Abstract
Individuals in distress emit audible vocalizations to either warn or inform conspecifics. The Indian short-nosed fruit bat, Cynopterus sphinx, emits distress calls soon after becoming entangled in mist nets, which appear to attract conspecifics. Phase I of these distress calls is longer and louder, and includes a secondary peak, compared to phase II. Activity-dependent expression of egr-1 was examined in free-ranging C. sphinx following the emissions and responses to a distress call. We found that the level of expression of egr-1 was higher in bats that emitted a distress call, in adults that responded, and in pups than in silent bats. Up-regulated cDNA was amplified to identify the target gene (TOE1) of the protein Egr-1. The observed expression pattern Toe1 was similar to that of egr-1. These findings suggest that the neuronal activity related to recognition of a distress call and an auditory feedback mechanism induces the expression of Egr-1. Co-expression of egr-1 with Toe1 may play a role in initial triggering of the genetic mechanism that could be involved in the consolidation or stabilization of distress call memories.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rare behavior of vocal learning has been documented only in humans, cetaceans, three avian taxa, and a few species of bats. Recall of a learned vocal signal completely depends on the auditory processing ability of the species, i.e., its ability to receive, interpret, and remember the order of signals (Funabiki and Konishi 2003). Individuals of the same species use learned vocal signals for communication (Zeigler and Marler 2004). Comparative anatomical studies have proven to be useful in understanding the organization of vocal system, which in birds involves song production, song perception, and discrimination (Perkel 2004). Jarvis et al. (2000) identified two brain areas (e.g., telencephalon, mesencephalon) involved in vocal communications, by monitoring expression of the transcriptional regulator ZENK in freely ranging birds after hearing and vocalizing behaviors. The activity-dependent expression of immediate-early gene (IEG) egr-1 (also known as zenk, z if268, N GF1A, and K rox 24) is the most commonly used marker to measure neuronal activity in avian species (Mello et al. 2004). The expression of zenk is transient and peaks within 30–40 min after hearing the song or after the onset of singing; it declines thereafter (Mello and Clayton 1994; Jarvis and Nottebohm 1997).
Early studies in mammalian systems have shown that expression of zenk is induced by synaptic plasticity, which plays a key role in the memory consolidation–reconsolidation process. Interestingly, a targeted mutation produced a zif268-/- mutant mouse that failed to show long-term memory in both spatial and non-spatial learning tasks (Jones et al. 2001). These experiments suggest that proteins encoded by zenk regulate the transcription of other downstream or late genes. Recently, Toe1 (the target of egr-1) has been identified as the target of Egr-1 (De Belle et al. 2003). Analysis of the genetic relationship of Egr-1 and its responsive gene Toe1 would constitute a significant step in understanding the activities associated with Egr-1.
Bats are the only mammals that are capable of active flight and known for echolocation. Most species live in social groups and use species-specific vocal signals for a variety of social interactions such as mother–infant communication, mating, group recognition, defending foraging area, and distress (Barclay et al. 1979; Esser and Schmidt 1989; Balcombe and McCracken 1992; Barlow and Jones 1997; Van Parijs and Corkeron 2002; Pfalzer and Kusch 2003; Russ et al. 2004). A distress call has been described as a vocalization of animals in a situation of distress; birds produce distress calls possibly to warn other individuals about predation, to attract a secondary predator to scare the original predator, and to request aid from kin or reciprocally altruistic individuals (Koenig et al. 1991; Conover 1994). The Indian short-nosed fruit bat, Cynopterus sphinx, roosts modified tents; the harem male constructs and defends the tent, and then recruits adult females (Bhat and Kunz 1995). Females of C. sphinx give birth to single young one in each breeding season. Newborn pup tenaciously cling their mother’s nipple up to 24 days following birth. During this postnatal period, mothers forage with their attached young ones (Elangovan et al. 2003). Mist net studies at foraging sites have reported that C. sphinx immediately after becoming entangled in the net, try to disentangle and flee from the mist net. Many of them produced distress call, which attracts conspecifics in the vicinity. In addition, the distress calls of juveniles attract lactating females to capture site (Nathan 2001).
The present study focused on understanding the emission of distress calls and behavioral responses associated with the expression of early responsive genes in C. sphinx. First, we observed the behavioral responses to the distress call. Second, we examined the induction of Egr-1 and expression of its target gene in free-ranging C. sphinx that emitted and responded to a distress call. Third, we analyzed the effect of the mother’s distress call on pups as pups developed by comparing expression at postnatal (PN) days 5 and 15. We chose PN-5 because it corresponds with the unfolding of pups’ pinnae and opening of eyes and PN-15 because the onset of hearing generally occurs by the second postnatal week (Rübsamen 1987). In order to examine distress call evoked responses on the day (PN-5) of pup’s unfolding of pinnae and opening of eyes and that on PN-15 were taken for analysis. To our knowledge, this is the first report demonstrating distress call-induced egr-1 expression and its co-expression of Toe1 in bats.
