Abstract
Like all animals, bees need to consume essential amino acids to maintain their body’s protein synthesis. Perception and discrimination of amino acids are, however, still poorly understood in bees (and insects in general). We used chemotactile conditioning of the proboscis extension response (PER) to examine (1) whether Bombus terrestris workers are able to perceive amino acids by means of their antennae and (if so) which ones, (2) whether they are able to differentiate between different amino acids, and (3) whether they are able to differentiate between different concentrations of the same amino acid. We found that workers perceived asparagine, cysteine, hydroxyproline, glutamic acid, lysine, phenylalanine, and serine, but not alanine, leucine, proline, or valine by means of their antennae. Surprisingly, they were unable to differentiate between different (perceivable) amino acids, but they distinguished between different concentrations of lysine. Consequently, bumblebees seem to possess amino acid receptors at the tip of their antennae, which enable a general perception of those solute amino acids that have an additional functional group (besides the common amino and carboxylic groups). They may thus have the ability to assess the overall amino acid content of pollen and nectar prior to ingestion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteins and specifically essential amino acids are important nutrients for all animals to maintain their own protein synthesis. Under- or overeating protein damages health by, e.g., weakening the immune system, reducing growth or impeding reproduction (Kropàcovà et al. 1968; Behmer 2009; Roche et al. 2011; Simpson and Raubenheimer 2012). For example, excess amounts of protein (DeGroot 1953; Standifer et al. 1960; Herbert et al. 1977; Iwasaki et al. 1988; Dourmad et al. 1994; Pirk et al. 2010; Helm et al. 2017) or of certain amino acids (Simpson and Raubenheimer 2009; Vrzal et al. 2010; Huang et al. 2011; Wu 2013) can shorten the lifespan. Consequently, animals would benefit from assessing the protein content of potential food resources, because this information would enable them to compose diets which best support their current metabolic needs.
As amino acids are hardly volatile substances, even though some degradation products may be volatile (Linander et al. 2012), assessment of their amount and composition requires chemotactile sensation, an inseparable combination of taste and tactile sensation (Ruedenauer et al. 2015). However, compared to olfaction, much less is known on chemotactile sensation, particularly in invertebrates (however see Amrein and Thorne 2005).
We here investigate chemotactile perception of amino acids via the antennae in a social bee, Bombus terrestris (Apidae; Fig. 1A), to better understand the sensory mechanisms underlying the perception of these essential nutrients in insects. Bees obtain all amino acids from floral resources, i.e., pollen and nectar, which are known to vary in the composition and particularly in the amount of amino acids (Roulston and Cane 2000; Somerville 2001; Petanidou et al. 2006; Weiner et al. 2010). Bees are known to detect differences in the nutritional composition of pollen and nectar and respond accordingly, e.g., through changing foraging patterns or reproductive investment (Brodschneider and Crailsheim 2010; Ruedenauer et al. 2015, 2016; Somme et al. 2015; Hendriksma and Shafir 2016; Muth et al. 2016; Zarchin et al. 2017). The sensory mechanisms underlying such nutrient assessment in bees have so far only been elucidated for carbohydrates, i.e., sugars, in nectar, which are detected via specific sugar receptors on the antenna (Slone et al. 2007; Jung et al. 2015). In contrast, the mechanisms underlying amino acid perception remain largely unclear.
In Drosophila, ionotropic receptors (IR) appear to be involved in amino acid reception (Benton et al. 2009; Croset et al. 2016; Ganguly et al. 2017). This recently described new receptor gene family most likely evolved from ionotropic glutamate receptors (iGluR), a large and conserved family of synaptic ligand-gated ion channels (Rytz et al. 2013). However, it remains unclear whether IRs are specialized on particular amino acids allowing to differentiate between them or if they act as general receptors sensitive to a subset of (or all) amino acids (Croset et al. 2016; Kudow et al. 2017).
