Abstract
The activity of four types of sound-sensitive descending brain neurons in the cricket Gryllus bimaculatus was recorded intracellularly while animals were standing or walking on an open-loop trackball system. In a neuron with a contralaterally descending axon, the male calling song elicited responses that copied the pulse pattern of the song during standing and walking. The accuracy of pulse copying increased during walking. Neurons with ipsilaterally descending axons responded weakly to sound only during standing. The responses were mainly to the first pulse of each chirp, whereas the complete pulse pattern of a chirp was not copied. During walking the auditory responses were suppressed in these neurons. The spiking activity of all four neuron types was significantly correlated to forward walking velocity, indicating their relevance for walking. Additionally, injection of depolarizing current elicited walking and/or steering in three of four neuron types described. In none of the neurons was the spiking activity both sufficient and necessary to elicit and maintain walking behaviour. Some neurons showed arborisations in the lateral accessory lobes, pointing to the relevance of this brain region for cricket audition and descending motor control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In animals, responses to external sensory stimuli often depend on the behavioural context during which a stimulus is presented. For example, air puffs to the cerci elicit escape runs in walking cockroaches, but initiate flying if cockroaches have lost tarsal contact with the ground (Ritzmann et al. 1980). The ultrasound avoidance reaction only occurs in flying, but not in walking crickets (Nolen and Hoy 1984; ter Hofstede et al. 2009).
The specific relationship between walking and neural processing of external stimuli was studied in several identified cricket interneurons. Staudacher and Schildberger (1998) and Staudacher (2001) describe that in Gryllus bimaculatus, the majority of descending brain neurons responded to various stimuli (such as moving, grating, artificial songs with ultrasound or conspecific sound frequency, air puffs to the cerci) only during walking. Such walking-mediated gating of sensory responses implies that significant information about the properties of sensory processing in higher order interneurons can only be gained from experiments in behaviourally relevant paradigms.
In cricket acoustic communication, the male stridulates while the female tries to locate the sound source performing phonotaxis (Popov and Shuvalov 1977; Stabel et al. 1989; Ulagarai and Walker 1973; Weber and Thorson 1989). On hearing the acoustic communication signal, the female faces two tasks: recognition of the signal and orientation towards it (Stabel et al. 1989; Wendler 1990). Processing of the stereotyped temporal pattern of sound pulses plays a crucial role in recognition of the conspecific song. Neural processing in the brain is vital for understanding phonotaxis since in a flying cricket preparation decapitation eliminated steering movements in response to sound (Pollack and Hoy 1981). In a study by Schildberger et al. (1988), hyperpolarization of a neuron AN1 that ascends from the prothoracic ganglion into the brain, while sound was presented to the ear that excites this neuron, caused the animals to reverse the direction of walking. Thus the paired ascending neurons AN1 (from here on referred to as TH1–AC1) may provide inputs to a central comparator that determines turning tendency in phonotaxis. However, TH1–AC1 has no temporal filtering properties; therefore, we may assume that the recognition of the signal takes place at a higher level of processing in the cricket’s brain (Kostarakos and Hedwig 2012).
While sensory responses of some higher order interneurons in crickets may be gated by walking, other neurons respond to auditory stimuli during both standing and walking. The responses, however, differ to a certain extent (usually additional action potentials occur during walking) between these two behavioural situations (Böhm and Schildberger 1992; Staudacher 2001). In a local interneuron, ON1 of G. bimaculatus for example, walking disrupted the representation of the conspecific auditory signal’s temporal pattern (Schildberger et al. 1988). Further, responses to artificial calling song of ascending, local and descending brain interneurons of G. bimaculatus differed between standing and walking in their mean spike rate, peak instantaneous spike rate and the song pattern coding properties (Zorović and Hedwig 2011).
In the present study, along with assessing the effect of walking on the responsiveness to conspecific songs of several descending auditory interneurons, we examined the impact of walking on the response intensity and the accuracy of the song pattern representation. Using a fast trackball system (Hedwig and Poulet 2005), we also aimed to determine the motor effects of descending interneurons by depolarizing them with current injection and examining whether activation of a single neuron was sufficient to trigger a behavioural response such as walking or steering.
Materials and methods
Animals
Experiments were performed on adult female crickets (G. bimaculatus de Geer) from our colony at the Department of Zoology, University of Cambridge, UK, maintained on a 12:12 h L:D photo cycle. Female larvae were isolated from the colony as last instars and raised individually in a separate room to ensure physical and acoustic isolation from males.