Materials and methods
Animal
For all the experiments, we used wild, free-ranging Indian short-nosed fruit bats, Cynopterus sphinx, which were captured during the period October 2007–September 2008 in a guava orchard located at 2 km from the Bharathidasan University campus (10°16′N; 78°15′E), Tiruchirappalli, India. The mist net (9 m × 2 m; Avinet-Dryden, USA) was placed near the regular flight paths of bats 1 h before sunset (Kunz and Brock 1975). Since C. sphinx flies 2 m above the ground level, we adjusted the height of the mist net based on our earlier study (Nathan et al. 2001). Behavior (call emitting, call responding, staying silent) of trapped bats was observed and noted by the observer. Trapped bats were removed from the mist net, categorized (male, female, female with pup, juvenile), and the morphological measurements (length of forearm, length of fifth digit, body mass) were recorded. Distress call responses were observed between 18:00 and 22:00 h for 21 days during the study period.
Distress call analysis
Distress calls were recorded from eight bats with a Sony WM-D6C Professional Walkman and Sony ECM-MS957 microphone (frequency response, 1–12 kHz ± 3 dB). Recordings were made on analogue tape and digitized with a Sigma Tel A/D card at a sampling rate of 44.1 in a computer (DELL, Pentium IV 1.60 GHz processor, 1 GB RAM). Temporal and spectral analyses of bat sound were carried out with Bat Sound software (V2.0, Pettersson Electronik, Upssala, Sweden); sequences of distress calls (n = 30) were selected on the basis of the quality of the sound recordings (signal-to-noise ratio). Temporal features of the distress calls, such as the duration of call and the interpulse interval were measured using an oscillogram and the peak frequency was determined from the power spectra. Maximum and minimum frequencies (for calculation of bandwidth) were recorded at the frequencies 10 dB below the peak frequency in the power spectra.
Behavioral analysis
Brain tissue obtained from animals subjected to behavioral experiments was categorized into three groups. For each experimental group, the trapped bat was allowed to remain at the mist net for 10 min. The bat that emitted the distress call was marked as a call emitter. Subsequently, bats in the vicinity that fell into the mist net within 2 min after they produced a distress call were considered as call responders.
Control
Two different controls were maintained in this group. After being entangled, bats that remained in the mist net for 10 min without emitting distress call were designated as silent bats (C1) and were decapitated 1 h after capture (n = 4). Bats that produced a distress call (C2) were removed from the mist net, tagged then housed in a free flight chamber (animal house bat chamber, 4.4 × 3.7 × 2.4 m conditioned for temperature and humidity) and allowed to rest quietly for 12 h. Commonly available fruits such as sapota (Achras sapota), papaya (Carica papaya), banana (Musa paradisiaca), and guava (Psidium guajava), along with water, were provided ad libitum. At the end of the rest period, bats were sacrificed and the brain was rapidly removed (n = 4).
Distress call emitting (DCE) mother–pup
This group comprised female bats with pups at PN-5 and PN-15. When the mother was captured in the net, and produced a distress call, we assumed that the pup heard its mother’s distress call. Four mother–pup pairs were taken for gene expression analysis. Age of free-ranging C. sphinx pups was calculated based on length of forearm (calipers, ±0.1 mm), epiphyseal gap in the fourth metacarpal-phalangeal joint (ocular micrometer, 1.0 μ) and body mass (Avinet-Dryden, spring balance, ±0.1 g) and then compared with an age-estimation (Elangovan et al. 2003).
Call emitter–call responder
The first male or female bat that produced a distress call in the mist net was considered as call emitter, and the one that immediately became trapped in the mist net was considered to be call responder. From this group, four call emitter and responders were taken for analysis of gene expression.
All groups of bats remained in the mist net for a maximum of 10 min. One hour after recording a call emission or response to the call, bats were decapitated and the brains were removed for analysis of gene expression driven by call emission/hearing. Previous studies have suggested that certain midbrain regions and the parabrachial nucleus control the call frequency in microchiropteran bats (Smotherman and Metzner 2003). In the present study, a region covering the auditory system and vocal motor system (CN, cochlear nucleus; SOC, superior olivary complex; NLL, nuclei of the lateral lemniscus; NCAT, nucleus of the central acoustic tract; IC, inferior colliculus; SC, superior colliculus) in complete hind brain and midbrain were dissected then cut transversely into two equal pieces for preparation of protein and total RNA.
Gene expression analysis
Total RNA was isolated from dissected pieces of brain homogenized with 0.5 mL of TRIzol (Invitrogen, USA) following manufacturer’s instructions. The total RNA was dissolved in diethyl-pyrocarbonate treated water containing RNase inhibitor (1 U/μL; Rnasin, Promega, Madison, USA). The concentration of RNA was quantified by measuring the optic absorbance at 260 nm with a spectrophotometer (Optima Inc, Japan). The specific 800 bp gene fragment of egr-1 was amplified with sense 5′ ATGGCAGCGGCCAAGGCCGA 3′ and antisense 5′ TAGGCAGGAGGCGGGT ACTGCA 3′ primers designed from the mouse egr-1 sequence (NM007913). Total RNA (2.0 μg/sample) was reverse-transcribed using the AccessQuick™ RT–PCR system (Promega, Madison, USA). First-strand cDNA was synthesized using AMV reverse transcriptase following the manufacturer’s instructions. Semi-quantitative RT–PCR was used to quantify the level of egr-1 expression together with β-actin as an internal control in a single PCR reaction. Moreover, the degree of expression of egr-1 was established by dividing the amount of egr-1 mRNA expression by the amount of β-actin mRNA expression (Chamizo et al. 2001). The expression level of β-actin was assessed using sense and anti-sense primers (McCaffrey et al. 2000). A 350-bp product was obtained under the following conditions: initial denaturation at 94°C for 2 min then denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, then final extension at 72°C for 10 min (MJ Mini Gradient Thermal Cycler, Bio-Rad). For semi-quantitative measurements, we amplified the egr-1 with β-actin and optimized the number of PCR cycles (27, 30, or 33 cycles) to maintain amplification within a linear range. Twenty microliters of each PCR product were electrophoresed on 1.0% (w/v) agarose gels containing ethidium bromide (0.5 μg/ml). Images of the amplified products were acquired with a Molecular Imager ChemiDoc XRS system. The intensity was estimated using image analysis software (Quantity one, Bio-Rad, USA). Band intensity was expressed as the relative peak density; egr-1/β-actin product ratios were calculated as indices of egr-1mRNA expression.