To investigate amino acid perception in B. terrestris, we used the conditioning of the proboscis extension response (PER) shown upon the perception of, e.g., an olfactory (e.g., Laloi et al. 1999; Matsumoto et al. 2012) or gustatory/chemotactile (Ruedenauer et al. 2015) cue associated with a sugar reward. A PER in the context of classical conditioning (Pavlov 1927) is typically shown when touching the bees’ antennae, tarsi, or parts of the mouth with a sugar solution (de Brito Sanchez et al. 2007). In a learning experiment, this so-called unconditioned stimulus (US) is evoked shortly after presenting a conditioned stimulus (CS, e.g., an olfactory or chemotactile cue) to associate both stimuli (i.e., US and CS). If the bees are able to learn this association, they will, after repeated exposure, extend their proboscis at the mere presentation of the CS (Bitterman et al. 1983; Matsumoto et al. 2012). Moreover, rewarding only one out of two different stimuli (i.e., differential conditioning) allows testing whether bees can differentiate between two stimuli. In insects, gustatory/chemotactile receptors are distributed over several body parts, including the antennae (Amrein and Thorne 2005; Montell 2009; de Brito Sanchez 2011). PER conditioning can thus be used to test whether bees can not only perceive, but also differentiate between different non-volatile cues by touching the antennae.
Using chemotactile PER conditioning, we tested whether bumblebees can perceive and differentiate between different amino acids as well as between different amounts/concentrations of the same amino acid. Given their nutritional importance, we hypothesized that bumblebees can perceive amino acids and differentiate between different amino acids and concentrations. We especially expected the essential amino acids [i.e., arginine, histidine, lysine, phenylalanine, tryptophan, leucine, isoleucine, methionine, threonine, and valine (DeGroot 1953)] as well as proline to be perceived by bumblebees. The latter is important as energy source of the flight muscles (Micheu et al. 2000) and seems to support colony growth in B. terrestris (Kämper et al. 2016).
Materials and methods
Study animals and test substances
Bombus terrestris colonies were purchased (Behr, Kampen, Germany) and kept in two-chambered wooden boxes (240 × 210 × 110 mm per chamber) in a climate chamber (25 °C, 50% humidity, 12/12 h light/dark-cycle) for ca. 3 weeks before starting the behavioral experiment. Bumblebees had ad libitum access to Apiinvert (a mixture of sucrose, fructose, and glucose; Südzucker AG, Mannheim, Germany) and bee-collected polyfloral pollen (Naturwaren Niederrhein GmBH, Goch-Asperden, Germany). For all learning experiments, individual workers were captured and chilled on ice for 15 min. As the size of a bumblebee determines its antennal sensitivity and differently sized workers may carry out different tasks (Spaethe et al. 2007), we randomly selected bumblebees of different sizes to cover the full size spectrum.
We tested the following proteinogenic essential and non-essential l-amino acids: alanine, asparagine, cysteine, glutamic acid, leucine, lysine, phenylalanine, proline, serine and valine as well as the non-proteinogenic hydroxyproline (Table 1, all Sigma-Aldrich, Taufkirchen, Germany). For stimulus presentation, all amino acids were solved in de-ionized water (henceforth only referred to as water) at a concentration of ~ 10 g/l, which was the highest possible concentration of the least water-soluble amino acid, i.e., glutamic acid. This concentration is higher than concentrations reported for single amino acids in pollen or nectar of several plant species (Weiner et al. 2010; Ruedenauer et al. 2015) and should, therefore, fall within the perception range of bees.
Experimental setup
The setup of the PER experiments followed Sommerlandt et al. (2014) and Ruedenauer et al. (2015). The chilled bumblebees were placed in a plastic tube (7 mm diameter, 35 mm long) and fixed with a “yoke” made of a paper clip, which allowed free movement of the bumblebees’ head and forelegs, thus enabling a proper PER. The fixed animals were fed ad libitum with a 0.5 M sucrose solution before being placed in a climate cabinet for 25 h, at 20 °C and 70% relative air humidity.
Prior to the actual experiment, we tested whether the animals showed PERs by touching their antennae with a toothpick soaked with a 0.5 M sucrose solution. Only bumblebees that properly responded to this stimulus by extending their proboscis were used for the learning experiments. We used a standard PER protocol for differential conditioning, which was established for honeybees (e.g., Bitterman et al. 1983; Laloi et al. 1999) and allows testing whether bees can differentiate between two stimuli, one rewarded (CS+) with sucrose solution (US) and the second one unrewarded (CS−). Each test animal was placed in a rack and allowed to rest for 15 s. For stimulus presentation, 5 µl of the solution was pipetted on a small filter paper, which was then placed on a copper plate and moved towards an antenna using a micromanipulator (Fig. 1B). The bee could then freely touch the stimulus with the tip of its left antenna. After the first touch, the stimulus was presented for 6 s. Three seconds after stimulus onset, a toothpick was presented to the right antenna, either as US (soaked with sucrose solution) or plain to equalize visual cues for the CS+ and CS− presentation. If the bumblebee extended its proboscis, it was allowed to lick on the toothpick. The US was removed together with CS offset after 3 s. Subsequently, the bumblebee was allowed to rest for another 15 s before being replaced by the next one. The time between trials (inter trial interval, ITI) was 8 min. The number of trials was 20 per individual (ten for CS+ and ten for CS−) presented in a pseudorandomized order (CS+, CS−, CS+, CS+, CS−, CS+, CS−, CS−, CS+, CS−, CS+, CS−, CS+, CS+, CS−, CS−, CS+, CS−, CS−, CS+). Each stimulus was tested once as CS+ and once as CS− (reversed meaning), and each animal was tested in one experiment only. The plates were cleaned with 70% ethanol after each usage.
