Abstract
Understanding the mechanistic links between individual variation in life history and behavior is a major challenge in evolutionary ecology. Glucocorticoids (GC) play a major role in this link through their baseline levels into the blood and their implication in stress responses to environmental perturbations. However, very few studies have investigated the long-term joint relationships between GC stress reactivity, life history, and behavior in natural conditions. Here, we took advantage of the behavioral and life history differences among individual males and females of a wild population of eastern chipmunks (Tamias striatus): We investigated how individual exploration, age, and reproduction were linked to level and intra-individual variability (IIV) of fecal cortisol metabolites over a 5-month period. Our analyses revealed that female cortisol levels decreased during gestation and lactation compared with non-reproductive females. We also found that slower exploring females and females with a smaller litter displayed higher IIV in fecal cortisol metabolites. For males, fecal cortisol metabolites level during the mating season increased with the number of offspring produced and decreased with age. Our study highlights the necessity of considering simultaneously seasonal fluctuations in GC level and the dynamics of stress reactivity in the study of life history and behavioral co-adaptations within natural populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In iteroparous species, individuals must balance resource allocation between current reproduction and future survival and reproduction (Williams 1966; van Noordwijk and de Jong 1986; Stearns 1989). Species adjust this allocation differently using suites of life history and behavior adaptations that are thought to be determined by ecological conditions (Stearns 1992; Ricklefs and Wikelski 2002; Wingfield 2005). For example, high predation pressures on adult stages leads to the evolution of fast life histories favoring current reproduction at the expense of future survival (Stearns 1992). Such “fast-living” species will typically have an early age at maturity, a high fecundity, but a shorter life span (Gaillard et al. 1989; Bielby et al. 2007). On the other hand, “slow-living” species, expressing a later age at maturity, a lower fecundity, but a longer life span, are often found in environments with low levels of resources (Wiersma et al. 2007). Fast-living species are predicted to display higher levels of aggressiveness, activity, and faster exploration than slow-living species (Réale et al. 2010). In contrast, slow species are predicted to be less aggressive, less active, and express a slower exploration (Réale et al. 2010). Similar relationships between behavior and life history have also been documented among populations within species (Dingemanse et al. 2007; see also Walsh and Reznick 2009; Torres Dowdall et al. 2012), and among individuals within populations (Harris et al. 2010; Dammhahn 2012; Krause and Liesenjohann 2012). A major challenge is currently to understand the mechanisms responsible for the relationships between life history and behavior (Stamps 2007; Biro and Stamps 2008; Réale et al. 2010; Dingemanse and Wolf 2010; Réale et al. 2010).
Glucocorticoids (GC) are hormones playing a major role in the regulation of both life history and behavior (Koolhaas et al. 1999; Ricklefs and Wikelski 2002; Reeder and Kramer 2005). For example, GC are constantly released into the blood at low (i.e., baseline) levels (Romero et al. 2009), where they are associated with the organism’s energetic state. Baseline GC increases in response to high energetic demands associated with reproduction or parental care (Romero et al. 1997; Romero 2002; Boonstra 2005; Kitaysky et al. 2010), and favors the expression of behaviors associated with resource acquisition, such as foraging activity or exploration (Kitaysky et al. 2001; Pravosudov 2003; Angelier et al. 2007; Gutman et al. 2011).
GC levels also regulate life history and behavior when the animal faces an acute perturbation of its environment (Wingfield 2005). During such perturbations, GC levels show a so-called stress response characterized by transient but intense increase over several minutes (Reeder and Kramer 2005). Stress responses shut down long-term reproductive functions, including parental behavior (Wingfield et al. 1994), foraging activity or exploration and redirect the resources to maximizing survival (Wingfield and Sapolsky 2003; Reeder and Kramer 2005; Romero et al. 2009). Species with faster life histories (Kitaysky et al. 1999b; Bókony et al. 2009) usually display a lower tendency to mount GC stress responses (i.e., they have a lower stress reactivity). Similarly, species where mating competition is high (Boonstra 2005) may even downregulate their stress reactivity during the reproductive season, thereby avoiding the inhibitory effects of GC stress responses on reproduction. Within populations, GC stress reactivity may vary as a function of how individuals value current reproduction against survival and future reproduction through different scenarios. First, individuals with higher reproductive investment (e.g., producing more numerous or heavier offspring) downregulate their stress reactivity more than others during the reproductive season (Lendvai and Chastel 2008; Bókony et al. 2009). Second, older individuals may reduce their stress reactivity, when age is associated with reduced chances of future reproduction (Otte et al. 2005; Heidinger et al. 2006; Wilcoxen et al. 2011). Third, faster exploring, more active, or more aggressive animals, which should also express faster life history trajectories (Réale et al. 2010), may display a lower GC stress reactivity (Koolhaas et al. 1999; Carere et al. 2005; Øverli et al. 2007; Atwell et al. 2012). No study has yet investigated jointly the relationship between GC, behavior, and life history at the within-population level. Such a study would require monitoring stress reactivity in known individuals over a period spanning a significant portion of the organism’s life history. Unfortunately most studies of stress reactivity investigate a limited number of experimentally caused stress responses (i.e., the challenge protocol used by Wingfield et al. 1994).