The brain tissue was homogenized in ice-cold lysis buffer (150 mM NaCl, 50 mM Tris–HCl pH 7.5, 5 mM EDTA, 0.1% v/v NP-40, 1 mM DTT, 0.2 mM sodium orthovanadate, 0.023 mM PMSF) and 4 μl/mL protease inhibitor cocktail (Sigma–Aldrich, USA) and incubated on ice for 30 min. The homogenate was centrifuged at 10,000×g for 30 min at 4°C, and the supernatant was collected in a fresh tube and again centrifuged at 12,000×g for 30 min at 4°C. The supernatants were collected and stored at −80°C until assayed. The protein concentration was determined by adopting Bradford’s Method (Bradford 1976). An equal concentration of protein (80 μg) was mixed with loading buffer (100% glycerol, 125 mM Tris–HCl pH 6.8, 4% SDS, 0.006% bromophenol blue, 2% mercaptoethanol) then boiled for 5 min and resolved on an 8% polyacrylamide gel. The separated proteins were transferred electrophoretically onto a Hybond-XL NT membrane (Amersham Pharmacia Biotech Ltd, Bangalore, India) then the membrane was blocked in PBS (5% non-fat dry milk; 0.1% Tween20) for 3 h at room temperature (RT) with gentle agitation. Immunodetection was performed by incubating the membrane with primary antibody (1:200; Egr-1 antibody, SC-189, Santa-Cruz Biotech) for 12 h in PBS (3% non-fat dry milk; 0.1% Tween20). The membrane was washed, and bound antibodies were detected by incubating for 6 h with mouse anti-rabbit antibody conjugated with alkaline phosphatase (1:2,000, SC-2358, Santa-Cruz Biotech). After a final wash, alkaline phosphatase activity was detected with 5-bromo-4-chloro-3-inolyl phosphate/nitroblue tetrazolium salt (BCIP/NBT) according to the manufacturer’s instructions (Invitrogen, USA). The level of expression was quantified using image analysis software (Quantity one, Bio-Rad, USA).
Egr-1 mediated co-expression of Toe1 was analyzed by semi-quantitative RT–PCR. Initially the specific 600 bp gene fragment of Toe1 was amplified with sense 5′ ATG GCCGCGGACAGTGACGA 3′ and antisense 5′ TAGACTTGG TGTTCCTGTA C-3′ primers designed from the mouse Toe1 sequence (NM026654). The amplified product was cloned in a pTZ57R vector (InsTAclone, PCR Cloning Kit, Fermentas International Inc, Canada) then confirmed by sequencing with universal M13 primers.
Northern blot analysis was used to identify the expression of Toe1 induced by Egr-1 in the distress call vocalized bats, responding adults, mother-dependent pups, and control bats. Total RNA (30 μg/sample) in sampling buffer (50% formamide, 2.2 M formaldehyde and 1× 4-morpholinopropane sulfonic acid (MOPS) buffer, pH 7.0) was loaded onto a 1% agarose gel containing 0.44 M formaldehyde and run in 1× MOPS buffer at constant voltage (50 V, 2 h). After electrophoresis, the total RNA was transferred onto a nitrocellulose membrane (Sigma–Aldrich, USA) and hybridized with a biotin-labeled specific probe for Toe1 prepared from C. sphinx (DecaLabel DNA Labeling Kit, Fermentas International Inc, Canada) at 42°C for 10 h. Non-specific hybridization was removed by subsequent washing in 2× SSC and 0.1% SDS at RT for 10 min (twice), followed by washing at 65°C in 0.1× SSC and 0.1% SDS for 20 min (twice). Specific hybridization of the Toe1 DNA probe with Toe1mRNA was visualized using a biotin chromogenic detection kit (Fermentas International Inc, Canada). Similarly, we estimated the level of Toe1 expression by adopting the aforementioned standardized protocol.
Statistical analysis
SigmaStat (ver.3.0) software package was used to compute one-way repeated measures ANOVA and one-way ANOVA to determine which group differed from others. Bonferroni’s post hoc tests were used for multiple comparisons. Prior to all ANOVAs the data was tested for normality. Values (mean ± SE) were plotted with KyPlot (version 1.0) for graphical representation.