We performed three different experiments:
-
1.
To determine whether bumblebees can perceive the amino acids, each amino acid (10 g/l water) was tested against pure water. Note that bumblebees, unlike honeybees (Page et al. 1998), do not show a PER to pure water. This experiment revealed that bumblebees could differentiate some amino acids (e.g., lysine, cysteine, and hydroxyproline), but not others (e.g., alanine, leucine, and proline) from water (Figs. 2, 3).
-
2.
To determine whether the bumblebees can further differentiate between different amino acids, we subsequently tested amino acids that could be differentiated from water against each other and amino acids that could not be differentiated from water against each other. We finally tested all amino acids that could be differentiated from water against one or two of the amino acids that could not be differentiated from water.
-
3.
To finally determine whether the bumblebees can also differentiate between different concentrations of the same amino acid, we presented the bumblebees with three different concentrations (i.e., 1 g/l, 10 g/l, and 20 g/l) of lysine (which the bumblebees could differentiate from water) and proline (which was not differentiated from water). These two amino acids were chosen because of their high water solubility, which allowed testing of higher and lower concentrations than the one previously used (i.e., 10 g/l).
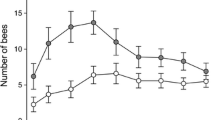
Proportions of proboscis extension responses (PER) shown by Bombus terrestris towards amino acids (all 10 mg/ml water), which were differentiated from water (H2O) in chemotactile conditioning: A cysteine, B lysine, C hydroxyproline, D glutamic acid, E phenylalanine, and F serine. CS+ represents the rewarded conditioned stimulus, CS− the unrewarded conditioned stimulus. Both substances (i.e., amino acid and water) were used as CS+ and CS− with no significant differences between the two stimulus groups (see Supplementary Material, Table S1). Different letters to the right of the learning curves indicate significant differences in learning performance between groups (Table 1)
Proportion of proboscis extension responses (PER) shown by Bombus terrestris towards asparagine (10 mg/ml water) vs. water (H2O): A asparagine used as CS+, B asparagine used as CS−. CS+ represents the rewarded conditioned stimulus, CS− represents the unrewarded conditioned stimulus. There was a significant difference between the two stimulus groups (Supplementary Material Table S1), preventing pooling of the two groups. Different letters to the right of the learning curves indicate significant differences in learning performance between groups (Table 1)
Statistical analysis
The numbers of responses to each CS+ and each CS− were counted for each animal (e.g., an animal may respond nine times to CS+ and one time to CS−) and used as response variable. To determine whether the bumblebees’ response behavior and thus learning performance depended on the type of rewarded substance, we first composed a Laplace generalized linear mixed effect model (GLMM) with Poisson distribution and individual included as random effect and tested for a significant effect of the interaction between stimulus type [i.e., rewarded (CS+) or unrewarded (CS−)] and substance (i.e., amino acids, water, and different concentrations). When the interaction was not significant (Tables S1–S3), we only tested for a significant effect of stimulus type. When the interaction was significant (i.e., in the case of asparagine), we tested for a significant effect of stimulus for both substance groups separately.
All statistical tests were performed using R v3.1.2 (R Foundation for Statistical Computing, Vienna, Austria). For data visualization, GraphPad Prism v6.01 was used.
Results
Differential conditioning of amino acids vs. water
Bumblebees differentiated asparagine, cysteine, glutamic acid, hydroxyproline, lysine, phenylalanine, and serine from water (Figs. 2, 3; Table 2). In case of asparagine, bumblebees only differentiated between asparagine used as CS+ and water used as CS−, but not vice versa (Fig. 3; Table 2). Learning performance did not depend on the type of rewarded substance for any of the other perceived amino acids (Table S1). However, bumblebees did not differentiate alanine, leucine, proline, or valine from water (Fig. 4; Table 2).
