Abstract
The eusocial bumblebees exhibit pronounced size variation among workers of the same colony. Differently sized workers engage in different tasks (alloethism); large individuals are found to have a higher probability to leave the colony and search for food, whereas small workers tend to stay inside the nest and attend to nest duties. We investigated the effect of size variation on morphology and physiology of the peripheral olfactory system and the behavioral response thresholds to odors in workers of Bombus terrestris. Number and density of olfactory sensilla on the antennae correlate significantly with worker size. Consistent with these morphological changes, we found that antennal sensitivity to odors increases with body size. Antennae of large individuals show higher electroantennogram responses to a given odor concentration than those of smaller nestmates. This finding indicates that large antennae exhibit an increased capability to catch odor molecules and thus are more sensitive to odors than small antennae. We confirmed this prediction in a dual choice behavioral experiment showing that large workers indeed are able to respond correctly to much lower odor concentrations than small workers. Learning performance in these experiments did not differ between small and large bumblebees. Our results clearly show that, in the social bumblebees, variation in olfactory sensilla number due to size differences among workers strongly affects individual odor sensitivity. We speculate that superior odor sensitivity of large workers has favored size-related division of labor in bumblebee colonies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foraging efficiency in pollinating insects strongly depends on their ability to detect and discriminate between flowers of different plant species (Chittka et al. 1999). The ability to identify and discriminate floral cues is limited by the sensory capabilities of the individual insect. Obviously, quantitative differences in size and number of sensory organs (i.e., number in sensory neurons) between individuals should result in differences in sensory capabilities (Chapman 1982; Dekker et al. 2006). There is evidence that this prediction holds for the visual system in bees (Spaethe and Chittka 2003), but so far, no experimental data exist whether it also applies for the olfactory system.
Eusocial bumblebees exhibit a pronounced size variation among workers of the same colony (Plowright and Jay 1968; Garófalo 1978). Large workers can reach up to 10 times the body weight of small workers (Cumber 1949). Worker size variation is caused by differences in feeding during larval development and, in turn, determines division of labor among adult workers (Brian 1952; Pereboom et al. 2003). Larger workers are predominantly engaged in foraging and smaller workers in performing nest tasks like provisioning of larvae or cell cleaning duties (Heinrich 1979; Michener 1974).
Previous studies of the olfactory system in bumblebees revealed a close morphological and anatomical similarity to that of honeybees (Fonta and Masson 1985; Agren and Hallberg 1996). As in honeybees, the pore plate sensilla are the most abundant antennal olfactory sensilla, which in bumblebees house 13–20, most commonly 14–15, sensory neurons (Esslen and Kaissling 1976; Agren and Hallberg 1996). Pore plate sensory neurons in honeybees were shown to be sensitive to flower odors as well as species-specific pheromones (Vareschi 1971; Brockmann et al. 1998).
In this study we address the question of whether and how the naturally occurring size variation among bumblebee workers affects the number of olfactory pore plate sensilla, antennal sensitivity, and behavioral thresholds to odors.
Materials and methods
Animals and body measurements
Bumblebee workers were derived from two Bombus terrestris colonies purchased from a commercial breeder (Koppert, Netherland). For size measurements, bees were randomly collected with respect to size; in the behavioral experiments, size variation among workers was smaller as we were limited to those individuals that were foraging. All size measurements were done at 20× magnification using a TM M3Z stereomicroscope (Wild, Gais, Switzerland). We determined largest head width (from eye to eye) and flagellum length of the right antenna of each worker. Head width correlates with body weight and other morphometric measures in bumblebees (Spaethe, unpublished data; Bullock 1999). For SEM examination, the right antennae (N = 11) were cut in two halves, air-dried, and gold–palladium-coated (Balzers sputter coater SCD 005, Liechtenstein). Total pore plate number of one antenna was determined on SEM photographs (Zeiss DSM 962, Oberkochen, Germany) counting all visible pore plates on flagellomers 2–10 of both flagellum halves. Pore plate density was calculated as pore plate number per flagellum surface. Flagellum surface was estimated by means of the equation for a cylinder [h × 2 × r × π; with h = flagellum length (flagellomere 2–10) and r = half of flagellum width]. As pore plates do not cover the whole flagellar surface, the value reflects an increase of the area containing pore plates rather than an increase of distance between single pore plates. Due to a beveled attachment of two antennae onto the SEM mount, we could not determine pore plate number and density for all 11 antennae.