Results
Behavioral responses to distress calls
The general approach of our experiment was to determine whether the emission of a distress call and its responses in conspecifics evoked genomic responses following neuronal stimulation in the Indian short-nosed fruit bat, C. sphinx. During the study period, we found that C. sphinx tried to escape from the mist net immediately after being captured, and then produced a distress call. Other C. sphinx foraging in the vicinity responded to the distress call by flying around the mist net continuously; a few bats that flew closer to the call-emitting bat and got captured in the mist net. The year-round (21 days) mist-netting study revealed that out of 373 trapped bats, 38 bats (22 individual adults; 16 mothers with pups) produced distress calls that attracted 214 bats, and 121 bats were captured either before or long time after producing a distress call. The distress call significantly (F 1,19 = 34.05, p < 0.01) attracts a large number of bats to the capture site than with the normal mist net. We did not observe any answering calls from the attracted bats.
Acoustic character of distress call
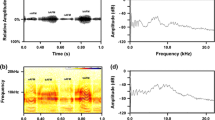
C. sphinx produced loud and audible distress calls with an irregular interval. The distress calls were continuous and consisted of two phases fused together so that phases I and II were alternated. The duration (ms) of phase I was 203.23 ± 2.9 (mean ± SD), which was longer than that of phase II, 123.33 ± 1.5 (repeated measures ANOVA, F1,29 = 4.48, p < 0.05), and phase I was louder than phase II (Fig. 1a). Both phases were characterized by multiple harmonics (Fig. 1b) with a bandwidth of 2 kHz, and the duration of a complete distress call ranged from 2.26 to 3.36 s. The minimum frequencies of phases I and II were 5.75 ± 0.1 and 5.28 ± 0.1 kHz and the maximum frequencies were 7.27 ± 0.01 and 7.09 ± 0.01 kHz, respectively. However, phase I was characterized by a secondary peak at 8.90 ± 0.11 kHz (Fig. 1c). The call structure did not vary significantly between individuals in band widths (repeated measures ANOVA, F1,29 = 1.36, p = 0.27), or peak frequencies (repeated measures ANOVA, F1,29 = 2.28, p = 0.11).
Distress call of free-ranging Cynopterus sphinx after becoming trapped in a mist net. a Oscillogram of a continuous distress call; note that phases I and II were fused together and alternate continuously. b Spectrogram of a continuous distress calls indicating harmonics. c Relative power spectrum depicting peak frequencies of the entire distress call. Arrow indicates the secondary peak; n = 30
Expression of egr-1 induced by the distress call
PCR amplicons of approximately 800 bp were obtained with the egr-1 degenerate primer and DNA sequence analysis confirmed that we amplified a part of the egr-1 gene from C. sphinx. The egr-1 expression patterns in all groups of bats were determined by semi-quantitative RT–PCR analysis (Fig. 2a). The level of egr-1 expression was determined by comparing the expression of housekeeping gene β-actin in control (C1), (C2), DCE mothers and their pups at the age of PN5, PN15, and then call emitter and call responder C. sphinx (Fig. 2b). The analysis showed that the level of egr-1 expression increased more than twofold in the call emitting and call responding bats compared to the control bats. Bonferroni’s post hoc test (C1 with each group) revealed significant differences in the level of egr-1 expression (F 7,31 = 5.31, p < 0.001). Interestingly, we observed distress call-induced expression in pups at PN-5 and PN-15. A significant difference was found in the expression level between PN-5 and PN-15 pups (F 1,6 = 5.31, p < 0.01). Difference in the level of expression was estimated in DCE mothers and their pups. Estimated level of egr-1 expression was not significantly different in the pups than their DCE mothers on PN-5 (F 1,6 = 6.21, p = 0.09). Subsequently, we found significant difference (F 1,6 = 64.89, p < 0.01) in the expression level at PN-15 pups as compared to their DCE mothers. Although we observed variation in the induction of egr-1 between pups (PN-5, PN-15) and call responders, variations were significantly lower only in PN-5 pups than call responders (F 1,6 = 34.75, p < 0.01), and the variation was not significant between PN-15 and call responders (F 1,6 = 1.94, p = 0.074). Likewise, no changes were found between the call emitter and responder (F 1,6 = 8.21, p = 0.19), as well as between controls (C1 vs. C2: F 1,6 = 0.61, p = 0.93). We did not observe similar induction of egr-1 in the olfactory bulb region (data not shown). We also performed immunoblot analysis with an antibody against Egr-1 to explore whether the induced mRNA translated as protein. The antibody recognized a protein with a molecular mass of 75 kDa (Fig. 3a) in all groups. We identified a higher level of Egr-1 protein in distress call emitting and responding C. sphinx. This suggested that the emission of distress calls and response through hearing induces expression of Egr-1 in C. sphinx. Comparing the expression of Egr-1 in all groups, we found differences in the level of Egr-1 expression when the trace value was measured. The level of expression of Egr-1 was more than twice as high in individuals that emitted calls and responded to the distress call than in the control group (Fig. 3b).