Preparation
Only a short description of the experimental procedures is given here, the experimental set-up and intracellular recording technique have been described in detail before (Zorović and Hedwig 2011). For dissection the animals were positioned upright on a block of Plasticine™ and all legs were restrained by metal clamps. After exposing the ventral surface of the brain, the leg restraints were removed and the cricket was placed on a trackball. Care was taken that all the legs were intact and moved freely. A small stainless-steel platform was placed underneath the brain for support and a metal ring was lowered onto the brain to stabilize it. The platform also served as a reference electrode for intracellular recordings.
Acoustic stimulation
We used a standard sound pattern that mimicked the natural calling song of the males. The pattern consisted of chirps that were repeated every 500 ms. Each chirp contained six sound pulses, each 21 ms long, with 2 ms rise and fall times, the intervals between pulses were also 21 ms long, yielding a 42 ms pulse period. The carrier frequency of acoustic stimuli was 4.8 kHz and intensity was set to 75 dB sound pressure level (SPL) RMS relative to 20 μPa.
Intracellular recording
Intracellular recordings of brain neurons were performed with sharp microelectrodes; for details see Zorović and Hedwig (2011). In female crickets, we recorded the responses of descending brain neurons to the male calling song during standing and walking, and also their activity in the absence of the auditory stimuli. Neurons were stained using iontophoretic injection of Lucifer yellow to reveal their morphology in the brain. After dissection, the brain was fixed in 4 % formaldehyde, dehydrated in an ethanol series and cleared in methyl-salicylate. The neurons were photographed and drawn as described in Zorović and Hedwig (2011).
Data sampling and analysis
Neuronal activity was sampled online to a PC using Spike 2 software of Cambridge Electronic Design (CED) and a CED 1401 plus data acquisition interface set to a sampling rate of 10 kHz/channel. Data evaluation was carried out with Spike 2 and NeuroLab software (Hedwig and Knepper 1992; Knepper and Hedwig 1997).
The responses of interneurons to auditory stimuli during standing and walking are presented as peri-stimulus time histogram (PSTH; bin size 5 ms) and raster plots. Where numbers of spikes per chirp are given, n refers to the number of chirps evaluated; in other cases n is the number of measurements of a certain parameter (e.g. response latency), while N refers to the number of animals. In case of spontaneous activity (i.e. spiking of neurons during standing and in the absence of the sound or other external stimuli), we subtracted the mean number of spikes in the 231 ms time window before the stimulus from the mean number of spikes elicited during the stimulus (taking into account the response latency) to get the number of spikes per stimulus.
The degree of synchronization of action potentials (APs) with the sound pulses was analysed for interneuron B-DC1(5) following the procedure described by Schildberger (1984); for additional details see Zorović and Hedwig (2011). After latency correction, the synchronization coefficient (SC) was determined; all spikes occurring during a pulse (SPu) and those occurring during a pause between pulses (SPa) were counted, and the ratio (SPu − SPa)/(SPu + SPa) was calculated.
While a cricket was walking on top of the trackball, an optical sensor recorded its left–right and forward–backward movement components. From the forward–backward component we calculated the cricket’s forward velocity and from the left–right component its lateral velocity. For details on evaluation of trackball data see Hedwig and Poulet (2005). The relation between walking and the spike rate of a neuron was analysed by calculating Pearson’s correlation coefficient between the forward velocity and the spike rate for consecutive 300 ms intervals over a 6–10 s period. The spike rate shown in recordings was averaged over 200–300 ms intervals.
Terminology
We followed the terminology outlined in our previous paper on cricket brain neurons (Zorović and Hedwig 2011). For instance, “B” stands for brain, “D” for a descending axon, and the following letters “I” or “C” signify an ipsi- or contralateral position of the axon with regard to the cell body. The addition of a bracketed number indicates the soma cluster in the brain (after Staudacher 1998). Terms ipsilateral and contralateral in figures, when referring to position of the sound source or direction of walking, are used following the same rule—they signify the position or direction with regard to the cell body.
The phrase spontaneous activity is used in the text to describe the neuronal activity in the absence of sound or other external stimuli. Similarly, spontaneous walking refers to walking in absence of sound or other external stimuli.
Results
In G. bimaculatus, the somata of approximately 200 bilateral pairs of descending neurons are located in different clusters of the brain (Staudacher 1998). The morphology and physiological properties of four of these neurons belonging to four different clusters of perikarya are presented here. With the exception of B-DI1(1), their morphology has been described previously (B-DI1(2) by Staudacher (1998); B-DI1(3) by Brodfuehrer and Hoy (1990) and B-DC1(5) by Staudacher (1998) and Zorović and Hedwig (2011)).
We recorded the responses of these neurons elicited by chirps of the male calling song in female crickets during standing and walking and found that all neurons responded to auditory stimuli either during standing or walking or both. We also recorded neuronal activity during standing and walking in the absence of sound and found that the spiking activity of all four neurons was in some way correlated to walking. Single-cell stimulation using intracellular current injection elicited walking and/or steering in case of all interneurons except B-DI1(3). Our findings indicate that these descending neurons may contribute to the control of phonotactic steering.