In this study, we assess the joint relationships between an individual’s exploration, reproduction, and age on both the average (i.e., baseline) level and intra-individual variability of fecal GC metabolites in a wild population of eastern chipmunks (Tamias striatus) in Southern Québec, Canada. Individuals from this population differ consistently in their exploration pattern in a novel environment throughout their entire life, ranging from slow to fast (Montiglio et al. 2010). Slower explorers display a later age at first reproduction and their highest reproductive success later in life compared with faster explorers (Montiglio et al. 2014). Slow explorers are also assumed to display higher fecal cortisol metabolites variability over the course of their yearly active season (Montiglio et al. 2012a). We expected that fecal cortisol metabolites levels during reproduction would increase with the number of juveniles produced. Such an increase would be observed during gestation and lactation for females, and during the mating season for males. We made the assumption that individuals with a higher reproductive output would show lower intra-individual variability in fecal cortisol metabolites, as a result of stress response downregulation. Older individuals, having reduced chances of future reproduction, should also display reduced intra-individual variability in fecal cortisol metabolites compared with younger ones. Finally, given our results from previous studies, we also expected that slow explorers with a slower life history would display higher intra-individual variability in fecal cortisol metabolites, even after accounting for reproduction.
Materials and methods
Study system and study site
Eastern chipmunks are sciurids feeding on seeds from masting trees (Landry-Cuerrier et al. 2008) that they hoard in their burrow for the winter (Snyder 1982). In Québec, chipmunks usually exhibit two mating seasons: A first one occurs in March while a second mating season occurs in June (Bergeron et al. 2011a). Mating seasons last from 3 to 4 weeks. During this period, males increase their space use and visit females on their home range prior to their estrus (Elliott 1978). A female’s estrus lasts for a day during which there is intense scramble competition between males for the access to and the fertilization of females (Elliott 1978). Litters produced in the summer typically display multiple paternities, with almost all juveniles within a given litter being sired by different males (Bergeron et al. 2011a). After ~4 weeks of gestation, females give birth to two to eight pups that spend the first 4–5 weeks of their life in the maternal burrow before emerging above ground and dispersing (Elliott 1978; Snyder 1982).
We followed a population of chipmunks located on a 25 ha grid in southern Québec (45°05′ N, 72°25′ E), Canada, from 2005 to 2009. The study site was characterized by a mixed forest dominated by red and sugar maples (Acer rubrum, Acer saccharum) and American beech (Fagus grandifolia). Although they feed on maple seeds and other food sources, chipmunks rely mostly on beech tree seeds for their reproduction and above-ground activity, and their whole life cycle is strongly linked to the contrasted inter-annual fluctuation in beech nut productions (Bergeron et al. 2011b; Montiglio et al. 2014). Every year between 2005 and 2009, from May to October, we trapped the chipmunks using Longworth traps (individuals were trapped between 5 and 60 times during the year). Traps were baited with peanut butter, opened at ~ 0800 in the morning, inspected every 2 h, and closed at dusk. Upon capture, individuals were systematically marked with metal ear tags and a passive integrated transponder (Eidap Inc., Alberta, Canada). We also sexed, weighed, and sampled each individual’s ear tissue for DNA analyses using a small punch (see next section). We determined reproductive status using testes position (abdominal or scrotal) for males. We determined female reproductive status by observing nipple aspects: Non-reproductive females have smaller nipples than either gestating, lactating, or post-lactating females; lactating females have darker colored nipples compared to gestating females. We also ascertained whether females were gestating by following their weight every other day during the reproductive season (Bergeron et al. 2011a). Females showing no change in nipple aspects and with a stable weight were considered as “non-reproductive” for the entire season and assigned a litter size of 0. Since the exact date of birth is unknown, we considered that all juveniles born in a given year were born 30 days prior to the peak in juvenile emergence (2006 birth cohort: August 1st; 2007 birth cohort: April 1st; 2008: August 1st; see Montiglio et al. 2014 for additional details). We considered that adults captured prior to the summer 2006 were born in 01 April 2005. In this population, most individuals die after 2 years, although a few chipmunks have been captured for up to 5 years on our grid (Bergeron et al. 2013). The population included ~ 200 individuals (Bergeron et al. 2011b).
Reproduction
Adult individuals were randomly equipped for a short period with radio transmitters to locate their burrow (84.32 % the individuals). To estimate litter size at weaning, we captured the juveniles directly at the mother’s burrow entry, before they dispersed (Bergeron et al. 2011a). We considered the number of offspring that emerged from the burrow (i.e., referred to as number of young produced, thereafter) as an index of a female reproductive success at each season. Observation of testes position suggested that all the males were reproductively active during the study period. Therefore, we considered all males as being reproductively active for a period of 6 weeks before the peak of estrus in the population (referred to as the “mating season”). During this period, males expand their home range to visit females (Elliott 1978; POM unpublished data). We confirmed maternal identity and determined the paternity of the juveniles each year using 11 microsatellite loci (see Chambers and Garant 2010; Bergeron et al. 2011a for details) and the software CERVUS 3.0.3 (Kalinowski et al. 2007) using a 95 % confidence level. We considered all males trapped on the study site during a year as potential fathers. Not all the juveniles produced on the study site could be assigned to a given male, and we did not consider unassigned juveniles (25 % of the juveniles with a known mother were not assigned) in the subsequent analyses on males. Note also that because males have wide home ranges during the reproductive season, we were unable to determine the absolute number of offspring produced by each male (see Bergeron et al. 2011a). Hence, we considered the number of offspring attributed to a male as an index of male reproductive success at each season (referred to as number of young produced, thereafter) and controlled for potential biases in the reproductive success of males by weighing each male’s observation as a function of its position on the study site (see statistical analyses). This metric is also a good proxy of the number of mating partners obtained by a male (Bergeron et al. 2012).