Distress call-induced expression of egr-1 (n = 4). a Semi-quantitative RT–PCR analysis shows the egr-1 expression at different behavioral states of bats; the amplification shows differential expression of egr-1 and a constant level of expression of the housekeeping gene β-actin. b The estimated level of egr-1 expression indicates that distress call vocalization and its responses induce egr-1 expression. The average expression rate of egr-1 was obtained by calculating the mean ± standard error; asterisks indicate significant differences
Distress call-induced expression of egr-1 completely translated as Egr-1 protein (n = 4). a The Egr-1 antibody recognized a protein of 70 kDa in all samples. b Expression of Egr-1 was induced in bats that vocalized or responded to a distress call, and in pups. Other samples show a basal level of expression
Co-expression of Egr-1 with other late gene
To investigate the relationship of the Egr-1 protein with other late-response genes, we performed northern blot analysis under conditions of high stringency to test whether the mouse Toe1 probe could hybridize to mRNA samples from bats that call emitted or responded to the distress call, and from control bats. We found a strong hybridization signal in individuals that emitted or responded to the distress call. We obtained PCR amplicons of 600 bp with the Toe1 degenerate primers; DNA sequence analysis confirmed that we had cloned a fragment of the Toe1 gene from C. sphinx. The sequence we obtained was submitted to GenBank (accession number FJ416156). Alignment of the translated Toe1 sequence showed that Toe1 in C. sphinx possesses an additional 15 amino acids in the C-terminal region compared to mouse Toe1, suggesting a different functional contribution of Toe1 in C. sphinx. Figure 4a shows a northern blot analysis of different groups, including bats that emitted calls or responded to the distress call and control bats; mRNA was hybridized with the C. sphinx probe. We found a stronger signal in adult bats that emitted calls or responded to the distress call, and in pups than in control bats (Fig. 4a). Furthermore, we estimated the variation in the expression of Toe1 in bats that emitted a call or responded to the call and control bats. Semi-quantitative RT–PCR analysis was used to estimate the level of Toe1 expression. The observed variation reflected the variation in the northern blot analysis (Fig. 4b). The level of Toe1 expression was estimated by comparing it with the expression of the housekeeping gene β-actin. The analysis showed that the level of Toe1 expression increased 2.3-fold in bats that call emitted or responded to the distress call in comparison to the control (C1) bats (Fig. 4c). We found a significant difference in the level of Toe1 expression (F 7,31 = 8.22, p < 0.001) with Bonferroni’s post hoc test which showed that C1 expression level was significantly different from all behavioral groups, when C1 was compared with each. Interestingly, the level of Toe1 expression was increased in the pups (PN-5, PN-15) while their mother produced a distress call, and level of expression varied during development. The level of expression was significantly higher on PN-15 pups than PN-5 pups (F 1,6 = 24.30, p < 0.01). Although we observed a notable difference in the level of Toe1 expression between DCE mothers and their pups (Fig. 4c), the observed variation was not statistically significant on PN-5 (F 1,6 = 1.33, p = 0.29). Subsequently, at PN-15, the expression level was significantly higher in pups (F 1,6 = 30.26, p < 0.01) as compared to their DCE mothers (Fig. 4c). Observed difference in the level of Toe1 expression between the distress call responder, in PN-5 and PN-15 pups, the level of variation was low on PN-5 pups and significantly different (F 1,6 = 48.97, p < 0.01). However, the difference in the level of expression was not statistically significant (F 1,6 = 0.348, p = 0.57) between call responder and PN-15 pups. In bats from the control group, the level of Toe1 mRNA in call emitted bats declined during the remaining 12 h in captivity (C2); the estimated value did not differ from that of silent (C1) bats (F 1,6 = 0.315, p = 0.59).
Egr-1 associated expression of Toe1 (n = 4). a Northern blot analysis shows the differential expression of Toe1. b Gel electrophoretic analysis of semi-quantitative RT–PCR products derived from different behavioral states of bats; the amplification shows differential expression of Toe1 and a constant level of expression of the housekeeping gene β-actin. c Semi-quantitative analysis of Toe1 expression. Asterisks indicate significant differences
Discussion
Behavioral response to the distress call
The zinc finger immediate-early gene egr-1 may be involved in an enormous number of dynamic processes such as fast cellular response to changes in neuronal activity, developmental events, and behavioral states (Long and Salbaum 1998). We have demonstrated a specific experimental paradigm for evaluating the relationship between gene expression and communication in Chiroptera. In the present study, our results have shown vocalization and behavioral responses to the induction of egr-1 associated with the distress call and expression of its target gene Toe1, in wild, free-ranging C. sphinx. However, we have not tested this by allowing a set of animals to listen to playbacks of the distress calls, complex non-distress calls or other neutral sound and then examining the induction of egr-1. Thus far, only a limited number of studies have investigated the distress calls of bats and their behavioral responses (Fenton et al. 1976; Avery et al. 1984; Russ et al. 1998, 2004). Ryan et al. (1985) observed that the distress call of the phyllostomid bat Artibeus jamaicensis attracted bat species belonging to the same family. During our study, we observed that bats foraging in the vicinity were attracted by the distress call irrespective of sex and only attract conspecifics. Also, we observed that another pteropodid bat, Rousettus leschenaulti, foraging in the same area, was not attracted by the distress call of C. sphinx. In contrast, the distress calls of the vespertilionid bat, Myotis lucifugus (Avery et al. 1984) and Pipistrellus pygmaeus (Russ et al. 1998) elicited intraspecific responses. In fact, R. leschenaulti has the ability to produce echolocation clicks with an acoustic energy between 18 and 32 kHz (Raghuram et al. 2007), which is close to the range of reported other pteropodid bat, R. aegyptiacus, and its maximum hearing sensitivity falls in the range of 8–10 kHz (Suthers and Summers 1980; Waters and Vollrath 2003). In relation to the echolocation clicks and hearing sensitivity of latter one, R. leschenaulti may hear the distress call of C. sphinx. However, C. sphinx distress call failed to attract R. leschenaulti. Our results demonstrated that C. sphinx produced a distress call possibly to attract conspecifics. Individuals of C. sphinx produced a distress call with a maximum frequency of 8.9 kHz at the end of the phase I call, which is optimized for long-distance communication.