Proportion of proboscis extension responses (PER) shown by Bombus terrestris towards amino acids (all in a 10 mg/ml concentration in water) vs. water, where bumblebees showed no discrimination: A alanine, B leucine, C proline, and D valine. CS+ represents the rewarded conditioned stimulus, and CS− represents the unrewarded conditioned stimulus. Both substances were used as CS+ and CS− with no significant differences between groups (see Supplementary Material, Table S1). The same letters to the right of the learning curves indicate no significant differences in learning performance between groups (Table 1), and letters with an asterisk indicate that the two (overlapping) curves had the same letter
Differential conditioning between different amino acids
Bumblebees did not differentiate between amino acids that could be differentiated from water, or between amino acids that could not be differentiated from water (Fig. 5; Table 3). They were, however, able to differentiate between amino acids that could be differentiated from water and amino acids that could not be differentiated from water (Fig. 6; Table 3).
PER of Bombus terrestris towards different amino acids (all 10 mg/ml water), which were or were not differentiated from water, tested against amino acids from the same group: A alanine vs. valine, B asparagine vs. glutamic acid, C asparagine vs. lysine, D cysteine vs. phenylalanine, E glutamic acid vs. lysine, F leucine vs. proline, and G lysine vs. serine. CS+ represents the rewarded conditioned stimulus, CS− represents the unrewarded conditioned stimulus. Both amino acids were used as CS+ and CS− with no significant differences between groups (Supplementary Material Table S2). Amino acids that were differentiated from water are marked with a hashtag. The same letters to the right of the learning curves indicate no significant differences in learning performance between groups (Table 2); letters with an asterisk indicate that the two (overlapping) curves had the same letter
Proportion of proboscis extension responses (PER) shown by Bombus terrestris towards amino acids (all 10 mg/ml water), which were or were not differentiated from water tested against amino acids from the other group: A alanine vs. phenylalanine and B glutamic acid vs. proline. CS+ represents the rewarded conditioned stimulus, CS− the unrewarded conditioned stimulus. Both amino acids were used as CS+ and CS− with no significant differences between groups (Supplementary Material Table S2). Amino acids that were differentiated from water are marked with a hashtag. Different letters to the right of the learning curves indicate significant differences in learning performance between groups (Table 2)
Differential conditioning between different concentrations of the same amino acid
Bumblebees differentiated between different concentrations of lysine (which could be differentiated from water), but not between different concentrations of proline (which could not be differentiated from water) (Fig. 7; Table 4).
Proportion of proboscis extension responses (PER) shown by Bombus terrestris towards different concentrations of amino acids which were or were not differentiated from water (concentrations given in mg/ml water): A lysine 1:1 vs. 10:1, B lysine 10:1 vs. 20:1, C proline 1:1 vs. 10:1 and D proline 10:1 vs. 20:1. CS+ represents the rewarded conditioned stimulus, CS− the unrewarded conditioned stimulus. Both concentrations were used as CS+ and CS− with no significant differences between groups (Supplementary Material Table S3). Amino acids that were differentiated from water are marked with a hashtag. Different letters to the right of the learning curves indicate significant differences in learning performance between groups (Table 3)
Discussion
Bumblebees can only perceive some amino acids by means of their antennae
Our results clearly show that, with access to chemotactile information by means of the antennae, bumblebee workers can differentiate some amino acids (i.e., asparagine, cysteine, glutamic acid, hydroxyproline, lysine, phenylalanine, and serine) from water, but not others (i.e., alanine, leucine, proline, and valine). Moreover, while bumblebees were perfectly able to differentiate different concentrations of the same (‘perceivable’) amino acid, they could not differentiate between different amino acids of the same group (‘perceivable’ or ‘non-perceivable’). These results were quite unexpected and contrast our expectations that all amino acids could be differentiated from water and against each other.
Interestingly, perception was not confined to those amino acids that are essential to bees (DeGroot 1953) (Table 1). Neither was perception related to polarity, charge, acidity, or the (subjective) human taste impression (participants were asked to eat amino acids and describe the taste, Schiffman et al. 1981) (Table 1). The only consistent difference between the two groups was that ‘perceivable’ amino acids possess a terminal functional group in addition to the amino acid-characteristic amino- and carboxyl group (Table 1). This difference is especially apparent for proline and hydroxyproline, which only differ in the terminal (hydroxyl) group (Table 1). While hydroxyproline was perceived by bumblebees, proline was not, indicating that this difference was sufficient to enable perception.