Fecal cortisol metabolites collection and assay
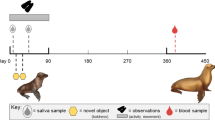
We previously validated an assay to quantify individual fecal cortisol (see Montiglio et al. 2012b for details). In brief, the assay detects a cortisol response to a standardized ACTH challenge test, as well as circadian variations in fecal cortisol metabolite levels in eastern chipmunks. Our validation also showed that fecal samples collected in this species represent an integrated measure of the cortisol produced over a period of ~8 h preceding defecation (Montiglio et al. 2012b). We collected fecal samples from the traps during each capture in 2009 (286 samples from 30 females, 9.57 samples/female, range = 2–26; 161 samples from 27 males, 5.96 samples/male, range = 1–19). Animals spent a maximum of 2 h in the traps. Fecal samples contaminated by urine were discarded. The samples were immediately transferred to test tubes and kept on ice before being transferred to a −20 °C at the end of the day. The samples were then stored into a −80 °C freezer within 2 weeks of collection. The fecal samples were first dried to constant mass in an oven (70 °C) and pulverized using a glass plunger. We vortexed ~35 mg of fecal matter in methanol (80 %) for 20 min (15,000 rpm), centrifuged the liquid and collected the supernatant for an enzyme immunoassay using a cortisol antibody with a horseradish peroxidase ligand (R4866, Coralie Munro, California, Riverside, see Young et al. 2004 for details). Samples were analyzed in duplicates, and we re-analyzed all samples showing a coefficient of variation higher than 20 %. Intra- and inter-assay coefficients of variation were 8.42 and 9.86 %, respectively.
Behavioral assays
Chipmunks were tested in the open-field test twice during their lifetime between 2006 and 2009. Prior to the test, we transferred the chipmunks from the trap to a handling bag and identified them without any manipulation with a handheld transponder reader. The individual was then transferred to a small chamber connected to the arena. We introduced the animal into the arena by lifting a small door connecting the chamber and the arena and videotaped its behavior for 90 s. The arena was a square white plastic box (80 × 80 × 40H cm) with a transparent plexiglas lid. Lines were drawn on the floor (spaced by 10 cm) to produce a grid. The arena offers no refuge (see also Montiglio et al. 2014). We quantified chipmunk exploration in the open-field with the software The Observer 5.0 (Noldus Information Technology 2003) as the number of lines crossed during the three consecutive 30-s intervals. Coding the behavior of chipmunks by intervals enabled us to determine the temporal patterns of exploration within the tests. Individuals expressed patterns ranging from slow, characterized by an intermediate but constant exploration level throughout the test, to fast, characterized by a high exploration level at the beginning of the test followed by a steep decline over the following seconds. The activity pattern in the open-field displays a repeatability of ~30 % (estimated using a linear mixed model, Montiglio et al. 2012b). Open-field tests predict the propensity of individuals to be captured or respond to handling over their entire life (see Montiglio et al. 2012b for a full details and analysis). We built a model accounting for the effects of date, age, sex, year, and trial order on exploration levels. The model also included individual identity as a random effect. We used the best unbiased linear predictors (BLUPs) from this model as individual indexes of exploration for the rest of the analysis. This index represents the deviation of each individual from the population’s predicted mean exploration pattern in standard deviation units (Pinheiro and Bates 2000). Additional details about the test and the validity of exploration BLUPs can be found in Montiglio et al. (2010, 2012b).
Statistical analyses
We analyzed fecal cortisol metabolites level for each sex using linear mixed models (Pinheiro and Bates 2000). Fecal cortisol metabolites level was log-transformed to reach normality. We initially included as fixed effects exploration, age in months, number of young produced, reproductive status (females: non-reproductive, pre-reproductive, gestating, lactating, or post-reproductive; males: reproductively active during the mating season or not reproductively active after the mating season), and all their two-way interactions in the model. Following Pinheiro and Bates (2000), we fitted individual identity as a categorical random effect. This effect enabled us to estimate variation in cortisol levels between individuals and to account for the potential pseudo-replication. We also included date as a categorical random effect to estimate day to day variation in cortisol levels. We privileged a categorical variable over a continuous one to avoid colinearity problems: Correcting for continuous time trend would also correct for the effects of reproductive status. Because we wanted to estimate day to day variability for each individual separately, we nested the date random effect within each individual (referred to as within-individual cortisol variability, thereafter). Doing so enabled us to estimate the daily average cortisol level of each individual separately. Since females are highly synchronous in their reproduction, we did not consider the date of sampling as a fixed effect for our analysis (in contrast to a previous study on the same dataset, Montiglio et al. 2012a). Reproductive status and date effects would be highly correlated, and we included the date random effect in the model accounting for temporal variation in cortisol levels. Hence, we still accounted for date effects while avoiding high correlation between explanatory variables.