Expression of egr-1 driven by distress call
Increased expression of zenk has been observed in telencephalon and mesencephalon regions of the brain, when song birds hear songs of their own species or vocalize the song (Chaudhuri 1997). Based on earlier studies of auditory feedback in bats (Smotherman and Metzner 2003), we used hindbrain and midbrain regions in the present study. We detected higher level expression of egr-1 by semi-quantitative RT–PCR analysis in distress call emitted/responded bats. This suggests that auditory input in the responding bats and the motor system involved in call emission possibly induces the expression of egr-1. We have not observed induction in silent bats (control I). Moreover, our results revealed that physical stress during their stay at mist net did not induce the expression, which provides additional support to our observation of distress call-induced egr-1 expression.
Various time courses of expression and declining levels of egr-1 mRNA have been demonstrated during different behavioral paradigms (Guzowski et al. 2001; Burmeister and Fernald 2005). Here we have shown that the emission of distress call and its response induce the expression and increase the level of egr-1 expression; this later declined to the basal level in 12 h under quiet conditions. A similar trend has been reported in song birds (Mello and Clayton 1994). We have not measured the decline in the level of the egr-1 during intermediate time periods. Previous studies in the zebra finch (Whitney and Johnson 2005) suggested that translation of zenk is regulated by social interaction. To date, zenk is one of the few genes for which post-translational modifications are regulated by social interaction (Pascale et al. 2004), which is essential for formation of long-term memory (Routtenberg and Rekart 2005). Long-term memory is a specific process that stabilizes memory after initial acquisition (consolidation) and, then later is recalled to maintain, strengthen or modify memories (reconsolidation) that was already stored (Tronson and Taylor 2007). Studies in rodents have reported that induction of zif-268 (a homolog of egr-1) is necessary for the consolidation and reconsolidation of memory that is required for long-term memory (Jones et al. 2001). If egr-1 expression regulates memory consolidation, our data would suggest that expression of egr-1 is required for acquiring the distress calls of a conspecific. Taken together, our observations provide the first step toward understanding distress call driven gene expression in bats and suggest that distress calls drive the expression of egr-1, which is faithfully translated to the Egr-1 protein for regulation of late genes.
Egr-1 regulating expression of the late gene
In recent years, numerous studies have identified various individual target genes of Egr-1 in diverse cells and tissue types (Liu et al. 1998). Toe1 has also been identified as an Egr-1 target gene; its promotor region is sensitive to the level of the Egr-1 protein and a proportional increase in Toe1 expression with increasing Egr-1 expression was found (De Belle et al. 2003). However, the Toe1 identified in C. sphinx differs slightly from the reported Toe1. The insertion of an additional 15 amino acids may be unique to Chiroptera. We have not confirmed the full length for additional differences.
The level of Toe1 was standardized to the level of the housekeeping gene β-actin, which was considered to show a constant rate of expression in the cell, independent of its state of activation. The observed basal level of expression of Toe1 in the control group was significantly lower than in other groups. All the different levels of expression of Toe1 were confirmed by hybridization with the C. sphinx Toe1 probe; this provides additional evidence for modulation of Toe1 expression. Overall, although we found a significantly higher level of Toe1 expression in the C. sphinx that responded to the distress call than in those that emitted, the differences were not statistically significant. This may be because the active participation of the motor system involved in call emission may also be involved in the auditory pathway. Interestingly, expression of zenk induced by singing occurs even in deafened birds, in which auditory feedback is absent (Jarvis and Nottebohm 1997). The observed expression of Toe1 and Egr-1 showed the genomic responses to the distress call and the subsequent behavioral responses in both sexes. The distress call is expected to be more familiar and sensitive in conspecifics, and perhaps this accounted for the induction of Egr-1 and its target gene Toe1.
Several studies in colonial bat species have shown that the mother emits directive calls during the process of reunion with their pup which are more easily heard by the pups. In response to the mother’s call, pups emit isolation calls, which facilitate the reunion of mother and pup, together with spatial memory and olfactory cues (Jones et al. 1991; Zhang et al. 2005). Developmental studies in C. sphinx (Elangovan et al. 2003) have reported that mothers forage with their attached pup until the pup becomes an independent forager/feeder. Expression of Egr-1 and its target gene Toe1 was observed in PN-5 and PN-15 pups, induced by their mother’s distress call. The observed level of expression of Toe1 in the pup was equal to the level of the expression induced by the distress call in adults. These results further indicate that pups receive auditory inputs that may trigger the genomic activity related to learning or other processes related to perception such as attention. We observed higher level of expression in PN-15 pups than PN-5 pups. Changes in expression level from PN-5 to PN-15 possibly occur with an ontogenetic increase in the auditory-evoked responses (Rübsamen 1987). Interestingly, the neotropical bat Phyllostomus hastatus uses distinctive social calls acquired by vocal learning to discriminate strangers (Boughman 1998; Boughman and Wilkinson 1998). Although we did not observe vocal learning in PN5 and PN15 pups, this does not exclude the possibility that species-specific distress call responses are acquired as a result of earlier experience in C. sphinx. Earlier studies have shown that induction of early response genes is necessary for memory consolidation and reconsolidation (Lee et al. 2004). If Egr-1 serves a similar function in C. sphinx, then our data would suggest that co-expression of Egr-1 with Toe1 could be involved in the consolidation or stabilization of distress call memories.