B-DI1(1)
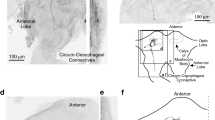
The B-DI1(1) neuron belongs to soma cluster i1 in the dorsal protocerebrum (Staudacher 1998). Its soma is located at the level of posterior margins of the optic nerves, close to the midline (Fig. 1a). B-DI1(1) shares a common feature with the other cells of this cluster by branching towards the dorsal lateral protocerebrum. Its morphology, however, differs substantially from previously identified cells of this cluster (Staudacher 1998; Staudacher 2001), which are all limited to the ipsilateral side, while in B-DI1(1) two branches from the dendritic tree extend across the midline. The dendrites occupy the area in the median posterior protocerebrum (the LAL region) and partly in the deutocerebrum. One prominent branch projects towards the optic nerve and one towards the contralateral side. A smaller part of the dendritic tree crosses the midline anteriorly. The descending axon projects into the ipsilateral circumoesophageal connective. Beaded collaterals suggest axonal arborisations and output synapses in the tritocerebrum. Data are derived from two B-DI1(1) neurons.
Structure and activity of interneuron B-DI1(1). a Structure of B-DI1(1) in the brain. b Responses of B-DI1(1) to chirps during standing and walking presented as PST histograms and raster plots. c Activity of B-DI1(1) upon sound presentation during standing and walking. During walking, the spike rate increased up to 100 Hz. Lateral and forward steering velocity. d Repeated activation of the interneuron by depolarizing current injections of 2.5 nA pulses reliably elicited walking towards the ipsilateral side. Depolarization initially elicited a strong turn to the ipsilateral side, which yielded a negative forward velocity component (arrows). Walking either stopped during a period of depolarization or outlasted it
During standing, presentation of calling song elicited responses that reliably represented the first pulse of the chirp (Fig. 1b, left). The number of spikes per chirp was 2.3 ± 1.1 (N = 2, n = 12). The response latency was 39.7 ± 12.8 ms (N = 2, n = 10). During walking, the membrane potential increased by 5–10 mV (Fig. 1c) and the spike activity of the neuron was between 40 and 100 AP/s. However, no acoustic response was apparent in the PSTH and raster plot (Fig. 1b, right). There was a highly significant correlation between spike rate and the forward–backward component of the walking velocity which oscillated between −1 and 4 cm/s (N = 1, r = 0.79; P < 0.001).
Injections of a 2.5 nA depolarizing current lasting from 3 to 6.5 s elicited strong turns to the ipsilateral side with a 180 ± 82 ms delay (n = 10), which was followed by walking mostly to the ipsilateral side (Fig. 1d). The initial negative values of forward velocity in this case were not a result of the cricket walking backwards, but the consequence of the strong rotation of the insect to the ipsilateral side without generating any forward movement (Fig. 1d, arrows). Motor responses occurred always at the beginning of current injection; walking then either stopped temporarily during the current injection or outlasted it by up to 1.6 s.
B-DI1(2)
B-DI1(2) belongs to soma cluster i2 of the dorsal protocerebrum (Staudacher 1998). It has relatively dense arborisations in the dorsomedial protocerebrum and one branch with extensive arborisations in the ventral posterior deutocerebrum (Fig. 2a). The neuron belongs to the group of descending antennal interneurons, responding to mechanical antennal stimulation and has recently been characterised by Schöneich et al. (2011) as DBNi2-1. We recorded from three and stained two B-DI1(2) cells.
Structure and activity of interneuron B-DI1(2). a Structure of B-DI1(2) in the brain. b Responses of B-DI1(2) to chirps during standing and walking presented as PST histograms and raster plots. The arrows indicate two spike doublets in the first sweep during walking. Note that spike doublets occur also in the consecutive sweeps. c Activity of B-DI1(2) upon presentation of sound from the ipsilateral side during standing and walking. During bouts of walking the membrane potential increased by up to 6 mV. Neuron activity was correlated to walking, but not to sound stimuli. d Repeated activation of the interneuron by depolarizing current injections of 4 nA pulses reliably elicited walking. Interneuron was depolarized by three consecutive pulses of 2–3 s duration. Walking either stopped during periods of depolarization or outlasted them
During standing, B-DI1(2) weakly responded to chirps. The number of spikes per chirp was 1.7 ± 1.3 (N = 2, n = 9) and as the neuron represented most often only the second pulse of the chirp (Fig. 2b, left), the response latency was 77 ± 28.1 ms (N = 2, n = 6). During walking, the response to calling song was not obvious from the histogram; however, mini-bursts of two action potentials following in a short interval appeared that were not present outside the acoustic stimulus (Fig. 2b, right, arrows). These spike doublets may indicate an auditory response to species-specific song during walking.