We also wanted to investigate how individual reproduction, exploration, and age affected intra-individual cortisol variability. In these conditions, we had to consider a model in which residual variance is allowed to vary between individuals and to be expressed as a function of reproduction, exploration, and age (Pinheiro and Bates 2000). We thus built a model estimating the effects of the number of juveniles produced, exploration, and age on the residual variance of observations available for each individual (these effects are called variance covariates thereafter). The model analyzed between-individual differences in cortisol (using conventional random effects), but also estimated the relationship between the “residual” intra-individual variability in fecal cortisol metabolites of each individual and the number of young it produced, its exploration, and its age. The model also took into account the fact that individuals were studied using different numbers of samples, thereby eliminating the need to control for the number of samples as a fixed effect (Pinheiro and Bates 2000). Because we included day as a random effect nested within individuals, the model also “corrected” the residual variation for the average cortisol level exhibited by each individual any given day. To investigate whether the relationship between reproduction and intra-individual variability in fecal cortisol metabolites varied with the individual exploration pattern, we also tested for an interaction between the two variance covariates: exploration and the number of young produced. We first simplified the fixed-effect structure of each model in a backward–forward stepwise manner using Akaike’s information criterion (AIC) and maximum likelihood optimization (Burnham and Anderson 2002, see Results section for the final model). We then tested for the significance of the variance covariates and random effects using likelihood ratio tests (LRT; Pinheiro and Bates 2000). This test uses one degree of freedom and compares the ratio between the log-likelihood of the model with the variance covariate of interest and a model without it. Preliminary analyses showed that a temporal autocorrelation structure and time of sampling (as a continuous variable) did not significantly improve the models (see Montiglio et al. 2012a, b). Thus, we are confident that the results presented here are not biased by circadian variation in fecal cortisol or by population level seasonal trends independent of their reproductive status. All linear mixed models were fitted in R 2.14.1 using the package nlme (Pinheiro and Bates 2000). An example of the code used to fit the models, including the variance covariates, is provided as Supplementary material (S3). We report means and estimates ± SE.
Results
Females
Model selection by AIC yielded two models with AIC closer than two units from each other (Supplementary material A). Since the two models presented nearly identical estimates and differed only by the inclusion of a litter size effect, which had a small effect (estimate = −0.02 ± 0.06), we present only the most parsimonious one. Cortisol level was lower in lactating females compared with non-reproductive ones (F4, 25 = 4.13, p = 0.01; Fig. 1a; Table 1). Fecal cortisol metabolites level increased as a function of age (F1, 27 = 13.66, p = 0.01; Fig. 1b). Exploration level was included in the final model but failed to reach significance (F1, 27 = 2.09, p = 0.16).
Factors affecting mean fecal cortisol metabolites level over one active season in female eastern chipmunks as estimated from the model presented in Table 1 (N = 286 samples from 30 females). a Female fecal cortisol metabolites level (mean ± standard error of the mean) as a function of their reproductive status (NON = non-reproductive, PRE = pre-reproductive stage, GEST = Gestating stage, LACT = Lactating stage, POST = post-reproduction stage). Number of samples available for each reproductive status class is given below the estimate, along with the number of individuals sampled, in parentheses. b Female fecal cortisol metabolites level as a function of age (in months). The line represents the estimated age effect presented in Table 1
Intra-individual variability in fecal cortisol metabolites was lower in slow-exploring females (LRT = 6.23, df = 1, p = 0.01; Fig. 2a; Table 1) and decreased with the number of young produced (LRT = 6.89, df = 1, p = 0.01; Fig. 2b). Female age (LRT = 0.04, df = 1, p = 0.84) and the interaction between exploration and number of young produced (LRT = 2.07, df = 1, p = 0.15) were not associated with intra-individual variability in fecal cortisol metabolites.
Factors affecting intra-individual variability in fecal cortisol metabolites over one active season in female eastern chipmunks. Dots represent the observed residual variance in fecal cortisol metabolites of each female, computed from a linear mixed model analyzing fecal cortisol metabolites as a function of reproductive status, age and exploration (see Table 1; N = 286 samples from 30 females). a Relationship between female exploration and intra-individual variability in log fecal cortisol metabolites. b Relationship between female litter size and intra-individual variability in log fecal cortisol metabolites
We also detected significant consistent variation in fecal cortisol metabolite levels among individuals (estimate = 0.215, LRT = 25.70, df = 1, p < 0.001) and days of sampling (estimate = 0.814, LRT = 15.43, df = 2, p < 0.001).
Males
Model selection yielded two models with similar AICs and identical effect sizes (Supplementary material B). The two models differed by only one term, with a negligible effect size (an interaction between age and siring success, with an estimate of 0.009 ± 0.001). We chose to present the most parsimonious model. Contrary to females, male fecal cortisol metabolite level was higher in reproductively active individuals (reproductive status main effect, F1, 131 = 19.06, p < 0.001; Table 2 for all model estimates) but less so in older individuals (interaction between reproductive status and age, F1, 131 = 4.68, p = 0.03; Fig. 3). Although they failed to reach significance, reproductive success (F1, 22 = 3.54, p = 0.06), exploration pattern (F1, 22 = 2.08, p = 0.16), as well as the interactions between age and exploration patterns (F1, 22 = 2.34, p = 0.14) and between reproductive status and reproductive success (F1, 131 = 1.99, p = 0.16) were kept in the model yielding the best AIC (Supplementary material B).
Fecal cortisol metabolites levels over one active season in reproductive (black dots, solid line) and non-reproductive male eastern chipmunks (empty dots, dashed line) as a function of age. Lines represent the estimates presented in Table 2 (N = 161 samples from 27 males)
Exploration, siring success, and age failed to predict intra-individual variability in fecal cortisol metabolites (all rejected with p > 0.4). Similarly, we did not detect any consistent variation among individuals or day of sampling (random effects, all p > 0.6).