The rather non-specific perception of amino acids with additional terminal functional groups contrasts with amino acid perception in vertebrates. For example, humans are able to differentiate between different amino acids and even between different enantiomers, i.e., d- and l-forms (Schiffman and Dackis 1975; Schiffman et al. 1981), which are received by different receptor types (as reviewed by Chandrashekar et al. 2006). Likewise, fish show different levels of attraction by different amino acids, suggesting that they also may be able to differentiate between different amino acids (Sutterlin 1975; Kasumyan and Morsi 1996), most likely by means of specific chemoreceptors (Marui and Kiyohara 1987; Mullin et al. 1994).
Interestingly, the proboscis of the hoverfly Eristalis tenax is sensitive to proline as a component of pollen (Wacht et al. 2000) and honeybees prefer nectar containing higher concentrations of proline (Carter et al. 2006). These findings suggest that bumblebees might also be able to perceive more amino acids and differentiate between them by means of receptors that are located on body parts other than the antennae, e.g., the proboscis, or after ingestion.
Potential antennal receptors
The lack of antennal differentiation in B. terrestris suggests that bumblebees possess generalistic receptors on their antennae, which are activated non-specifically by amino acids that possess an additional functional group. Such a reception system may resemble the mammalian amino acid receptor heteromer (T1R1+3) or the Drosophila ionotropic co-receptor IR76b, which both respond to a broad spectrum of l-amino acids (Nelson et al. 2002; Ganguly et al. 2017), including amino acids that could not be perceived by bumblebees. Moreover, IR76b is highly conserved among insects and also present in Apis mellifera (Croset et al. 2010), rendering it a potential amino acid co-receptor also in B. terrestris. In fact, the IR76b receptor gene is present in the B. terrestris genome, but it is distinct from the A. mellifera IR76b in its amino acid sequence (Sadd et al. 2015). Alternatively, the additional functional groups may be received non-specifically by non-amino acid-specific receptors responding to functional groups in general.
Essentiality of amino acids is not important for perception
Interestingly, some essential amino acids (i.e., leucine and valine) were not perceived, indicating that antennal amino acid perception in B. terrestris does not reflect the importance of a particular amino acid or its effect on the bumblebees’ health. However, essential amino acids seem to be present in every pollen species and proline often dominates bee resources (Weiner et al. 2010). Consequently, these amino acids either do not need to be assessed specifically, are perceived via receptors on other body parts or are perceived only post-ingestive.
Do bumblebees use antennal perception to infer information on protein content?
Although bumblebees cannot differentiate between different amino acids and are, therefore, not able to assess qualitative differences in amino acid profiles of nectar and pollen by means of their antennae, they can use antennal perception to infer concentration differences of specific amino acids. In both pollen and nectar, proportions of different amino acids correlate with each other and with overall amino acid content (r ≥ 0.5, P < 0.01 for the data set composed by Weiner et al. 2010). Therefore, bumblebees could easily infer the overall quantity based on the content of only some amino acids and use this information for their foraging decisions. This would provide them with sufficient information on the amino acid content of floral resources. It would further enable bumblebees to avoid high concentrations of (free) amino acids potentially detrimental to bees (Huang et al. 2011).
It remains open why our tested bumblebees could only differentiate between asparagine and water when asparagine was presented as CS+. One possible explanation for this finding is that asparagine needs reinforcement by the reward (CS+) to be learned, whereas it cannot be learned when it is not rewarded (CS−).
Conclusion
Summarizing our results, we suggest that antennal perception of dissolved amino acids enables bumblebees to assess the free amino acid content of floral resources and thus to assess their overall amino acid content, but most likely not their qualitative composition. Moreover, the overall protein content of pollen could be easily inferred via this free amino acid assessment, as free amino acids seem to be positively correlated with overall protein (i.e., the sum of protein-bound and free amino acids) in pollen (Weiner et al. 2010). Although most nutrients in pollen are located inside the grain and, therefore, inaccessible to bees (Stanley and Linskens 1974), nutritional information could still be obtained from small nutrients (e.g., free amino acids) leaking through pores onto the pollen surface or through occasionally damaged pollen grains. Following antennal perception of overall amino acid content, more precise information on amino acid composition might be obtained via receptor on other body parts or post-ingestive. In future studies, electrophysiological and molecular methods as well as tests for proboscis or internal (via feeding) perception of amino acids will help to further elucidate the mechanisms underlying amino acid reception and perception in bees.