The spike rate of B-DI1(2) neurons during standing was around 15 Hz. When crickets started walking, the firing rate increased to 30–50 Hz (Fig. 2c). The correlation between the firing rate and the animal’s forward velocity, which reached up to 4 cm/s during walking proved to be highly significant (N = 1, r = 0.79, P < 0.001).
Injections of a 4 nA depolarizing current lasting 2–3 s increased the neuron’s discharge rate to ca. 125 Hz and elicited walking towards the contralateral side with a delay of 405 ± 80 ms (n = 10); (Fig. 2d). Crickets walked throughout the duration of increased neural activity; walking occasionally outlasted the current injection by a maximum of 600 ms.
B-DI1(3)
The soma of B-DI1(3) is positioned in the medial pars intercerebralis. The sparse dendritic arborisations extend laterally in the dorsal parts of the nonglomerular proto- and deutocerebrum and do not enter the contralateral half of the brain or the optic lobes. The axon projects ipsilaterally into the circumoesophageal connective (Fig. 3a). Data are derived from two B-DI1 (3) neurons.
Structure and activity of interneuron B-DI1(3). a Structure of B-DI1(3) in the brain. b Responses of B-DI1(3) to chirps during standing and walking presented as PST histograms and raster plots. c Activity of B-DI1(3) upon presentation of chirps from ipsi- and contralateral sides during standing and walking. During walking there was no response to sound, the spike rate, however, increased to 50 Hz and was correlated with forward velocity
During standing there was some background neural activity, which could contribute to any auditory responses. Auditory responses to calling song stimulation during standing occurred only at the onset of the chirp (Fig. 3b, left). The PSTH of responses to 15 chirps shows a marked increase in the number of spikes in the time window between 30 and 75 ms after the start of the chirp. The number of spikes per chirp was 3.9 ± 1.7 (N = 3, n = 15) and the average response latency was 51.3 ± 9 ms (N = 3, n = 15). During walking the sound stimuli caused no changes in the neuron’s ongoing spiking activity (Fig. 3b, right). The spike rate increased from 15 Hz during rest to 50 Hz during walking irrespective of the presentation of sound (Fig. 3c). While the cricket was walking, the spike rate oscillated between 15 and 50 Hz while the forward–backward walking velocity component was between 1.9 and –2.6 cm/s. This correlation between the firing rate and forward velocity was highly significant (N = 1, r = 0.72, P < 0.001). Injections of 5 nA depolarizing current had no effect on cricket’s walking behaviour (data not shown).
B-DC1(5)
The cell body of B-DC1(5) is located in the ventro-medial protocerebrum near the border to the deutocerebrum. Main morphological characteristics of these neurons of cluster 5 (Staudacher 1998) are extensive bilateral arborisations in the lateral accessory lobes (LALs). Dendritic ipsilateral branches run in the medial to dorsal protocerebrum near the border to the deutocerebrum whereas the contralateral axonal branches of these neurons are restricted to the dorsal parts in the proto- and deutocerebrum. The axon descends from the brain contralaterally in the medial third of the connective. We recorded from seven and stained five B-DC1(5) cells.
During standing B-DC1(5) responded to chirps, the mean number of spikes was 7 ± 2.2 (N = 2, n = 12). Note that in this neuron, we show the responses elicited by the sound from the contralateral side, because the sound from the ipsilateral side elicited weaker responses. The neurons copied all six pulses (SC = 0.41) (Fig. 4b, left) with the response to the first pulse being slightly stronger than the following. The response latency was 47.1 ± 8.7 ms (N = 2, n = 2). While the number of action potentials per chirp during walking was similar [6 ± 1.3 (N = 2, n = 12)] as during standing, the coding of the pulses improved (SC = 0.63) as less spikes occurred in the pulse intervals (Fig. 4b, right).
Structure and activity of interneuron B-DC1(5). a Structure of B-DC1(5) in the brain. b Responses of B-DI1(5) to chirps during standing and walking presented as PST histograms and raster plots. Pulse copying was better during walking [synchronization coefficient (SC) = 0.63] than during standing (SC = 0.41). c Dendritic recording during phonotactic steering; recording of lateral and forward steering velocity. d Activation of the interneuron by depolarizing current injection of 4 nA pulses elicited walking towards the contralateral side
Figure 4c shows activity of B-DC1(5) that was recorded while the female reliably performed phonotaxis. The animal was always steering to the side of the active speaker. When the sound stimulus stopped, the cricket stopped steering and at the onset of the chirps from the opposite speaker started steering to the other side. During the presentation of a train of 12 chirps the steering velocity usually increased until the 4th or 5th chirp and then started slowly decreasing again. Analysis of the lateral steering velocity, which oscillated between +1 and −1 cm/s, revealed a highly significant correlation between the spike rate and the contralateral steering velocity (N = 1, r = 0.34, P < 0.001), whereas there was no correlation between the spike rate and the ipsilateral steering velocity (N = 1, r = 0.028, P > 0.1). There was also a significant positive correlation between forward velocity and the firing rate of the neuron (N = 1, r = 0.59, P < 0.002).