Abbreviations
- IEG:
-
Immediate-early gene
- Egr-1:
-
Early growth response 1 gene
- TOE1:
-
Target of Egr1
- PN:
-
Postnatal day
- RT–PCR:
-
Reverse transcriptase-polymerase chain reaction
References
Avery MI, Racey PA, Fenton MB (1984) Short distance location of hibernaculum by little brown bats. J Zool 204:588–590
Balcombe JP, McCracken GF (1992) Vocal recognition in Mexican free-tailed bats: do pups recognize mothers? Anim Behav 43:79–88. doi:10.1016/S0003-3472(05)80073-9
Barclay RMR, Fenton MB, Thomas DW (1979) Social behaviour of the little brown bat, Myotis lucifugus. Behav Ecol Sociobiol 6:137–146. doi:10.1007/BF00292559
Barlow KE, Jones G (1997) Function of pipistrelle social calls: field data and a play back experiment. Anim Behav 53:991–999. doi:10.1006/anbe.1996.0398
Bhat HR, Kunz TH (1995) Altered flower/fruit clusters of the kitul palm used as roosts by the short-nosed fruit bat, Cynopterus sphinx (Chiroptera: Pteropodidae). J Zool (Lond) 235:363–770
Boughman JW (1998) Vocal learning by greater spear-nosed bats. Proc R Soc Lond B 265:227–233
Boughman JW, Wilkinson GS (1998) Greater spear-nosed bats discriminate group mates by vocalization. Anim Behav 55:1717–1732. doi:10.1006/anbe.1997.0721
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Burmeister SS, Fernald RD (2005) Evolutionary conservation of the Egr-1 immediate-early gene response in a teleost. J Comp Neurol 481:220–232
Chamizo C, Rubio JM, Moreno J, Alvar J (2001) Semi-quantitative analysis of multiple cytokines in canine peripheral blood mononuclear cells by a single tube RT-PCR. Vet Immunol Immunopathol 83:191–202. doi:10.1016/S0165-2427(01)00385-3
Chaudhuri A (1997) Neural activity mapping with inducible transcription factors. Neuroreport 8:5–9
Conover MR (1994) Stimuli eliciting distress call in adult passerines and response of predators and birds to their broadcast. Behavior 131:19–37
De Belle I, Wu JX, Sperandio S, Mercola D, Adamson ED (2003) In vivo cloning and characterization of a new growth suppressor protein TOE1 as a direct target gene of Egr1. J Biol Chem 278:14306–14312. doi:10.1074/jbc.M210502200
Elangovan V, Yuvana SPE, Raghuram H, Marimuthu G (2003) Postnatal development in the Indian short-nosed fruit bat Cynopterus sphinx: growth rate and age estimation. Acta Chiropterol 5:107–116
Esser KH, Schmidt U (1989) Mother-Infant communication in the lesser spear-nosed bat, Phyllostomus discolor (Chiroptera, Phyllostomidae): evidence for acoustic learning. Ethology 82:156–168
Fenton MB, Belwood JJ, Fullard JH, Kunz TH (1976) Responses of Myotis lucifugus (Chiroptera: Vespertilionidae) to calls of conspecifics and to other sounds. Can J Zool 54:1443–1448
Funabiki Y, Konishi M (2003) Long memory in song learning by zebra finches. J Neurosci 23:6928–6935
Guzowski JF, Setlow B, Wagner EK, McGaugh JL (2001) Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci 21:5089–5098. doi:0270-6474/01/215089-10$15.00/0
Jarvis ED, Nottebohm F (1997) Motor-driven gene expression. Proc Natl Acad Sci USA 94:4097–4102
Jarvis ED, Ribeiro S, Vielliard J, DaSilva M, Ventura D, Mello CV (2000) Behaviorally-driven gene expression reveals hummingbird brain vocal nuclei. Nature 406:628–632. doi:10.1038/35020570
Jones G, Hughes PM, Rayner JMV (1991) The development of vocalizations in Pipistrellus pipistrellus (Chiroptera: Vespertilionidae) during post-natal growth and the maintenance of individual vocal signatures. J Zool 225:71–84
Jones MW et al (2001) A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci 4:289–296. doi:10.1038/85138
Koenig WD, Stanback MT, Hooge PN, Mumme RL (1991) Distress calls in the acorn woodpecker. Condor 93:637–643
Kunz TH, Brock CE (1975) A comparison of mist-nets and ultrasonic detectors for monitoring flight activity of bats. J Mammal 56:907–911
Lee J, Everitt BJ, Thomas KL (2004) Independent cellular processes for hippo campal memory consolidation and reconsolidation. Science 304:839–843. doi:10.1126/science.1095760
Liu C, Rangnekar VM, Adamson ED, Mercola D (1998) Suppression of growth and transformation and induction of apoptosis by EGR-1. Cancer Gene Ther 5:3–28
Long KD, Salbaum JS (1998) Evolutionary conservation of the immediate-early gene ZENK. Mol Biol Evol 15:284–292
McCaffrey TA et al (2000) High-level expression of Egr-1 and Egr-1-inducible genes in mouse and human atherosclerosis. J Clin Invest 105:653–662. doi:10.1172/JCI8592