Discussion
In this study, we quantified the relationship between an animal’s exploration profile and current reproductive investment on GC levels and their intra-individual variability in a natural, uncontrolled environment. Our predictions were based on the assumption that a higher stress reactivity should translate into more intense or frequent stress responses, and so in a higher intra-individual variability in fecal cortisol metabolites in natural environments. In accordance with previous results (Montiglio et al. 2012a), we found that females with a slower exploration during open-field tests also displayed increased within-individual cortisol variability over the summer through which we collected fecal samples. Our results suggest that individuals are likely to display consistent differences in intra-individual variability in fecal cortisol metabolites over their life. Our results also suggest that we may expect individuals to show variation in intra-individual variability in fecal cortisol metabolites between years, as a function of their reproduction. Litter size varies widely over an individual’s life time in this population (Montiglio et al. 2014), and we hypothesize that consistent individual variation in GC stress reactivity as well as plasticity in GC stress reactivity mediate the expression of alternative life history and behavioral trajectories, balancing the trade-off between current reproduction and future reproduction or survival differently. Contrary to our predictions, however, we found that females displayed reduced fecal cortisol metabolites levels during gestation and lactation. Male fecal cortisol metabolite levels increased during the mating season as a function of age and the number of juveniles sired, potentially reflecting a male’s reproductive effort. We did not find consistent differences in cortisol in males.
Within-individual cortisol variability and stress reactivity
In contrast to short-term restraint tests (e.g., Wingfield et al. 1994), our study investigated stress reactivity by monitoring the intra-individual variability in fecal cortisol metabolites levels in individuals over many months in a natural environment. Because individuals are free to adjust their behavior to the changing environmental conditions, this method takes into account how they cope with their environment, thereby providing a more realistic biological context to the study of the ecological function of glucocorticoids.
In accordance with our predictions, we found that females with larger litters displayed reduced intra-individual variability in fecal cortisol metabolites. If the variability reported here is mostly affected by individual differences in stress reactivity, females with larger litters would be maximizing their current reproduction by limiting the amount of energy invested in survival functions (Stearns 1992). Downregulation of stress reactivity is associated with a higher reproductive performance in other species as well. For example, black-legged kittiwakes (Rissa tridactyla) display reduced stress responses when submitted to a standardized blood sampling challenge during the egg-laying phase. The authors hypothesized this decreased stress reactivity would increase their fecundity (Kitaysky et al. 1999a, b, 2010; Jessop 2001). In the same species, birds in better condition were even able to show a stronger decrease in stress reactivity, which lends support to this hypothesis (Kitaysky et al. 1999b). Conversely, increased stress reactivity has been associated with lower reproductive success in other species (reviewed in Breuner et al. 2008). For instance, high variability in fecal GC is correlated with reproductive cycle disruption in captive black (Diceros bicornis) and white (Ceratotherium simum) female rhinoceroses (Carlstead and Brown 2005). The stress response induces the degradation of protein and glycogen stores (Sapolsky et al. 2000), and higher stress reactivity is associated with lower fitness in conditions of starvation in some systems (Romero and Wikelski 2001, 2010). Thus, stress reactivity may reduce reproductive success also by increasing energy expenditure, associated with energy conversion. In the studied population, food availability fluctuates dramatically from one year to the next and strongly shapes torpor patterns, above-ground activity, reproduction, and survival of individual chipmunks (Landry-Cuerrier et al. 2008; Munro et al. 2008; Bergeron et al. 2011b; Montiglio et al. 2014). Hence, downregulating stress reactivity and lowering energy expenditure is likely to have an important effect on individual fitness in this system. Alternatively, some female chipmunks may show lower within-individual variability in cortisol because they experience less/smaller environmental perturbations. As such, females with bigger litter sizes or slow exploration (having lower fecal cortisol metabolites variability) may avoid riskier or less predictable habitats to a larger extent than females with smaller litter sizes or slower exploration. Further studies on this population should thus focus on investigating the behavior and habitat use of individuals, as a function of their reproduction and exploration across years with differing food abundance.
In contrast, exploration profile and reproductive effort were not associated with intra-individual variability in fecal cortisol metabolites levels of males. Males also failed to show any significant individual differences in cortisol levels. We acknowledge that the lower sample size available for males, in addition to the difficulty of estimating their absolute reproductive effort, may have limited our capacity to detect these effects. However, it has been suggested previously that males in many squirrel species could either maximize their reproductive success through mate-searching or mate-guarding (Koford 1982; Koprowski 1993; Schulte-Hostedde et al. 2002). Bigger or more aggressive males may thus invest more in mate-guarding (see Schulte-Hostedde et al. 2002). In our study, older males expressed lower mean fecal cortisol metabolites levels compared with younger ones during the mating season (see previous section). Mate-guarding may be associated with lower energy expenditure, but further studies, quantifying the energetic costs of male mating tactics in eastern chipmunks, are necessary to shed light on the (lack of) pattern reported here.