Injection of a 4 nA depolarizing current lasting between 2.5 and 4 s increased the neuron’s discharge rate to 300 Hz and elicited walking and steering to the contralateral side with 230 ± 70 ms latency (Fig. 4d). The lateral steering velocity was higher than in normal walking, reaching 3 cm/s. Walking either stopped during depolarization or outlasted it. In two crickets, we were able to terminate walking by hyperpolarization of B-DC1(5). Such cessation of walking by injection of negative current (3, 4 and 5 nA) was repeated twice in one and eight times in the other cricket.
Discussion
The experimental setup used was developed to allow behaviourally relevant conditions for investigating the auditory responses of descending brain neurons during standing and walking in crickets and the possibility to observe potential motor effects of their activation. We investigated the responsiveness to conspecific calling song of four interneurons belonging to four different clusters of cell bodies. By applying a depolarizing current, we successfully elicited walking in three of the four recorded interneurons.
Characteristic arborisation patterns
Interneurons B-DI1(1) and B-DC1(5) share characteristic arborisation areas in the lateral accessory lobes (LALs). This morphological trait of cricket auditory interneurons was already observed in our previous study, describing the flow of auditory information from the ascending via local towards descending brain neurons (Zorović and Hedwig 2011). The local and descending neurons, which may be putative elements of the phonotaxis sensory-to-motor pathway, displayed dense arborisations in the bilateral LAL regions. The importance for insect motor activity of the LALs in the inferior protocerebrum has been described in connection to locust flight control (Homberg 1994), visual control of flight orientation in dragonflies (Olberg 1986) and control of pheromone-guided flight in moths (Kanzaki and Shibuya 1986; Kanzaki et al. 1991a, b). None of the descending neurons involved in walking, however, showed dendrites in the central body (CB). So far there is no evidence that the CB is involved in the control of cricket phonotaxis, although numerous studies imply its strong connection to walking and turning activity in other insect taxa (Homberg 1987; Strauss and Heisenberg 1993; Popov et al. 2004; Ridgel et al. 2007). Two other neurons with a similar morphology, DBIN7 and DBNc5-5, were identified by Brodfuehrer and Hoy (1990) and Staudacher (2001), respectively. Dendritic trees of both neurons are nearly identical to B-DC1(5), but the contralateral axonal branches are lacking.
The major dendrite of B-DI1(2) lies in the antennal mechanosensory neuropil of the lateral protocerebrum. This arborisation pattern points to its primary role in the antennal mechanosensory pathway; B-DI1(2) receives information on antennal stimulation and/or movements from antennal mechanosensory afferents and conveys it to the ventral nerve cord (Gebhardt and Honegger 2001; Schöneich et al. 2011).
B-DI1(3) was described first by Brodfuehrer and Hoy (1990) as an ultrasound sensitive neuron and later identified morphologically once more by retrograde labelling from cervical connectives by Staudacher (1998).
Based on the morphology and response latencies of all descending neurons characterized here, we conclude that none of them receive auditory inputs directly from ascending interneurons TH1–AC1 or TH1–AC2, but rather from local brain neurons with output regions in medial and posterior proto- and anterior deutocerebrum.
Responding to the temporal structure of the song
The results of behavioural experiments performed by Hedwig and Poulet (2004) show that the pulse structure of chirps is preserved from the auditory input to the motor output as it is reflected in rapid phonotactic steering movements of female crickets. However, reactive steering to sound pulses is controlled by recognition of the species-specific pattern in a way that is not yet understood (Poulet and Hedwig 2005). Whatever the mechanism of pattern recognition underlying phonotaxis, there has to be a sensory-to-motor pathway that preserves the pulse structure of chirps from the auditory stimulus to stimulus-induced phonotactic movements.