Electroantennogram recordings
The electroantennogram (EAG) response amplitude represents the summed dendritic potentials elicited by an odor stimulus and correlates with the overall number of sensory neurons that are sensitive to that odor (Schneider 1957; Kaissling and Thorson 1980; Kaissling 1995). We measured summed antennal sensitivity at three different odor concentrations.
For the EAG recordings, bumblebees were restrained in a metal tube. The most distal flagellomere of the stimulated antenna was removed and a saline-filled glass electrode was placed over the tip. From the contralateral antenna, all flagellomeres with olfactory sensory sensilla were removed and a saline-filled electrode was placed over the remaining pedicellus and scape serving as the reference electrode. Electrodes were connected to a combined DC-coupled amplifier and A/D converter (IDAC-4-USB Data Aquisition System, Syntech, Hilversum, The Netherlands). During recordings, charcoal-filtered humidified air was continuously blown over the antennae (1.5 l/min) and the odor stimulus was pulsed (0.53 l/min) into the continuous air stream for 200 ms using a stimulus control system (CS-01, Syntech).
We used jasmine, which contains many common flower odor components, as the odor stimulus (extract of Jasminum grandiflorum, Primavera Life, Sulzberg, Germany). The jasmine fragrance comprises benzyl acetate (ca. 60%), linalool (15%), geraniol (10%), α-terpineol (5%), cis-jasmone (3%), indole (2.5%), methyl anthranilate (1.5%), methyl jasmonate (<1%), and more than 15 other components in traces (Van der Gen 1972). Filter papers (5 mm diameter) were loaded with 5 μl of three different jasmine/ethanol dilutions containing 0.5, 0.1, and 0.01 μl, respectively, of the pure jasmine extract. Prior to stimulation, the solvent was allowed to evaporate for 10 min. Each antenna was tested at all odor concentrations, and stimuli were presented in a random order. Each concentration was tested four to five times, with a minimum of 20 s between stimulations. Maximum EAG-response amplitude was automatically determined by the software (EAG2000, Syntech, see dash–dotted line in Fig. 2a) and could be manually corrected if necessary. For each concentration, the mean maximum EAG-response was calculated and plotted against flagellum length. We measured the length of the removed flagellum using a SEM photograph (see above).
Behavioral experiment
A bumblebee colony was connected to a foraging arena (1.10 × 0.70 m, 0.3 m height). A Y-maze made up of Plexiglas tubes and two clear plastic vials (10 × 5 cm) was accessible from the flight cage (Fig. 3a). Small bottle caps (1 cm diameter) filled with 1 M sucrose solution (rewarded arm) or plain water (control arm) were used as feeders. We covered the bottom of each container with filter paper to exclude possible scent marking. At the back of each container, a filter paper (1 cm diameter) was placed and loaded with either 10 μl solution of different jasmine extract concentrations (1:1,000, 1:10,000, 1:50,000; 1:75,000; 1:100,000, 1:250,000; diluted in ethanol) in the arm where sucrose solution was presented, or with 10 μl solvent in the control arm. A pump circulated the air out of the Y-maze to prevent saturation (Fig. 3a).