Average fecal cortisol metabolites levels
Mean levels of fecal cortisol metabolites are thought to fluctuate in relation to the energetic state of individuals, increasing when food availability is low or when energy expenditure is high (Romero 2004). In rodents, energy expenditure is typically higher during lactation than during any other phase of the life cycle (reviewed in Naya et al. 2008). In a closely related chipmunk species (Tamias amoenus), trapped females also display higher plasma cortisol levels during lactation, potentially reflecting a higher baseline level (Kenagy and Place 2000). In contrast, we showed that female eastern chipmunks displayed lower fecal cortisol metabolites levels during gestation and lactation. Plasma and fecal cortisol measurements may differ for a number of reasons. For example, plasma measurements are affected by the animal’s stress response to the sampling procedure itself (Palme 2005), while fecal measurements may be affected by the diet or food consumption rate of the animals (reviewed in Goyman 2012). Females are indeed likely to increase food intake rate during gestation and lactation, which should lead to lower fecal cortisol metabolite concentration in fecal samples (as cortisol metabolites get diluted in a higher volume of fecal matter; Goymann 2005, 2012). Metabolites from the degradation of gonadal steroids could also interact with the cortisol antibody we used (Goyman 2012). However, these potential confounding effects do not account for the systematic differences in intra-individual variability among chipmunks of differing exploration, or with different litter sizes we detected over the full active season. Interestingly, female yellow-bellied marmots (Marmota flaviventris) also displayed lower fecal cortisol metabolites levels during lactation (Smith et al. 2012). We also found that mean fecal cortisol metabolites levels increased with female age. Previous studies also documented heightened mean GC levels in older individuals during the reproductive season, which may be associated with the higher reproductive output of such individuals (Angelier et al. 2006; but see Wilcoxen et al. 2011). In our analyses, however, litter size failed to explain female average cortisol level. Furthermore, in a previous study (Montiglio et al. 2014), we found that females decreased the number of young they produced after 20 to 30 months of age, and that fast explorers did so at an earlier age than slow explorers. The confounding effect of exploration profiles and senescence may thus explain the absence of link between litter size and cortisol level.
We found that cortisol levels during the mating season increased with the number of offspring sired by a male. Mate searching (Elliott 1978; Lane et al. 2010) may involve extremely high energy expenditure, potentially as important as maternal expenditure during gestation and lactation (see Lane et al. 2010 for an example in a related species). During the mating season, older males displayed lowered cortisol levels. As suggested in closely related species, it is possible that older and potentially bigger males spend less energy on mate search but instead guard a limited number of females (Koford 1982; Koprowski 1993; Schulte-Hostedde et al. 2002). Guarding a limited set of burrows potentially incurs less energy expenditure than mate-searching. Older males could also maintain a more positive energy balance by securing higher-quality burrows. Alternatively, this pattern may arise from the disappearance of older males with higher cortisol levels (van de Pol and Verhulst 2006). Such potential biases could be addressed in future studies with longitudinal data, following the cortisol level of individuals over more than 1 year.
In conclusion, our study, by documenting the relationships between fecal cortisol metabolites, reproduction, age, and exploration in a free-ranging chipmunk population, suggests that quantifying both the fluctuations in the mean levels of fecal cortisol metabolites and its intra-individual variability may provide important insights on how animals regulate their reproduction and integrate their behavior and life history. Such an approach may provide an interesting way of analyzing the functional role of GC in an ecological context. Future studies should complement this approach by investigating how environmental fluctuations interact with the endocrinology of both females and males over extended periods of time and at different spatial scales in the wild.
References
Angelier F, Shaffer SA, Weimerskirch H, Chastel O (2006) Effect of age, breeding experience and senescence on corticosterone and prolactin levels in a long-lived seabird: the wandering albatross. Gen Comp Endocrinol 149:1–9
Angelier F, Moe B, Weimerskirch H, Chastel O (2007) Age-specific reproductive success in a long-lived bird: do older parents resist stress better? J Anim Ecol 76:1181–1191
Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED (2012) Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol 23:960–969
Bergeron P, Réale D, Humphries MM, Garant D (2011a) Evidence of multiple paternity and mate selection for inbreeding avoidance in wild eastern chipmunks. J Evol Biol 24:1685–1694
Bergeron P, Réale D, Humphries MM, Garant D (2011b) Anticipation and tracking of pulsed resources drive population dynamics in eastern chipmunks. Ecology 92:2027–2034
Bergeron P, Montiglio P-O, Réale D, Humphries MM, Gimenez O, Garant D (2012) Bateman gradients in a promiscuous mating system. Behav Ecol Sociobiol 66:1125–1130
Bergeron P, Montiglio P-O, Réale D, Humphries MM, Gimenez O, Garant D (2013) Disruptive viability selection on adult exploratory behaviour in eastern chipmunks. J Evol Biol 26:766–774
Bielby J, Mace GM, Bininda-Emonds ORP, Cardillo M, Gittleman JL, Jones KE, Orme CDL, Purvis A (2007) The fast-slow continuum in mammalian life history: an empirical reevaluation. Am Nat 169:748–757
Biro P, Stamps J (2008) Are animal personality traits linked to life-history productivity? Trends Ecol Evol 23:361–368