Of the neurons described here, only B-DC1(5) is potentially involved in rapid steering. Its SC value during standing was 0.41, and increased to 0.63 during walking. Furthermore intracellular stimulation of B-DC1(5) reliably elicited walking and steering to the contralateral side, i.e. the side of the axon. Thus any acoustically evoked spike activity in this neuron will have an impact on the animals’ walking and steering behaviour. In B-DI1(1) and B-DI1(3) the strongest auditory responses were elicited by the first pulse of the chirps, while in B-DI1(2) the strongest response occurred to the second pulse. A similar characteristic was described for a local brain neuron B-LC2 with even a stronger response to the second pulse (Zorović and Hedwig 2011). This effect is also seen in local brain neurons, which are tuned to the pattern of the species-specific song (Kostarakos and Hedwig 2012). It is based on an inhibition triggered by the first pulse of a chirp and may reflect a delay coincidence detector mechanism (Reiss 1964; Grothe 1994). The fact that a stronger response to the second pulse of a chirp is not exhibited in other descending brain neurons may point to parallel channels in the auditory-to-motor pathways of cricket phonotaxis.
Gating of auditory responses during walking
A modulation of motor activity in activity-dependent fashion is well established in both vertebrates and invertebrates (for review see Pearson 1995). Most of these modulations, however, are confined to low level mechanosensory responses closely related to the animal’s locomotor systems. An example where gating of responses occurs on a level not directly coupled with locomotor behaviour in crickets was first reported by Brodfuehrer and Hoy (1989). In Teleogryllus oceanicus, flight activity gated the ultrasound elicited descending steering commands in the neck connectives.
The first study in which intracellular recordings were performed in the brain of walking crickets (Böhm and Schildberger 1992) showed that during bouts of walking, responses of local and descending brain neurons to stimuli of the conspecific calling song frequency were usually different (with additional action potentials) from those when the animal was standing. Two studies in which the recordings were made from neck connectives (Staudacher and Schildberger 1998; Staudacher 2001) show walking-dependent gating of auditory responses in most descending brain neurons, while Schildberger et al. (1988) demonstrated walking-induced suppression of auditory responses in a local prothoracic neuron ON1. Zorović and Hedwig (2011) compared the responses to artificial calling song during standing and walking along three levels of the auditory pathway: in ascending, local, and descending brain neurons. At the level of ascending and local brain neurons, walking generally resulted in an increase of the auditory response intensity, while at the level of descending neurons the auditory response decreased with walking. Along the processing pathway, the accuracy of song pattern copying seemed to deteriorate during walking, with an exception of one local and one descending neuron where walking apparently improved the accuracy of the auditory response.
The present study showed that none of the three ipsilaterally descending neurons (B-DI1(1), B-DI1(2) and B-DI1(3)) responded to conspecific calling song during walking. Whether the response was actively suppressed or just concealed by some other activity remains unclear. Only the contralaterally descending B-DC1(5) responded to stimuli with a similar response intensity (expressed as the number of spikes per chirp) irrespective of the cricket’s behaviour.
From the perspective of phonotactic behaviour in crickets, it is important that the female is able to process auditory information during standing and walking. The relatively weak responses to chirps and additional sensitivity to visual and mechanosensory stimuli (air puffs, touches with a paintbrush; personal observation) of B-DI1(1), B-DI1(2) and B-DI1(3) may point to multimodality of these three descending neurons and may indicate only an accessory role in phonotaxis. Certainly B-DI1(2) has also a role as antennal mechanosensory interneuron. Staining the neurons all the way to their output regions might indicate possible links to motor regions and provide additional cues to their putative role in the phonotaxis.
Correlation with and control of walking
Highly significant correlations of neural activity and behavioural parameters are commonly accepted as evidence that the activity of a given neuron could be relevant for a specific behaviour (Staudacher 2001). In all four descending neurons described here, there was a significant correlation between spike rate and forward walking velocity, indicating a relevance of the neurons for the control of walking.
In general, eliciting rhythmic and/or stereotyped motor activity seems to be controlled by command neurons that activate dedicated pattern generator networks for specific motor programs (Kupfermann and Weiss 1978; Edwards et al. 1999; Hedwig 2000). The command neuron concept implies that the activity of a command neuron must be both sufficient and necessary to start and maintain the rhythmic motor pattern.
Depolarizing current injections in neurons B-DI1(1), B-DI1(2) and B-DC1(5) elicited walking. The specific effects of depolarization varied for different neuron types, causing walking to either ipsilateral or contralateral side, sometimes with strong turns, which resulted in transient negative values of the forward velocity component. Although walking was successfully elicited by depolarization, it occasionally stopped during depolarization or it outlasted it, i.e. the animal stopped walking despite the active neuron or continued walking despite the inactive neuron. The hyperpolarization of B-DC1(5) in a walking cricket resulted in cessation of walking. The activity of B-DC1(5) was therefore necessary for maintenance of walking; however, none of the neurons strictly fulfilled the sufficiency criteria for command neurons, as walking sometimes stopped during depolarization.