We trained individual bumblebees to enter the Y-maze and the arm where the jasmine stimulus was presented. At the beginning, we allowed all foragers of the colony to freely explore the arena. After a couple of hours, some of the foragers discovered the sucrose containers in the Y-maze by chance. Those foragers were individually marked with numbered plastic tags (“Opalithplättchen”). During testing, only one bee at a time was allowed to forage in the arena. After the bee fed on the sucrose solution and returned to the colony, we removed both containers, cleaned each with 80% ethanol, exchanged the paper covering the bottom of the containers, replaced both filter papers (one with jasmine and one with solvent), refilled both screw caps, and reattached the containers to the Y-maze in a random order. During a pretraining phase, each bee was tested with a jasmine concentration of 1:1,000 until it reached a level of at least 80% of correct choices. A choice was counted as correct when the bee entered the container where the odor stimulus was presented. Only the first choice of each foraging bout was used for data analysis. A bee was then tested 30 times (= 30 foraging bouts) at each odor concentration in a descending order beginning with the highest concentration (1:1,000). The experiment was terminated when an odor concentration was reached at which the bee chose both arms at random. For each bee (N = 14) we calculated the percentage of correct choices as a function of odor concentration. A regression analysis was used to determine the threshold concentration, defined as the odor concentration at which the bee was still able to achieve 65% correct choices. Individual threshold concentrations were plotted against flagellum length.
Data analysis
Correlations between morphological characters (antennal size, pore plate number, and pore plate density) and worker size as well as correlations between EAG response or behavioral threshold and flagellum length were tested by a nonparametric test for association (Spearman’s rank correlation). To test whether odor concentration affects mean EAG response, we used Friedman’s nonparametric repeated measures comparisons. A Wilcoxon signed-rank test was applied to test for specific differences in EAG response between any two of the three odor concentration groups. Alpha was set at 0.05. When we performed multiple significance tests, we adjusted α-values by using the sequential Bonferroni correction to control for type 1 error (Rice 1989).
Results
Peripheral olfactory system
Size variation of workers within a single B. terrestris colony was found to be substantial; head widths of workers used for morphometric measurements ranged from 2.7 to 4.3 mm (Fig. 1). Pore plate number ranged from 708 to 2,594, density from 2,377 to 3,168 pp/mm2. We found a significant correlation between head width and flagellum length (N = 11, r s = 0.88, p < 0.001), pore plate number (N = 10, r s = 0.93, p < 0.001), and pore plate density (N = 9, r s = 0.86, p < 0.01; all correlations significant after sequential Bonferroni correction) (Fig. 1a–c). In our study, the largest workers exhibited large antennae with about 3.5 times more pore plates compared to their smallest nestmates.
Size-related antennal sensitivity measured by EAGs
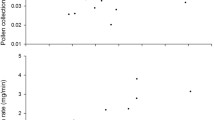
The EAG responses for jasmine exhibited the typical properties of an EAG response (see Fig. 2a; Schneider 1957; Nishino and Kuwabara 1983; White 1991). For each concentration we found a significant correlation between EAG responses and flagellum length (and, thus, pore plate number); the higher flagellum length, the larger the EAG responses (Fig. 2b–d).
a Typical EAG responses with a sharp negative deflection followed by a slower recovery phase for three different jasmine concentrations and the control stimulus. During the experiment, the stimuli were presented in a randomized order. Maximum EAG-response amplitude was automatically determined by the software (EAG2000, Syntech) and is shown as dash–dotted lines. Short bold horizontal bar, 200-ms odor stimulus pulse; vertical units = 1 mV. b–d EAG responses in millivolts as a function of flagellum length. (b) Jasmin oil 1:10 dilution (y = – 0.26+0.00048x), (c) Jasmin oil 1:50 dilution (y = – 0.53+0.00049x), (d) Jasmin oil 1:500 dilution (y = –0.78+0.00051x), (e) Control stimulus
As the EAG amplitude might also be affected by nonspecific changes in the electrical conductance due to size, we additionally tested whether the response to the control stimulus correlates with antennal size. We found no size effect on EAG response to the control stimulus (see results, Fig. 2e). Therefore, variation of EAG responses among different antennae at a given odor concentration was affected by sensillum length, and thus most likely by sensilla number, but not by size-related properties of the antenna itself.
