Abstract
This study was designed to test whether Cynopterus sphinx distress calls influence olfactory learning and memory in conspecifics. Bats were exposed to distress calls/playbacks (PBs) of distress calls/modified calls and were then trained to novel odors. Bats exposed to distress calls/PBs made significantly fewer feeding attempts and bouts of PBs exposed to modified calls, which significantly induced the expression of c-Fos in the caudomedial neostriatum (NCM) and the amygdala compared to bats exposed to modified calls and trained controls. However, the expression of c-Fos in the hippocampus was not significantly different between the experimental groups. Further, protein phosphatase-1 (PP-1) expression was significantly lower, and the expression levels of E1A homologue of CREB-binding protein (CBP) (P300), brain-derived neurotrophic factor (BDNF) and its tyrosine kinase B1 (TrkB1) receptor were significantly higher in the hippocampus of control/bats exposed to modified calls compared to distress calls/PBs of distress call-exposed bats. Exposure to the call possibly alters the reciprocal interaction between the amygdala and the hippocampus, accordingly regulating the expression levels of PP1, P300 and BDNF and its receptor TrkB1 following training to the novel odor. Thus, the learning and memory consolidation processes were disrupted and showed fewer feeding attempts and bouts. This model may be helpful for understanding the contributions of stressful social communications to human disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acoustic communication plays a significant role in information exchange between conspecifics (Fenton 2003; Gladziola et al. 2012; Hörmann et al. 2020). Conspecific communications are discrete acoustic structures that readily discriminate and transmit the intentional state of emitters to potential receivers, termed “social calls”. Earlier studies reported that social calls’ acoustic structures are context-specific, such as mother–pup reunion (Knörnschild et al. 2013), foraging coordination (Wright et al. 2014), group recognition (Budenz et al. 2009), mate attraction (Knörnschild et al. 2014) and distress (Huang et al. 2015). Distress calls are produced during stressful situations such as extreme physical stress (Russ et al. 2005; Carter et al. 2015; Walter and Schnitzler 2019) or when being threatened/attacked by a predator (Lima and Ó Keefe 2013; Huang et al. 2015). The distress call structure is often in the range of low-frequency, high-intensity broadband and is very similar among species, manifesting as “repeated sequences” (Luo et al. 2013; Hörmann et al. 2020). Earlier studies showed that the amygdala plays a central role in neural circuits involved in social vocalization and response (Naumann and Kanwal 2011; Tressler et al. 2011; Gadziola et al. 2012). It is well known that the amygdala receives intrinsically rewarding and aversive input stimuli, which may be associated with acoustic features within calls and context or experience (Ma et al. 2010; Sengupta et al. 2018).
Earlier studies demonstrated that olfactory information is transferred from the olfactory bulb to the amygdala and the hippocampus (Wilson et al. 2004). Depending on the context, olfactory learning activates a signalling cascade through protein kinase A (PKA), extracellular-signal-regulated kinase-1/2 (ERK-1/2), and cyclic AMP response element binding protein-1 (CREB-1) (Peng et al. 2010; Ganesh et al. 2010a). Activated CREB requires CBP/CREB-binding protein (CBP) and its homologue E1A binding protein (p300) to induce a network of genes that regulate learning and memory. Studies have shown that p300 acetylates lysine residues on histones and enhances memory formation (Maddox et al. 2013). Conversely, serine/threonine (Ser/Thr) protein phosphatases (PPs: PP1, PP2A) dephosphorylate CREB to suppress memory formation (Koshibu et al. 2009; Mauna et al. 2011). Activated CREB influences cellular processes and enhances synaptic plasticity (Minatohara et al. 2016) through the induction of C-fos. Training-dependent expression of C-fos in the amygdala and the hippocampus has been reported as an indication of memory formation and leads to the transcription of late-response genes (Luscher Dias et al. 2016; Minatohara et al. 2016; Mukilan et al. 2018). Furthermore, activation/inhibition of brain-derived neurotrophic factor (BDNF) and its tyrosine kinase B1 (TrkB1) receptor has been known to influence long-term potentiation (LTP), which is likely to contribute to synaptic plasticity, learning and memory (de Deus et al. 2020).