The latency between the onset of spike bursts and the start of ‘spontaneous’ walking, i.e. walking in absence of sound or other external stimuli, was ca. 30–50 ms. Compared to this, the latency from the time of the current injection to the start of walking was much longer, above 150 ms. We assume that although the stimulation of a single neuron may be sufficient to drive a complex behaviour such as walking, the same neuron may normally work in consensus with others (Kien 1983); the initiation of walking in an intact animal may rather be the result of an orchestrated activity of many neurons, which may explain the difference in latencies. When it comes to phonotactic walking and steering, however, only a small number of neurons may be involved in the sensory-to-motor pathway to facilitate the rapid steering response (Hedwig and Poulet 2005). Böhm and Schildberger (1992) also quantified the correlation between spike rate of two descending neurons and their rotational and translational velocities. They found significant correlations between spike activity of descending neurons and the translational walking velocity, which is equivalent to our forward velocity. Furthermore, they also managed to elicit walking by injecting current into one of descending interneurons. So far, none of these neurons could be ascribed to any of the clusters described by Staudacher (1998).
Based on the response properties described in the previous chapters together with a significant positive correlation between forward and contralateral steering velocities and the neuron’s firing rate, B-DC1(5) seems to be the best potential candidate for an integral part of the phonotaxis pathway. Its response latency (47.1 ± 8.7 ms) implies that this neuron may potentially be involved in the rapid auditory steering responses which occur with a latency of only 55–60 ms (Hedwig and Poulet 2005).
Conclusions
Our data expand the conclusions of Staudacher and Schildberger (1998) and Staudacher (2001) as we find descending auditory neurons, which do not exhibit walking-dependent gating of auditory responses. Moreover, neurons responded to sound only during standing, while their auditory responses were suppressed during walking. In the only neuron which copied the temporal pattern of the chirp both during standing and walking, B-DC1(5), the pulse copying accuracy even increased with walking. As this interneuron also elicited walking upon intracellular stimulation, we speculate that this type of neurons may contribute to phonotactic steering.
References
Böhm H, Schildberger K (1992) Brain neurons involved in the control of walking in the cricket Gryllus bimaculatus. J Exp Biol 166:113–130
Brodfuehrer PD, Hoy RR (1989) Integration of ultrasound and flight inputs on descending neurons in the cricket brain. J Exp Biol 145:157–171
Brodfuehrer PD, Hoy RR (1990) Ultrasound sensitive neurons in the cricket brain. J Comp Physiol A 166:651–662
Edwards DH, Heitler WJ, Krasne FB (1999) Fifty years of a command neuron: the neurobiology of escape behaviour in the crayfish. Trends Neurosci 22:153-160
Gebhardt M, Honegger HW (2001) Physiological characterisation of antennal mechanosensory descending interneurons in an insect (Gryllus bimaculatus, Gryllus campestris) brain. J Exp Biol 204:2265–2275
Grothe B (1994) Interaction of excitation and inhibition in processing of pure tone and amplitude-modulated stimuli in the medial superior olive of the moustached bat. J Neurophysiol 71:706–721
Hedwig B (2000) Control of cricket stridulation by a command neuron: efficacy depends on the behavioral state. J Neurophysiol 83:712–722
Hedwig B, Knepper M (1992) NEUROLAB, a comprehensive program for the analysis of neurophysiological and behavioral data. J Neurosci Methods 45:135–148
Hedwig B, Poulet J (2004) Complex auditory behaviour emerges from simple reactive steering. Nature 430:781–785
Hedwig B, Poulet J (2005) Mechanisms underlying phonotactic steering in the cricket Gryllus bimaculatus revealed with a fast trackball system. J Exp Biol 208:915–927
Homberg U (1987) Structure and functions of the central complex in insects. In: Gupta AP (ed) Arthropod brain: its evolution, development, structure, and functions. Wiley, New York, pp 347–367
Homberg U (1994) Flight-correlated activity changes in neurons of the lateral accessory lobes in the brain of the locust Schistocerca gregaria. J Comp Physiol A 175:597–610
Kanzaki R, Shibuya T (1986) Descending protocerebral neurons related to the mating dance of the male silkworm moth. Brain Res 377:378–382
Kanzaki R, Arbas EA, Hildebrand JG (1991a) Physiology and morphology of protocerebral olfactory neurons in the male moth Manduca sexta. J Comp Physiol A 168:281–298