Odor concentration significantly affected mean EAG response (Friedman’s test; Chi2 = 44.9, p < 0.001). We found a significant increase of the EAG response with increasing stimulus concentration (1:500 vs 1:50: Z = −3,8, p < 0.001; 1:50 vs 1:10: Z = −4.3, p < 0.001).
To summarize, the EAG recordings show that flagellum length, and thus, most likely, pore plate number, affects the olfactory signal input in bumblebee workers. Larger antennae with a higher number of pore plate sensilla generate a stronger sensory response than smaller antennae.
Behavioral thresholds
We tested 14 individual bumblebees with head widths between 3.6 and 4.3 mm. Behavioral threshold concentrations ranged from 7.6 × 10−4 to 2.1 × 10−5 μl jasmine per filter paper. We found a significantly negative correlation between flagellum length (i.e., bumblebee size) and behavioral threshold concentration (Fig. 3b). The largest bees in our experiment were able to detect a 36-times lower jasmine concentration compared to the smallest ones. For the training phase, when each bee was trained to associate a sucrose reward with the jasmine stimulus until she reached a level of 80% correct choices, we found no size-related differences in learning performance (data not shown).
a Behavioral set-up of the dual choice experiment. The Y maze was connected to a flight arena (for a detail description, see “Materials and methods” section). b Behavioral response thresholds of individual bumblebee worker (black dots) plotted against flagellum length. The behavioral response threshold was defined as the calculated odor concentration at which the individual bees would perform 65% correct choices (see “Materials and methods” section for details)
Discussion
Our study demonstrates that, in bumblebee workers, (1) number and density of olfactory pore plate sensilla increase with size and (2) these changes in the peripheral olfactory system give rise to an increased summed antennal sensitivity and correlate with a decrease in behavioral threshold to odors. Consequently, within a bumblebee colony, the larger workers have a more sensitive olfactory system and a lower behavioral threshold to odors than their smaller nest mates.
The summed antennal olfactory sensitivity depends on two factors: the sensitivity of the single sensory neuron and the total number of sensory neurons sensitive to a given odor. Multiplication of olfactory sensory sensilla (and, thus, sensory neurons) increases the probability that a certain number of neurons per antenna catch and bind an odor molecule from the environment and become excited (Ochieng and Hansson 1999; Chapman 1998). Thus, if stimulated with a specific odor above threshold, an increase in sensilla number results in a higher total activity within the respective population of similarly tuned sensory neurons (Dekker et al. 2006). Consistent with this prediction, we found that, for all three odor concentrations tested, a given concentration elicited higher EAG amplitudes (i.e., larger total dendritic potentials) in antennae with higher numbers of pore plate sensilla. This finding shows that the increase in pore plate number indeed implicates a higher sensitivity of larger antennae to odors. In addition, as olfactory systems are organized in such a way that the majority of similarly tuned sensory neurons converge onto a few second-order interneurons (Rospars 1988; Hildebrand and Shepherd 1997), an increase of neural activity among the specific population of sensory neurons directly results in a higher signal input to the next level of signal processing (Dekker et al. 2006).
Finally, the behavioral choice experiments demonstrate that the differences in the olfactory sensory system between large and small bumblebees limit the ability to respond to low ambient odor concentrations. However, the animal’s behavioral threshold is not only determined by the peripheral sensory system, and the sensory system in turn is not the only part of the nervous system that is controlled by size. Thus, it is highly likely that there are additional modifications, potentially at all levels of the neural pathway controlling olfactory behavior, that contribute to the differences in behavioral response threshold between differently sized bumblebee workers. For example, a recent study on the mushroom bodies, a higher olfactory and multimodal integration center in insect brains, revealed that volumes of these brain neuropils correlate with size in bumblebees (Mares et al. 2005). This finding suggests that not only sensitivity to odors but also more complex odor and multimodal processing capabilities might be affected by size (Heisenberg 2003; Farris and Roberts 2005). However, it is interesting to note that we did not find differences in the acquisition of operant odor conditioning in our experiments.