Mello CV, Clayton DF (1994) Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci 14:6652–6666
Mello CV, Velho TA, Pinaud R (2004) Song-induced gene expression: a window on song auditory processing and perception. Ann NY Acad Sci 1016:263–281
Nathan PT (2001) Behavior of Indian short nosed fruit bat Cynopterus sphinx (Vahl 1797), field and semi naturalistic ethological studies. PhD thesis, MS University, India
Nathan PT et al (2001) Mist-net capture and field observations on the short-nosed fruit bat (Chiroptera: Pteropodidae) Cynopterus sphinx (Vahl). J Bombay Nat Hist Soc 98:373–378
Pascale A et al (2004) Increase of the RNA binding protein HuD and post transcriptional up-regulation of the GAP-43 gene during spatial memory. Proc Natl Acad Sci USA 101:1217–1222
Perkel DJ (2004) Origin of the anterior forebrain pathway. Ann NY Acad Sci 1016:736–748
Pfalzer G, Kusch J (2003) Structure and variability of bat social calls: implications for specificity and individual recognition. J Zool 261:21–33. doi:10.1017/S0952836903003935
Raghuram H, Gopukumar N, Sripathi K (2007) Presence of single as well as double clicks in the echolocation signals of a fruit bat, Rousettus leschenaulti (Chiroptera: Pteropodidae). Folia Zool 56:33–38
Routtenberg A, Rekart JL (2005) Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci 28:12–19. doi:10.1016/j.tins.2004.11.006
Rübsamen R (1987) Ontogenesis of the echolocation system in the rufous horseshoe bat, Rhinolophus rouxi (audition and vocalization in early postnatal development). J Comp Physiol A 161:899–913
Russ JM, Jones G, Racey PA (1998) Intraspecific responses to distress calls of the pipistrelle bat, Pipistrellus pipistrellus. Anim Behav 55:705–713. doi:10.1006/anbe.997.0665
Russ JM, Jones G, Mackie IJ, Racey PA (2004) Interspecific responses to distress calls in bats (Chiroptera: Vespertilionidae): a function for convergence in call design? Anim Behav 67:1005–1014. doi:10.1016/j.anbehav.2003.09.003
Ryan MJ, Clark DB, Lackey JA (1985) Response of Artibeus lituratus (Chiroptera: Phyllostomidae) to distress calls of conspecifics. J Mammal 66:179–181
Smotherman M, Metzner W (2003) Effects of echo intensity on Doppler-shift compensation behavior in horseshoe bats. J Neurophysiol 89:814–821. doi:10.1152/jn.00246.2002
Suthers RA, Summers CA (1980) Behavioural audiogram and masked thresholds of the megachiropteran echolocation bat, Rousettus. J Comp Physiol 136:223–227
Tronson NC, Taylor JR (2007) Molecular mechanisms of memory consolidation. Nat Rev Neurosci 8:262–275
Van Parijs SM, Corkeron PJ (2002) Ontogeny of vocalisations in infant black flying foxes, Pteropus alecto. Behavior 139:1111–1124
Waters DA, Vollrath C (2003) Echolocation performance and call structure in the megachiropteran fruit-bat Rosettus aegyptiacus. Acta Chiropterol 5:209–219
Whitney O, Johnson F (2005) Motor-induced transcription but sensory-regulated translation of ZENK in socially interactive songbirds. J Neurobiol 65:251–259. doi:10.1002/neu.20187
Zeigler HP, Marler P (2004) Behavioral neurobiology of bird song. New York Academy of Sciences, New York
Zhang L, Jones G, Parsons S, Liang B, Zhang S (2005) Development of vocalizations in the flat-headed bats, Tylonycteris pachypus and T. robustula (Chiroptera: Vespertilionidae). Acta Chiropterol 7:91–99
Acknowledgments
We thank Prof. Thomas H. Kunz and two anonymous referees for their valuable comments and suggestions that improved the final version of this manuscript. This research was supported by the Department of Biotechnology (grant no. BT/PR7442/BRB/10/479/2006), Government of India, and a DAE young scientist grant to KER. We thank the UGC-SAP programme for supporting the Department of Animal Science instrumentation facility. Additional support was provided by the UGC-CAS of the SBS-MKU. Experimental protocol used in this study were approved by Institutional Animal Ethical Committee (IAEC) of Bharathidasan University and complied with the current laws of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganesh, A., Raghuram, H., Nathan, P.T. et al. Distress call-induced gene expression in the brain of the Indian short-nosed fruit bat, Cynopterus sphinx . J Comp Physiol A 196, 155–164 (2010). https://doi.org/10.1007/s00359-009-0502-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-009-0502-z