The Indian greater short-nosed fruit bat Cynopterus sphinx feeds on a variety of fruits, flowers and leaves, and they can identify and learn about palatability based on compounds originating from food sources (Elangovan et al. 2006). Earlier studies from my laboratory showed that C. sphinx produces multiharmonic audible distress calls as a narrowband arched frequency modulation (nAFM) and a broadband arched frequency modulation (bAFM). The specific structural sequence of the call elicits a behavioural response and alters the levels of stress hormones, neurotransmitters and other molecules involved in the stress response in conspecifics (Ganesh et al. 2010b; Mariappan et al. 2013, 2016). The present study was designed to test whether olfactory learning and memory were influenced by a distress call of conspecifics. To test the hypothesis, individuals were exposed to the distress call/PB of the distress call/modified call and were then trained to a novel odor to test their learning ability. Subsequently, the expression patterns of c-Fos in the caudomedial neostriatum (NCM) region of the auditory nuclei, the amygdala and the expression levels of PP1, P300, BDNF and its receptor TrkB1 in the hippocampus were examined.
Materials and methods
Animals
Male Cynopterus sphinx (forearm length 65 ± 7 mm, body mass 54.3 ± 7.0 g) was captured using a mist net (9 m × 2 m; Avinet-Dryden, USA) in a guava orchard located 2 km away from the Bharathidasan University Campus, Tiruchirappalli, India (10° 16′ N; 78° 15′ E). Bat’s morphometric details were recorded and tagged with plastic neck collars consisting of light-reflective colored tapes (Rajan and Marimuthu 1999) and then transferred to the animal house facility. Bats were maintained in the free-flight chamber (of 2.2 × 1.3 × 2.1 m) under constant conditions (temperature 30 °C ± 3 °C, relative humidity 85 ± 3%; light:dark 12 h:12 h) at animal house facility and allowed to acclimatize for 5 days. Bats were feed with the commonly available fruits [sapota (Achras sapota), papaya (Carica papaya), banana (Musa paradisiaca) and guava (Psidium guajava)] and water ad libitum. The health status of animals was assessed by inspecting fur, bite wounds and infections. Bats that were inactive and declining to eat during the study were excluded and released at their capture site.
Behavioural analysis
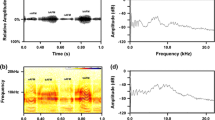
Bats (n = 6 for each group) were randomly classified into four groups; (1) control (CON); (2) distress call group—bats were exposed to the distress call of conspecifics; (3) playback (PB) of distress call—bats were exposed to playback of distress call and (4) modified call—bats were exposed to PB of modified call. To test whether responses were specific to the distress call, the original distress call as well as the call structure altered by changing the call sequence “modified call” (Mariappan et al. 2016) was used in this study. The distress call was composed of a specific sequence of two types of syllables [narrow band arched frequency modulation (nAFM: 123.33 ± 1.5 ms; 5.75 ± 0.1 kHz) and broadband arched frequency modulation (bAFM: 203.23 ± 2.9 ms; 7.27 ± 0.01 kHz)] alternatively. In the modified call, the sequence of syllables was changed to: nAFM, nAFM, bAFM, and bAFM. Distress and modified calls (Figs. 1, 2) used in this experiment were reported earlier from my laboratory (Mariappan et al. 2016).
Free-ranging C. sphinx distress call after becoming trapped in a mist net. a Oscillogram of a continuous distress call, with narrowband arched frequency modulation (nAFM) and broadband arched frequency modulation (bAFM) fused together and alternating continuously. b Spectrogram of continuous distress calls indicating harmonics. c Relative power spectrum depicting peak frequencies of the distress call
The modified distress call of C. sphinx shows variation in a the oscillogram, with nAFM and nAFM fused together and then bAFM and bAFM alternating continuously. b Spectrogram of modified calls indicating altered harmonics. c Relative power spectrum depicting peak frequencies of the modified distress call
Playback experiment
The experimental facility consists of two chambers: (1) free-flight chamber (2.2 m × 1.3 m × 2.1 m), (2) experimental chamber (2.1 m × 2.4 m × 2.4 m), and the window between chambers facilitates to transfer the bats without disturbances. An hour before the experiment bats were transferred from the free-flight chamber to experimental chamber. Then, distress calls or modified calls were played back (20 s/day) 15 min before the olfactory training (Mariappan et al. 2016). All the experiments were performed under red light (0.09 ± 0.02 lx) to minimize the visual cue (Shafie et al. 2014).