Kanzaki R, Arbas EA, Hildebrand JG (1991b) Physiology and morphology of descending neurons in pheromone-processing olfactory pathways in the male moth Manduca sexta. J Comp Physiol A 169:1–14
Kien J (1983) The initiation and maintenance of walking in the locust: An alternative to the command concept. Proc R Soc Lond B 219:137–174
Knepper M, Hedwig B (1997) NEUROLAB, a PC-program for the processing of neurobiological data. Comput Methods Prog Biomed 52:75–77
Kostarakos K, Hedwig B (2012) Calling song recognition in female crickets: temporal tuning of indentified brain neurons matches behavior. J Neurosci 32(28):9601–9612
Kupfermann I, Weiss KR (1978) The command neuron concept. Behav Brain Sci 1:3–39
Nolen TG, Hoy RR (1984) Initiation of behaviour by single neurons: the role of behavioural context. Science 4677(226):992–994
Olberg RM (1986) Identified target-selective visual interneurons descending from the dragonfly brain. J Comp Physiol A 159:827–840
Pearson KG (1995) Proprioceptive regulation of locomotion. Curr Opin Neurobiol 5:786–791
Pollack GS, Hoy RR (1981) Phonotaxis in flying crickets: neural correlates. J Insect Physiol 27:41–45
Popov AV, Shuvalov VF (1977) Phonotactic behaviour of crickets. J Comp Physiol A 119:111–126
Popov AV, Peresleni AI, Savvateeva-Popova EV, Wolf R, Heisenberg M (2004) The role of the mushroom bodies and of the central complex of Drosophila melanogaster. Brain in the organization of courtship behavior and communicative sound production. J Evol Biochem Phys 40(6):641–652
Poulet J, Hedwig B (2005) Auditory orientation in crickets: pattern recognition controls reactive steering. Proc Natl Acad Sci USA 102:15665–15669
Reiss RF (1964) A theory of resonant networks. In: Reiss RF (ed) Neural theory and modelling. Stanford University Press, Stanford, pp 105–137
Ridgel AL, Alexander BE, Ritzmann RE (2007) Descending control of turning behaviour in the cockroach, Blaberus discoidalis. J Comp Physiol A 193:385–402
Ritzmann RE, Tobias ML, Fourtner CR (1980) Flight activity initiated via giant interneurons of the cockroach: evidence for bifunctional trigger interneurons. Science 210:443–445
Schildberger K (1984) Temporal selectivity of identified auditory neurons in the cricket brain. J Comp Physiol A 155:171–185
Schildberger K, Milde JJ, Hörner M (1988) The function of the auditory neurons in cricket phonotaxis. II. Modulation of auditory responses during locomotion. J Comp Physiol A 163:633–640
Schöneich S, Schildberger K, Stevenson PA (2011) Neuronal organization of a fast-mediating cephalothoracic pathway for antennal-tactile information in the cricket (Gryllus bimaculatus DeGeer). J Comp Neurol 519:1677–1690
Stabel J, Wendler G, Scharstein H (1989) Cricket phonotaxis: localization depends on recognition of the calling song pattern. J Comp Physiol A 165:165–177
Staudacher E (1998) Distribution and morphology of descending brain neurons in the cricket Gryllus bimaculatus. Cell Tissue Res 294:187–202
Staudacher E (2001) Sensory responses of descending brain neurons in the walking cricket, Gryllus bimaculatus. J Comp Physiol A 187:1–17
Staudacher E, Schildberger K (1998) Gating of sensory responses of descending brain neurons during walking in crickets. J Exp Biol 201:559–572
Strauss R, Heisenberg M (1993) A higher control centre of locomotor behaviour in the Drosophila brain. J Neurosci 13(5):1852–1891
ter Hofstede HM, Killow J, Fullard JH (2009) Gleaning bat echolocation calls do not elicit antipredator behaviour in the Pacific field cricket, Teleogryllus oceanicus (Orthoptera: Gryllidae). J Comp Physiol A 195:769–776
Ulagarai SM, Walker TJ (1973) Phonotaxis of crickets in flight: attraction of male and female crickets to male calling songs. Science 182:1278–1279
Weber T, Thorson J (1989) Phonotactic behaviour of walking crickets. In: Huber F, Moore TE, Loher W (eds) Cricket behavior and neurobiology. Cornell University Press, Ithaca, pp 310–339
Wendler G (1990) Pattern recognition and localization in cricket phonotaxis. In: Gribakin G, Wiese K, Popov A (eds) Sensory systems and communication in arthropods. Birkhäuser, Basel, pp 387–394
Zorović M, Hedwig B (2011) Processing of species-specific auditory patterns in the cricket brain by ascending, local, and descending neurons during standing and walking. J Neurophysiol 105:2181–2194
Acknowledgments
This work was supported by the BBSRC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zorović, M., Hedwig, B. Descending brain neurons in the cricket Gryllus bimaculatus (de Geer): auditory responses and impact on walking. J Comp Physiol A 199, 25–34 (2013). https://doi.org/10.1007/s00359-012-0765-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-012-0765-7