Bókony V, Lendvai AZ, Liker A, Angelier F, Wingfield JC, Chastel O (2009) Stress response and the value of reproduction: are birds prudent parents? Am Nat 173:589–598
Boonstra R (2005) Equipped for life: the adaptive role of the stress axis in male mammals. J Mammal 86:236–247
Breuner CW, Patterson SH, Hahn TP (2008) In search of relationships between the acute adrenocortical response and fitness. Gen Comp Endocrinol 157:288–295
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach. Springer, New York
Carere C, Drent PJ, Privitera L, Koolhaas JM, Groothuis TGG (2005) Personalities in great tits, Parus major: stability and consistency. Anim Behav 70:795–805
Carlstead K, Brown JL (2005) Relationships between patterns of fecal corticoid excretion and behavior, reproduction, and environmental factors in captive black (Diceros bicornis) and white (Ceratotherium simum) rhinoceros. Zoo Biol 24:215–232
Chambers J, Garant D (2010) Determinants of population genetic structure in eastern chipmunks (Tamias striatus): the role of landscape barriers and sex-biased dispersal. J Hered 101:413–422
Dammhahn M (2012) Are personality differences in a small iteroparous mammal maintained by a life-history trade-off? Proc R Soc B 279:2645–2651
Dingemanse NJ, Wolf M (2010) Recent models for adaptive personality differences: a review. Phil Trans R Soc B 365:3947–3958
Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R, Dawnay N (2007) Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol 76:1128–1138
Elliott L (1978) Social behavior and foraging ecology of the eastern chipmunk (Tamias striatus) in the Adirondack Mountains. Smithson, Cont Zool, p 265
Gaillard JM, Pontier D, Allainé D, Lebreton JD, Trouvilliez J, Clobert J (1989) An analysis of demographic tactics in birds and mammals. Oikos 56:59–76
Goyman W (2012) On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol Evol 3:757–765
Goymann W (2005) Non-invasive monitoring of hormones in bird droppings: biological validations, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann N Y Acad Sci 1046:35–53
Gutman R, Dayan T, Levy O, Schubert I, Kronfeld-Schor N (2011) The effect of the lunar cycle on fecal cortisol metabolite levels and foraging ecology of nocturnally and diurnally active spiny mice. PLoS One 6:e23446
Harris S, Ramnarine IW, Smith HG, Pettersson LB (2010) Picking personalities apart: estimating the influence of predation, sex and body size on boldness in the guppy Poecilia reticulata. Oikos 119:1711–1718
Heidinger BJ, Nisbet ICT, Ketterson ED (2006) Older parents are less responsive to a stressor in a long-lived seabird: a mechanism for increased reproductive performance with age? Proc R Soc B 273:2227–2231
Jessop TS (2001) Modulation of the adrenocortical stress response in marine turtles (Cheloniidae): evidence for a hormonal tactic maximizing maternal reproductive investment. J Zool 254:57–65
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
Kenagy GJ, Place NJ (2000) Seasonal changes in plasma glucocorticosteroids of free-living female yellow-pine chipmunks: effects of reproduction and capture and handling. Gen Comp Endocrinol 117:189–199
Kitaysky AS, Piatt JF, Wingfield JC, Romano M (1999a) The adrenocortical stress-response of black-legged kittiwake chicks in relation to dietary restrictions. J Comp Physiol B 169:303–310
Kitaysky AS, Wingfield JC, Piatt JF (1999b) Dynamics of food availability, body condition and physiological stress response in breeding black-legged kittiwakes. Funct Ecol 13:577–584
Kitaysky AS, Wingfield JC, Piatt JF (2001) Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav Ecol 12:619–625
Kitaysky AS, Piatt JF, Hatch SA, Kitaiskaia EV, Benowitz-Fredericks ZM, Shultz MT, Wingfield JC (2010) Food availability and population processes: severity of nutritional stress during reproduction predicts survival of long-lived seabirds. Funct Ecol 24:625–637
Koford RR (1982) Mating system of a territorial tree squirrel (Tamiasciurus douglasii) in California. J Mammal 63:274–283
Koolhaas JM, Korte SM, de Boer SF, van der Vegt BJ, van Reenen CG, Hopster H, de Jong IC, Ruis MAW, Blokhuis HJ (1999) Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev 23:925–935
Koprowski JL (1993) Alternative reproductive tactics in male eastern gray squirrels: “making the best of a bad job.”. Behav Ecol 4:165–171
Krause ET, Liesenjohann T (2012) Predation pressure and food abundance during early life alter risk-taking behaviour and growth of guppies (Poecilia reticulata). Behaviour 149:1–14
Landry-Cuerrier M, Munro D, Thomas DW, Humphries MM (2008) Climate and resource determinants of fundamental and realized metabolic niches of hibernating chipmunks. Ecology 89:3306–3316
Lane JE, Boutin S, Speakman JR, Humphries MM (2010) Energetic costs of male reproduction in a scramble competition mating system. J Anim Ecol 79:27–34
Lendvai A, Chastel O (2008) Experimental mate-removal increases thestress response of female house sparrows: the effects of offspring value? Horm Behav 53:395–401
Montiglio P-O, Garant D, Thomas DW, Réale D (2010) Individual variation in temporal activity patterns in open-field tests. Anim Behav 80:905–912
Montiglio P-O, Garant D, Pelletier F, Réale D (2012a) Personality differences are related to long-term stress reactivity in a population of wild eastern chipmunks, Tamias striatus. Anim Behav 84:1071–1079
Montiglio P-O, Pelletier F, Palme R, Garant D, Réale D, Boonstra R (2012b) Noninvasive monitoring of fecal cortisol metabolites in the eastern chipmunk (Tamias striatus): validation and comparison of two enzyme immunoassays. Physiol Biochem Zool 85:183–193
Montiglio P-O, Garant D, Bergeron P, Dubuc Messier G, Réale D (2014) Pulsed resources and the coupling between life history strategies and exploration patterns in eastern chipmunks (Tamias striatus). J Anim Ecol. doi:10.1111/1365-2656.12174
Munro D, Thomas DW, Humphries MM (2008) Extreme suppression of above-ground activity by a food-storing hibernator, the eastern chipmunk Tamias striatus. Can J Zool 86:364–370
Naya DE, Ebensperger LA, Sabat P, Bzinovic F (2008) Digestive and metabolic flexibility allows female degus to cope with lactation costs. Physiol Biochem Zool 81:186–194