Olfactory training
Bats were individually trained to the novel odor [pieces of chopped apple mixed with freshly prepared cinnamon powder (0.8% wt/wt)] (Ratcliffe and ter Hofstede 2005) and their activities were recorded using a computerized-activity monitor (Electronic Engineering Corporation Inc., India). The activity monitor consists of an infra-red (IR) receiver–transmitter and a mass-sensitive platform (food tray). Their olfactory learning was tested by proving control fruit (fresh pieces of chopped apple) on one platform and fruit with novel odor in the other platform. The constant distance (1.8 m) was maintained between two platforms, as wells between the platforms to perch and location of platforms were changed randomly every day to prevent spatial learning. After acclimatization, bats were individually trained to the novel odor for 5 days (20 min/day), rested for 5 days and then tested their memory for 5 days. Individual bat’s responses to novel odor in terms of: (1) flights out—short flights from the perch (indicating bats are active); (2) attempts—approaches to the food tray (novel odor), but returning without the piece of fruit being picked up; (3) feeding bouts—landings on the food tray and returning with the piece of fruit (Ganesh et al. 2010a, b; Mukilan et al. 2018).
Sample preparation, RNA isolation, and cDNA synthesis
Animals (n = 4 from each group) were euthanized and the whole brain was dissected out. The caudomedial neostriatum (NCM), amygdala and hippocampal region were dissected as described elsewhere (Kalin et al. 1994), then bisected for preparation of total RNA and protein. Total RNA was isolated using TRIzol (Merck Specialties Pvt. Ltd., Mumbai, India) and stored with RNase inhibitor (GeNei™; Merck Specialties Pvt. Ltd.) at − 80 °C. Total RNA (2.0 µg/sample) was reverse transcribed into cDNA (I Script Reverse Transcription Kit; Bio-Rad Laboratories, Hercules, CA) in accordance with the manufacturer’s instructions.
Quantitative real-time PCR
The quantitative real-time PCR (qRT-PCR) reactions were performed using the reaction mixure (20 µL; SSoAdvanced™ SYBR® green supermix; Bio-Rad Laboratories) with specific primers (10 µM) and cDNA (0.1 µg). The specific primers used were: TrkB1 (For 5′-CCAAGAGGCTAAATCCAGTCC-3′ and Rev 5′-CCAGGTTACCAACATGCTAATA-3′); and GAPDH (glyceraldehydes-3-phosphate dehydrogenase; For 5′-CGGGAAGCTCA CTGGCATGG-3′ and Rev 5′-CCTGCTTCACCACCTTCTTG-3′). The qRT-PCR reactions were performed using the CFX-96 Touch™ Real-time PCR Detection System (Bio-Rad Laboratories Inc.) following the conditions: initial denaturation (92 °C, 30 S); denaturation (92 °C, 5 S), annealing (TrkB1: 61 °C; GAPDH: 58 °C for 5 S), extension (72 °C, 5 S), with 39 cycle repeats and melt-curve analysis (65–95 °C, 0.5 °C increment for 0:05 S). Reactions were performed in triplicates with three fold serial dilution of cDNA to verify the consistency and then normalized with the internal control GAPDH. The data are presented as mean fold change of the relative expression (CFX Manager™ version 2 software, CFX-96 Touch™ Real-time PCR Detection System; Bio-Rad Laboratories Inc).