Abbreviations
- CS:
-
Conditioned stimulus
- CS+:
-
Rewarded conditioned stimulus
- CS−:
-
Unrewarded conditioned stimulus
- GLMM:
-
Generalized linear mixed effect model
- iGluR:
-
Ionotropic glutamate receptor
- ITI:
-
Inter trial interval
- IR:
-
Ionotropic receptor
- PER:
-
Proboscis extension response
- US:
-
Unconditioned stimulus
References
Amrein H, Thorne N (2005) Gustatory perception and behavior in Drosophila melanogaster. Curr Biol 15(17):R673–R684. https://doi.org/10.1016/j.cub.2005.08.021
Behmer ST (2009) Insect herbivore nutrient regulation. Annu Rev Entomol 54:165–187. https://doi.org/10.1146/annurev.ento.54.110807.090537
Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB (2009) Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136(1):149–162. https://doi.org/10.1016/j.cell.2008.12.001
Bitterman ME, Menzel R, Fietz A, Schäfer S (1983) Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97(2):107–119
Brodschneider R, Crailsheim K (2010) Nutrition and health in honey bees. Apidologie 41(3):278–294. https://doi.org/10.1051/apido/2010012
Carter C, Shafir S, Yehonatan L, Palmer RG, Thornburg R (2006) A novel role for proline in plant floral nectars. Naturwissenschaften 93(2):72–79. https://doi.org/10.1007/s00114-005-0062-1
Chandrashekar J, Hoon MA, Ryba NJP, Zuker CS (2006) The receptors and cells for mammalian taste. Nature 444:288. https://doi.org/10.1038/nature05401
Croset V, Rytz R, Cummins SF, Budd A, Brawand D, Kaessmann H, Gibson TJ, Benton R (2010) Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Gen 6(8):e1001064. https://doi.org/10.1371/journal.pgen.1001064
Croset V, Schleyer M, Arguello JR, Gerber B, Benton R (2016) A molecular and neuronal basis for amino acid sensing in the Drosophila larva. Sci Rep 6:34871. https://doi.org/10.1038/srep34871
de Brito Sanchez MG (2011) Taste perception in honey bees. Chem Senses 36(8):675–692. https://doi.org/10.1093/chemse/bjr040
de Brito Sanchez MG, Ortigao-Farias JR, Gauthier M, Liu FL, Giurfa M (2007) Taste perception in honeybees: just a taste of honey? Arthropod Plant Interact 1(2):69–76. https://doi.org/10.1007/s11829-007-9012-5
DeGroot AP (1953) Protein and amino acid requirements of the honey bee (Apis mellifica L.). Phys Comp Oecol 3:197–285
Dourmad JY, Etienne M, Prunier A, Noblet J (1994) The effect of energy and protein intake of sows on their longevity: a review. Livest Prod Sci 40(2):87–97. https://doi.org/10.1016/0301-6226(94)90039-6
Ganguly A, Pang L, Duong V-K, Lee A, Schoniger H, Varady E, Dahanukar A (2017) A molecular and cellular context-dependent role for Ir76b in detection of amino acid taste. Cell Rep 18(3):737–750. https://doi.org/10.1016/j.celrep.2016.12.071
Helm BR, Slater G, Rajamohan A, Yocum GD, Greenlee KJ, Bowsher JH (2017) The geometric framework for nutrition reveals interactions between protein and carbohydrate during larval growth in honey bees. Biol Open. https://doi.org/10.1242/bio.022582
Hendriksma HP, Shafir S (2016) Honey bee foragers balance colony nutritional deficiencies. Behav Ecol Sociobiol 70(4):509–517. https://doi.org/10.1007/s00265-016-2067-5
Herbert EW, Shimanuki H, Caron D (1977) Optimum protein levels required by honey bees (Hymenoptera, Apidae) to initiate and maintain brood rearing. Apidologie 8(2):141–146. https://doi.org/10.1051/apido:19770204
Huang T, Jander G, de Vos M (2011) Non-protein amino acids in plant defense against insect herbivores: representative cases and opportunities for further functional analysis. Phytochemistry 72(13):1531–1537
Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo E-J, Yu BP (1988) The influence of dietary protein source on longevity and age-related disease processes of fischer rats. J Gerontol 43(1):B5–B12. https://doi.org/10.1093/geronj/43.1.B5
Jung JW, Park KW, Ahn Y-J, Kwon HW (2015) Functional characterization of sugar receptors in the western honeybee, Apis mellifera. J Asia Pac Entomol 18(1):19–26. https://doi.org/10.1016/j.aspen.2014.10.011