Noldus Information Technologies (2003) The Observer 5.0
Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC (2005) A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology 30:80–91
Øverli Ø, Sørensen C, Pulman KGT, Pottinger TG, Korzan W, Summers CH, Nilsson GE (2007) Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci Biobehav Rev 31:396–412
Palme R (2005) Measuring fecal steroids - guidelines for practical application. Ann NY Acad Sci 1046:75–80
Pinheiro JC, Bates DM (2000) Mixed effects models in S and S-plus. Springer, New York
Pravosudov VV (2003) Long-term moderate elevation of corticosterone facilitates avian food-caching behaviour and enhances spatial memory. Proc R Soc Lond B 274:2599–2605
Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O (2010) Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil Trans R Soc B 365:4051–4063
Reeder DM, Kramer KM (2005) Stress in free-ranging mammals: integrating physiology, ecology, and natural history. J Mammal 86:225–235
Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17:462–468
Romero LM (2002) Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128:1–24
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255
Romero LM, Wikelski M (2001) Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Nino events. Proc Natl Acad Sci U S A 98:7366–7370
Romero LM, Wikelski M (2010) Stress physiology as a predictor of survival in Galapagos marine iguanas. Proc R Soc Lond 277:3157–3162
Romero LM, Ramenofsky M, Wingfield JC (1997) Season and migration alters the corticosterone response to capture and handling in an Arctic migrant, the white-crowned sparrow (Zonotrichia leucophrys gambelii). Comp Biochem Physiol C 116:171–177
Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav 55:375–389
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrinol Rev 21:55–89
Schulte-Hostedde AI, Millar JS, Gibbs HL (2002) Female-biased sexual size dimorphism in the yellow-pine chipmunk (Tamias amoenus): sex-specific patterns of annual reproductive success and survival. Evolution 56:2519–2529
Smith JE, Monclús R, Wantuck D, Florant GL, Blumstein DT (2012) Fecal glucocorticoid metabolites in wild yellow-bellied marmots: experimental validation, individual differences and ecological correlates. Gen Comp Endocrinol 178:417–426
Snyder DP (1982) Tamias striatus. Mamm Species 168:1–8
Stamps J (2007) Growth-mortality tradeoffs and "personality traits" in animals. Ecol Lett 10:355–363
Stearns SC (1989) Trade-offs in life-history evolution. Funct Ecol 3:259–268
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Torres Dowdall J, Handelsman CA, Ruell EW, Auer SK, Reznick DN, Ghalambor CK (2012) Fine-scale local adaptation in life histories along a continuous environmental gradient in Trinidadian guppies. Funct Ecol 26:616–627
van de Pol MV, Verhulst S (2006) Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am Nat 167:766–773
van Noordwijk AJ, de Jong G (1986) Acquisition and allocation of resources: their influence on variation in life history tactics. Am Nat 128:137–142
Walsh MR, Reznick DN (2009) Phenotypic diversification across an environmental gradient: a role for predators and resource availability on the evolution of life histories. Evolution 63:3201–3213
Wiersma P, Munoz-Garcia A, Walker A, Williams JB (2007) Tropical birds have a slow pace of life. Proc Natl Acad Sci U S A 104:9340–9345
Wilcoxen TE, Boughton RK, Bridge ES, Rensel MA, Schoech SJ (2011) Age-related differences in baseline and stress-induced corticosterone in Florida scrub-jays. Gen Comp Endocrinol 173:461–466
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100:687–690
Wingfield JC (2005) The concept of allostasis: coping with a capricious environment. J Mammal 86:248–254
Wingfield JC, Sapolsky RM (2003) Reproduction and resistance to stress: when and how. J Endocrinol 15:711–724
Wingfield JC, Deviche P, Sharbaugh S, Astheimer LB, Holberton R, Suydam R, Hunt K (1994) Seasonal changes of the adrenocortical responses to stress in redpolls, Acanthis flammea, in Alaska. J Exp Zool 270:372–380
Young KM, Walker SL, Lanthier C, Waddell WT, Monfort SL, Brown JL (2004) Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Gen Comp Endocrinol 137:148–165
Acknowledgments
We thank Nature Conservancy of Canada and Ruiter Valley Land Trust for access to study site. We also thank Hélène Presseault-Gauvin for valuable assistance during the fecal cortisol metabolites assays and all graduate students and research assistants that have contributed to the data collection. The authors also thank William Vickery, Albrecht Schulte-Hostedde, Jean-François Giroux, and two anonymous reviewers for comments on a previous version of this manuscript. This work was funded by Natural Sciences and Engineering Research Council of Canada Discovery Grants (DG, DR, and FP) and Canada Research Chairs program (DR and FP) and by a team research project grant from the Fonds Québécois de la Recherche sur la Nature et les Technologies (DR, DG, Murray Humphries, Don Kramer, and the late Don Thomas). POM was supported by a scholarship from the Fonds Québécois de la Recherche sur la Nature et les Technologies. The authors declare no conflict of interest.
Ethical standards
All manipulations, captures, and tests were conducted following the guidelines of the Canadian Council on Animal Care via Université du Québec at Montréal (permit numbers CIPA 0603-462-0607 and 0507-613-0509).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Soulsbury
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material A
(DOC 45.0 kb)
Supplementary material B
(DOC 40.5 kb)
Supplementary material C
(DOC 38.5 kb)
Rights and permissions
About this article
Cite this article
Montiglio, PO., Garant, D., Pelletier, F. et al. Intra-individual variability in fecal cortisol metabolites varies with lifetime exploration and reproductive life history in eastern chipmunks (Tamias striatus). Behav Ecol Sociobiol 69, 1–11 (2015). https://doi.org/10.1007/s00265-014-1812-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1812-x