beissinger S, Berovides V, Gálvez XA, Brice AT, Joyner K, Eberhard J, Martuscelli P, Gilardi J, Koening SE, Stoleson S, Meyers M, Renton K, Rodriguez AA, Sosa-Asanza A, Vilella FJ, Wiley JW (2001) Nest poaching in neotropical parrots. Conserv Biol 15:710–720
Acknowledgments
We thank the staff at the Curitiba Zoo for all their helpful assistance, and Pedro Scherer Neto and Elenise A. B. Sipinski, who shared their knowledge of the species and provided constructive criticisms of the manuscript. This research was funded by Fundação Araucária, Federal University of Paraná and Society for Wildlife Research and Environmental Education (SPVS). Experimental procedures were in compliance with Brazilian laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Popp, L.G., Serafini, P.P., Reghelin, A.L.S. et al. Annual pattern of fecal corticoid excretion in captive Red-tailed parrots (Amazona brasiliensis). J Comp Physiol B 178, 487–493 (2008). https://doi.org/10.1007/s00360-007-0241-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-007-0241-9