Kämper W, Werner PK, Hilpert A, Westphal C, Blüthgen N, Eltz T, Leonhardt SD (2016) How landscape, pollen intake and pollen quality affect colony growth in Bombus terrestris. Landscape Ecol. https://doi.org/10.1007/s10980-016-0395-5
Kasumyan A, Morsi AK (1996) Taste sensitivity of common carp Cyprinus carpio to free amino acids and classical taste substances. J Ichthyol 36(5):391–403
Kropàcovà S, Haslbachova H, Novàk V (1968) Development of honeybee ovaries as affected by narcosis and injections of certain substances. Sborn Vys zemed v Brne 16:537–543
Kudow N, Miura D, Schleyer M, Toshima N, Gerber B, Tanimura T (2017) Preference for and learning of amino acids in larval Drosophila. Biol Open 6(3):365–369. https://doi.org/10.1242/bio.020412
Laloi D, Sandoz JC, Picard-Nizou AL, Pham-Delegue MH (1999) Olfactory conditioning of the proboscis extension reflex in the bumble bee Bombus terrestris. Ann Soc Entomol Fr 35:154–158
Linander N, de Ibarra NH, Laska M (2012) Olfactory detectability of l-amino acids in the European honeybee (Apis mellifera). Chem Senses 37(7):631–638. https://doi.org/10.1093/chemse/bjs044
Marui T, Kiyohara S (1987) Structure–activity relationships and response features for amino acids in fish taste. Chem Senses 12(2):265–275. https://doi.org/10.1093/chemse/12.2.265
Matsumoto Y, Menzel R, Sandoz JC, Giurfa M (2012) Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J Neurosci Methods 211(1):159–167. https://doi.org/10.1016/j.jneumeth.2012.08.018
Micheu S, Crailsheim K, Leonhard B (2000) Importance of proline and other amino acids during honeybee flight. Amino Acids 18(2):157–175. https://doi.org/10.1007/s007260050014
Montell C (2009) A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol 19(4):345–353
Mullin CA, Chyb S, Eichenseer H, Hollister B, Frazier JL (1994) Neuroreceptor mechanisms in insect gustation: a pharmacological approach. J Insect Physiol 40(11):913–931. https://doi.org/10.1016/0022-1910(94)90130-9
Muth F, Francis JS, Leonard AS (2016) Bees use the taste of pollen to determine which flowers to visit. Biol Lett. https://doi.org/10.1098/rsbl.2016.0356
Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJP, Zuker CS (2002) An amino-acid taste receptor. Nature 416(6877):199–202
Page RE, Erber J, Fondrk MK (1998) The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A 182(4):489–500. https://doi.org/10.1007/s003590050196
Pavlov IP (1927) Conditioned reflexes. Oxford University Press, Oxford
Petanidou T, Van Laere A, Ellis WN, Smets E (2006) What shapes amino acid and sugar composition in Mediterranean floral nectars? Oikos 115(1):155–169. https://doi.org/10.1111/j.2006.0030-1299.14487.x
Pirk CWW, Boodhoo C, Human H, Nicolson S (2010) The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutellata). Apidologie 41(1):62–72. https://doi.org/10.1051/apido/2009055
Roche J, Burke C, Meier S, Walker C (2011) Nutrition × reproduction interaction in pasture-based systems: is nutrition a factor in reproductive failure? Anim Prod Sci 51(12):1045–1066
Roulston TH, Cane JH (2000) Pollen nutritional content and digestibility for animals. Plant Syst Evol 222(1–4):187–209. https://doi.org/10.1007/bf00984102
Ruedenauer FA, Spaethe J, Leonhardt SD (2015) How to know which food is good for you: bumblebees use taste to discriminate between different concentrations of food differing in nutrient content. J Exp Biol 218(Pt 14):2233–2240. https://doi.org/10.1242/jeb.118554
Ruedenauer FA, Spaethe J, Leonhardt SD (2016) Hungry for quality—individual bumblebees forage flexibly to collect high-quality pollen. Behav Ecol Sociobiol 70(8):1209–1217. https://doi.org/10.1007/s00265-016-2129-8
Rytz R, Croset V, Benton R (2013) Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem Molec 43(9):888–897. https://doi.org/10.1016/j.ibmb.2013.02.007
Sadd BM, Barribeau SM, Bloch G, De Graaf DC, Dearden P, Elsik CG, Gadau J, Grimmelikhuijzen CJ, Hasselmann M, Lozier JD (2015) The genomes of two key bumblebee species with primitive eusocial organization. Genome Biol 16(1):1–32