Western blotting
Samples were homogenised in ice-cold lysis buffer (150 mM NaCl, 50 mM Tris–Hcl pH 7.5, 5 mM EDTA, 0.1% V/V NP-40, 1.0 mM DTT, 0.2 mM sodium orthovanadate, 0.23 mM PMSF) with protease inhibitor cocktail (Sigma-Aldrich, USA). Subsequently, homogenates were incubated on ice for 30 min and then centrifuged (10,000×g) for 30 min at 4 °C. Finally, clear supernatants were collected by centrifuged at 12,000×g for 15 min at 4 °C, and then stored at − 80 °C as aliquots. Protein concentration was estimated using a Biophotometer (Eppendorf Inc., Germany). An equal concentration of protein (60 μg) was mixed with a loading buffer (100% glycerol, 125 mM Tris–HCL pH 6.8, 4% SDS, 0.006% bromophenol blue, 2% mercaptoethanol) and boiled for 5 min, then resolved on 10% polyacrylamide gel (PAGE). Separated proteins were transferred electrophoretically onto the polyvinylidenedifluoride (PVDF) membrane using Turbo-Mini PVDF Transfer Packs (Cat #1704156, Bio-Rad Laboratories Inc, USA) with Trans-Blot® Turbo Transfer System (Cat # 1704150; -Rad Laboratories Inc, USA). The membranes were then pre-blocked with Tris-buffered saline [(10 mM Tris-base pH-7.5, 150 mM Nacl (TBS) containing non-fat dried milk (5.0%) and Tween-20 (0.1%)] for 3 h at room temperature. Membranes were incubated with the any one of the primary antibody, rabbit monoclonal anti-C-fos (CST—Cat # 4384, 1:2000)/rabbit monoclonal anti-phospho-C-fos (Ser 32) (CST—Cat # 5348, 1:2000)/rabbit monoclonal anti-PP-1α (CST—Cat # 2582, 1:2000)/rabbit polyclonal anti-p300(SC-584; 1:500)/rabbit polyclonal mature anti-BDNF [SC-546 (N-20); 1:500] or rabbit polyclonal anti-β-actin (SC-130656; 1:2000) antibody. The membrane was washed with 1X TBS-T and bound antibodies were detected by incubating for 4 h with goat anti-rabbit (MERK Cat # 62110080011730; 1:2000) alkaline phosphatase (ALP) conjugated antibody. The membrane was washed with 1X TBS-T, and ALP activity was detected with 5-bromo-4-chloro-3-indolylphosphate disodium salt (BCIP)/nitro-blue tetrazoliumchloride (NBT) (AP conjugate substrate kit Cat # 1706432, Bio-Rad Laboratories Inc, USA). The western blot images were acquired using Image Lab 2 software (Molecular Imager ChemiDoc XRS system, Bio-Rad Laboratories, Inc, USA), then the trace quantity of each band was measured and normalized with β-actin.
Statistical analysis
The significant difference was tested using two-way ANOVA for behavioural data and one-way ANOVA for gene expression data and subsequently Bonferroni post hoc test was performed [Sigma Stat software (Ver 22.0)]. Data were presented as a mean ± standard error of the mean (SEM), and plotted with KyPlot (Ver 5.0).
Results
Exposure to conspecific distress calls can impair olfactory learning and memory
The observed behavioural response provided evidence that the distress call affects novel odor learning in C. sphinx (Fig. 3 During olfactory learning, the C. sphinx behavioural response to the novel odor differed significantly between groups (F4,71 = 82.86; P < 0.001) and between behavioural responses (F2,71 = 87.18; P < 0.001), as did the interaction between group × behavioural response (F8,71 = 9.26; P < 0.001). Bonferroni post hoc comparisons revealed significant differences between groups in feeding attempts (P < 0.001) and feeding bouts (P < 0.001), but not in out-flies (P = 0.84) (Fig. 3a).
The specific structure of distress calls influences the olfactory learning and memory of C. sphinx conspecifics. Behavioural responses of C. sphinx to the novel odour during a training and b retention tests. Data are shown as the mean ± SEM, *indicates a significant difference (***P 0.001) with respect to comparisons within groups (a Con vs Distress call; b Con vs PB distress call; c modified call vs PB distress call; d modified call vs distress call)
Similarly, during testing, bat responses to the novel odour were significantly different between groups (F4,71 = 91.62; P < 0.001) and between their behavioural responses (F2,71 = 78.92; P < 0.001), as was the interaction between group × behavioural response (F8,71 = 11.42; P < 0.001). A post hoc test revealed significant differences between groups in feeding attempts (P < 0.001) and feeding bouts (P < 0.001) but no difference between groups in out-flies (P = 1.000) (Fig. 3b). The observed behavioural data demonstrate that the specific structure of the distress call possibly suppresses novel odour learning.
Exposure to conspecific distress calls promotes c-Fos expression in caudomedial neostriatum (NCM)
Expression of c-Fos in the NCM region in the experimental groups was detected, as shown in Fig. 4a. There was a significant difference in the c-Fos expression level between groups (F3,15 = 246.12; P < 0.001). In addition, post hoc analysis revealed that the levels of c-Fos were significantly higher in bats exposed to distress (P < 0.001) and PB calls (P < 0.001) than in the control group, but there was no difference between the control and bats exposed to modified calls (P = 0.87) (Fig. 4b). Similarly, the c-Fos expression levels were significantly higher in bats exposed to distress calls (P < 0.001) and PB of distress calls (P < 0.001) than in the group exposed to modified calls. The observed c-Fos expression in the NCM of conspecifics suggests that exposure to distress/PB of distress possibly activates neuron in the NCM region.