Selection for size-related division of labor in bumblebees has previously been ascribed to differences in efficiency between small and large workers, due to variation in physiological properties such as thermoregulation and flight speed that enable larger bumblebee workers to be active at lower temperatures and to fly faster (Heinrich 1979). Our results on the olfactory system, together with those for the visual system by Spaethe and Chittka (2003), suggest that differences in the sensory systems may also contribute to size-related variation in efficiency, and hence to the evolution of size-related division of labor. In this case, behavioral thresholds are lower for large than for small workers, suggesting that the larger workers could be more efficient at components of foraging such as detection of flower patches and choices among or among flowers (Goulson et al. 2002; Spaethe and Weidenmüller 2002).
Variation in response thresholds among workers is hypothesized to be a proximate mechanism that results in division of labor among workers (Beshers et al. 1999; Bonabeau and Theraulaz 1999; reviewed in Beshers and Fewell 2001). Response thresholds are thought of as being located in the central nervous system (Beshers et al. 1999). Our results suggest that peripheral effects may also contribute to variation in responsiveness among individual workers, and hence to division of labor.
As size effects are not limited to the peripheral sensory system but also affect central brain neuron populations, bumblebee workers are a promising model to study developmental mechanisms and functional consequences of size related brain plasticity in insects in general and in social insects in particular.
References
Agren L, Hallberg E (1996) Flagellar sensilla of bumble bee males (Hymenoptera, Apidae, Bombus). Apidologie 27:433–444
Beshers SN, Fewell JH (2001) Models of division of labor in social insects. Annu Rev Entomol 46:413–440
Beshers SN, Robinson GE, Mittenthal JE (1999) Response thresholds and division of labor in insect colonies. In: Detrain C, Deneubourg JL, Pasteels JM, (eds) Information processing in social insects. Birkhäuser, Basel, pp 115–139
Brian AD (1952) Division of labor and foraging in Bombus agrorum Fabricius. J Anim Ecol 21:223–240
Bonabeau E, Theraulaz G (1999) Role and variability of response thresholds in the regulation of division of labor in insect colonies. In: Detrain C, Deneubourg JL, Pasteels JM, (eds) Information processing in social insects. Birkhäuser, Basel, pp 141–163
Brockmann A, Brückner D, Crewe RM (1998) The EAG response spectra of workers and drones to queen honeybee mandibular gland components: the evolution of a social signal. Naturwissenschaften 85:283–285
Bullock SH (1999) Relationships among body size, wing size and mass in bees from a tropical dry forest in México. J Kans Entomol Soc 72:426–439
Chapman RF (1982) Chemoreception: the significance of receptor numbers. In: Berridge MJ, Treherne JE, Wigglesworth VB (eds) Advances in insect physiology, vol. 16. Academic Press, New York, pp 247–356
Chapman RF (1998) The insects. Cambridge University Press, Cambridge
Chittka L, Thomson J, Waser NM (1999) Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 8:361–377
Cumber RA (1949) The biology of humble-bees, with special reference to the production of the worker caste. Trans R Entomol Soc Lond 100:1–45
Dekker T, Ibba I, Siju KP, Stensmeyr MC, Hansson BS (2006) Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr Biol 16:101–109
Esslen J, Kaissling KE (1976) Zahl und Verteiling antennaler Sensillen bei der Honigbiene (Apis mellifera L.). Zoomorphol 83:227–251
Farris SM, Roberts NS (2005) Coevolution of generalists feeding ecologies and gyrencephalic mushroom bodies in insects. Proc Natl Acad Sci USA 102:17394–17399
Fonta C, Masson C (1985) Organisation neuroanatomique de la voie afférente antennaire chez les Bourdons mâles et femelles (Bombus sp.). C R Acad Sci Paris 3:437–442