Schiffman SS, Dackis C (1975) Taste of nutrients: amino acids, vitamins, and fatty acids. Percept Psychophys 17(2):140–146. https://doi.org/10.3758/bf03203878
Schiffman SS, Sennewald K, Gagnon J (1981) Comparison of taste qualities and thresholds of d- and l-amino acids. Phys Behav 27(1):51–59. https://doi.org/10.1016/0031-9384(81)90298-5
Simpson SJ, Raubenheimer D (2009) Macronutrient balance and lifespan. Aging US 1(10):875–880
Simpson SJ, Raubenheimer D (2012) The nature of nutrition: a unifying framework from animal adaptation to human obesity. Princeton University Press, Princeton
Slone J, Daniels J, Amrein H (2007) Sugar receptors in Drosophila. Curr Biol 17(20):1809–1816
Somerville D (2001) Nutritional value of bee collected pollens—a report for the rural industries research and development corporation, Report DAN 134A. A report for the Rural Industries Research and Development Corporation, May 2001
Somme L, Vanderplanck M, Michez D, Lombaerde I, Moerman R, Wathelet B, Wattiez R, Lognay G, Jacquemart A-L (2015) Pollen and nectar quality drive the major and minor floral choices of bumble bees. Apidologie 46(1):92–106. https://doi.org/10.1007/s13592-014-0307-0
Sommerlandt FMJ, Rossler W, Spaethe J (2014) Elemental and non-elemental olfactory learning using PER conditioning in the bumblebee, Bombus terrestris. Apidologie 45(1):106–115. https://doi.org/10.1007/s13592-013-0227-4
Spaethe J, Brockmann A, Halbig C, Tautz J (2007) Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften 94(9):733–739. https://doi.org/10.1007/s00114-007-0251-1
Standifer LN, McCaughey WF, Todd FE, Kemmerer AR (1960) Relative availability of various proteins to the honey bee. Ann Entomol Soc Am 53(5):618–625
Stanley RG, Linskens HF (1974) Pollen: biology biochemistry management. Springer, New York
Sutterlin AM (1975) Chemical attraction of some marine fish in their natural habitat. J Fish Res Board Can 32(6):729–738. https://doi.org/10.1139/f75-095
Vrzal EM, Allan SA, Hahn DA (2010) Amino acids in nectar enhance longevity of female Culex quinquefasciatus mosquitoes. J Insect Physiol 56(11):1659–1664. https://doi.org/10.1016/j.jinsphys.2010.06.011
Wacht S, Lunau K, Hansen K (2000) Chemosensory control of pollen ingestion in the hoverfly Eristalis tenax by labellar taste hairs. J Comp Physiol A 186(2):193–203. https://doi.org/10.1007/s003590050019
Weiner CN, Hilpert A, Werner M, Linsenmair KE, Blüthgen N (2010) Pollen amino acids and flower specialisation in solitary bees. Apidologie 41(4):476–487. https://doi.org/10.1051/apido/2009083
Wu G (2013) Functional amino acids in nutrition and health. Amino Acids 45(3):407–411. https://doi.org/10.1007/s00726-013-1500-6
Zarchin S, Dag A, Salomon M, Hendriksma HP, Shafir S (2017) Honey bees dance faster for pollen that complements colony essential fatty acid deficiency. Behav Ecol Sociobiol 71(12):172. https://doi.org/10.1007/s00265-017-2394-1
Acknowledgements
We would like to thank Martin Strube-Bloss for useful comments on the experimental setup and helpful discussions of the results. We would also like to thank two anonymous reviewers for their helpful comments on the manuscript. Funding was provided by the Deutsche Forschungsgemeinschaft (DFG project: LE 2750/5-1 to SDL and SP1380/1-1 to JS).
Author information
Authors and Affiliations
Contributions
JS, SDL, FAR, and KL conceived the experimental concept. FAR performed the experiments. FAR and SDL analyzed the data. All authors wrote the manuscript, discussed the results, commented on the paper and agreed to the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human/animal rights statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruedenauer, F.A., Leonhardt, S.D., Lunau, K. et al. Bumblebees are able to perceive amino acids via chemotactile antennal stimulation. J Comp Physiol A 205, 321–331 (2019). https://doi.org/10.1007/s00359-019-01321-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-019-01321-9