The specific structure of the distress call promotes c-Fos expression in the caudomedial neostriatum (NCM) of C. sphinx. Bats were allowed to listen to the distress call/PB of the distress call/modified call and were trained to a novel odour after 15 min. a Representative western blots showing the expression patterns of c-Fos in the NCM regions of experimental groups. Estimated levels of b c-Fos expression showing significant differences (*P < 0.05; ***P < 0.001) between groups (a Con vs Distress call; b Con vs PB distress call; c distress call vs modified call; d PB distress calls vs modified call; e Con vs Modified call)
Exposure to conspecific distress calls promotes c-Fos expression in the amygdala
As shown in Fig. 5a, the level of c-Fos expression in the amygdala differed significantly between the experimental groups (F3,15 = 120.88; P < 0.001). The post hoc analysis showed that the c-Fos expression levels were significantly higher in bats exposed to distress calls (P < 0.001) and PB of distress calls (P < 0.001), but there was no significant difference between the control and bats exposed to modified calls (P = 0.246). Interestingly, when compared with the modified call, the level of c-Fos expression was significantly higher in the distress call- (P < 0.001)- and the PB of distress call (P < 0.001)-exposed groups (Fig. 5b). The observed expression pattern of c-Fos in the amygdala region suggests that the call structure specifically activates the amygdala.
Conspecific distress calls with specific structures induce the expression of c-Fos in the amygdala of C. sphinx. Bats were allowed to listen to the distress call/PB of the distress call/modified call and were trained to a novel odour after 15 min. a Representative western blots showing the expression patterns of c-Fos in the amygdala in the experimental groups. Estimated levels of b c-Fos expression showing significant differences (*P < 0.05; ***P < 0.001) between groups (a Con vs distress call; b Con vs PB distress call; c distress call vs modified call; d PB distress calls vs modified call; e Con vs modified call)
Exposure to conspecific distress calls induces c-Fos expression in the hippocampus
Further analysis showed that the level of c-Fos expression (Fig. 6a) in the hippocampus was not significantly different between the groups (F3,15 = 3.12; P = 0.076). The results of the post hoc analysis demonstrated that there was no significant difference in c-Fos between the control and bats exposed to modified calls (P = 0.781). In comparison, there was no significant difference after the bats were exposed to distress calls (P = 0.12) or PB of distress calls (P = 0.23) compared with the trained group. Similarly, estimated c-Fos expression levels in bats exposed to a distress call (P = 0.46) or the PB of a distress call (P = 0.48) were not significantly different from bats exposed to modified calls (Fig. 6b). This observation demonstrates that a specific call structure differentially induces c-Fos expression in the hippocampus; however, the observed differences in expression were not significantly different.
The specific structure of the distress call induces the expression of c-Fos in the hippocampus of C. sphinx. Bats were allowed to listen to the distress call/PB of the distress call/modified calls and were trained to a novel odour after 15 min. a Representative western blots showing the expression patterns of c-Fos in the hippocampus of the experimental groups. Estimated levels of b c-Fos expression showing no significant difference in any comparison between groups
Exposure to conspecific distress calls selectively regulates protein phosphatase-1 (PP-1) and p300 expression in the hippocampus
A contrasting pattern of PP-1 and p300 expression was observed between the groups (Fig. 7a). The estimated level of PP1 reached significance with respect to the groups (F3,15 = 285.48; P < 0.01). The post hoc analysis also demonstrated that PP-1 expression was significantly higher after the bats were exposed to distress calls (P < 0.001) and PBs of distress calls (P < 0.001) than after the bats were exposed to the trained control group. In comparison, in the modified call-exposed group, the PP-1 expression levels were significantly higher in both the distress call (P < 0.001)- and the PB of the distress call (P < 0.001)-exposed groups, but there was no significant difference between the control and modified call-exposed groups (P = 0.084) (Fig. 7b). In parallel, the estimated expression levels of p300 were significantly different between groups (F3,15 = 132.46; P < 0.001). Interestingly, post hoc analysis showed no significant difference between the control and modified call-exposed groups (P = 0.652). In comparison, p300 levels were significantly lower in the distress call (P < 0.001) and the PB of the distress call (P < 0.001) groups compared to the control group. Similarly, when compared with the modified distress call group, the p300 levels were significantly lower in the distress call (P < 0.001) and the PB of the distress call (P < 0.001) groups (Fig. 7c). Evidently, PP1 and p300 were differentially altered in the hippocampus of conspecifics by the specific structure of the distress call.