Garófalo C (1978) Bionomics of Bombus (fervidobombus) morio: 2. body size and length of life of workers. J Apic Res 17:130–136
Goulson D, Peat J, Stout JC, Tucker J, Darvill B, Derwent LC, Hughes WOH (2002) Can alloethism in workers of the bumblebee Bombus terrestris be explained in terms of foraging efficiency? Anim Behav 64:123–130
Heinrich B (1979) Bumblebee economics. Havard University Press, Cambridge
Heisenberg M (2003) Mushroom body memoir: from maps to models. Nat Rev Neurosci 4:266–275
Hildebrand JG, Shepherd GM (1997) Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci 20:595–631
Kaissling K-E (1995) Single unit and electroantennogram recordings in insect olfactory organs. In: Spielman AI, Brand JG (eds) Experimental cell biology of taste and olfaction. CRC Press, Boca Raton, pp 361–377
Kaissling K-E, Thorson J (1980) Insect olfactory sensilla: structural, chemical and electrical aspects of the functional organization. In: Sattelle DB, Hall LM, Hildebrand JG (eds) Receptors for neurotransmitters, hormones and pheromones in insects. Elsevier, Amsterdam, pp 261–282
Mares S, Ash L, Gronenberg W (2005) Brain allometry in bumblebee and honey bee workers. Brain Behav Evol 66:50–61
Michener CD (1974) The social behavior of the bees. Harvard University Press, Cambridge
Nishino C, Kuwabara K (1983) Threshold dose values for sex pheromones of the American cockroach in electroantennogram and behavioural responses. Comp Biochem Physiol 74:909–914
Ochieng SA, Hansson BS (1999) Responses of olfactory receptor neurons to behaviourally important odours in gregarious and solitary desert locust, Schistocerca gregaria. Physiol Entomol 24:28–36
Pereboom JJM, Velthuis HHM, Duchateau MJ, et al. (2003) The organisation of larval feeding in bumblebees (Hymenoptera, Apidae) and its significance to caste differentiation. Insectes Soc 50:127–133
Plowright RC, Jay SC (1968) Caste differentiation in bumblebees (Bombus latr.: Hym.) I. The determination of female size. Insectes Soc 2:171–192
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rospars JP (1988) Structure and development of the insect antennodeutocerebral system. Int J Insect Morphol Embryol 17:243–294
Schneider D (1957) Eletrophysiologische Untersuchungen von Chemo- und Mechanorezeptoren der Antenne des Seidenspinners Bombyx mori. Z Vergl Physiol 40:8–41
Spaethe J, Chittka L (2003) Interindividual variation of eye optics and single object resolution in bumblebees. J Exp Biol 206:3447–3453
Spaethe J, Weidenmüller A (2002) Size variation and foraging rate in bumblebees (Bombus terrestris). Insectes Soc 49:142–146
Van der Gen A (1972) Corps olfactifs a l’odeau al jasmin. Parfum Cosmet Savon 2:356–370
Vander Meer RK, Breed MD, Espelie KE, Winston ML (1998) Pheromone communication in social insects. Westview Press, Oxford
Vareschi E (1971) Duftunterscheidung bei der Honigbiene - Einzelzell-Ableitungen und Verhaltensreaktionen. Z Vergl Physiol 75:142–173
White PR (1991) The electroantennogram response: effects of varying sensillum numbers and recording electrode position in a clubbed antenna. J Insect Physiol 37:145–152
Acknowledgment
We thank R. F. Chapman, J. G. Hildebrand, and J. Schachtner for their valuable comments on an earlier version of the manuscript and C. Lutz and S. Beshers for comments on the present version. J.S. and A.B. were supported by the German Research Foundation DFG (SFB554 and Graduiertenkolleg 200). The experiments in this study comply with the current laws of Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Spaethe and Brockmann both contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Spaethe, J., Brockmann, A., Halbig, C. et al. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften 94, 733–739 (2007). https://doi.org/10.1007/s00114-007-0251-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-007-0251-1