Conspecific distress calls with specific structures differentially regulate the expression levels of PP1/P300 in the hippocampus of C. sphinx. Bats were allowed to listen to the distress call/PB of the distress call/modified call and were trained to a novel odour after 15 min. a Representative western blots showing the expression patterns of PP1 and P300 in the hippocampus of the experimental groups. Estimated levels of b PP1 and c P300 showing contrasting patterns of expression and significant differences (***P < 0.001) between groups (a Con vs Distress call; b Con vs PB distress call; c distress call vs modified call; d PB distress calls vs modified call; e Con vs modified call)
Exposure to conspecific distress calls suppresses the novel odour training-induced expression of brain-derived neurotrophic factor (BDNF) and its receptor tyrosine kinase (Trk) B1 in the hippocampus
Further, the BDNF expression (Fig. 8a) levels were significantly different between the groups (F3,15 = 305.57; P < 0.001). Post hoc analysis revealed that the BDNF levels in the distress call (P < 0.001)- and the PB of the distress call (P < 0.001)-exposed groups were significantly lower than that of the control group. Furthermore, the observed differences were significantly lower in bats exposed to distress calls (P < 0.001) and the PB of distress calls (P < 0.001) compared to the modified call-exposed group. However, the expression was not significantly different between the control and modified call-exposed groups (P = 0.068) (Fig. 8b). Expression of its receptor TrkB1, which responds to the activation of BDNF, was also significantly different between the groups (F3,15 = 2 86.17; P < 0.001). Interestingly, post hoc analysis revealed significantly lower expression in individuals exposed to distress calls (P < 0.001) and PB distress calls (P < 0.001) than in the control group. Similarly, the expression of TrkB1 levels in bats exposed to distress calls (P < 0.001) and PB distress calls (P < 0.001) were significantly lower than that in the modified call-exposed group. However, the difference in expression between the control and modified call-exposed groups reached significance (P < 0.001) (Fig. 8c). The expression pattern of BDNF in the experimental groups showed that different call structures differently activated the expression of BDNF and its receptor.
C. Sphinx distress call with a specific structure differentially regulates the expression of BDNF and its receptor TrkB1 in the hippocampus of conspecifics. Bats were allowed to listen to the distress call/PB of the distress call/modified call and were trained to a novel odour after 15 min. a Representative western blots showing the expression patterns of BDNF in the hippocampus of the experimental groups. Estimated levels of b BDNF and c the mRNA levels of TrkB1, showing significant differences (***P < 0.001) between groups (a Con vs distress call; b Con vs PB distress call; c distress call vs modified call; d PB distress calls vs modified call; e Con vs modified call)
Schematic representation of the intracellular signalling molecules (Egr-1—early growth response 1 gene; TOE 1—target of Egr 1; 5-Hydroxytryptamine (5-HT-serotonin); ACTH—adrenocorticotropic hormone; CORT—corticosterone; DA—dopamine; DAT—dopamine transporter; GR—glucocorticoid receptor; SRC-1—steroid receptor co-activator; D1DR—dopamine receptor; Nurr-1—nuclear receptor-related factor-1; TH—tyrosine hydroxylase; PP1α—protein phosphatase 1; BDNF—brain-derived neurotrophic factor; TrkB—tropomyosin receptor kinase B) altered by exposure to the distress call or the modified distress call. Observed changes in this study marked with * green arrows indicate the effects of modified calls, and red arrows indicate the effects of distress calls
Discussion
Earlier studies from my laboratory demonstrated that C. sphinx produces distress calls, which trigger call-specific changes in the behavioural, physiological/autonomic state of the receiver (Ganesh et al. 2010b; Mariappan et al. 2013, 2016). The distress call of C. sphinx could be a unique syllabi possibly emitted with a distinct temporal pattern (Bohn et al. 2008), could be context-specific and could encode an emotional state (Pfalzer and Kusch 2003; Carter et al. 2015; Walter and Schnitzler 2019). This study was designed to test whether exposure to conspecific distress calls affects olfactory learning in C. sphinx.
Conspecific distress call and olfactory learning
After the bats were exposed to the conspecific distress call/PB of the distress call, they made few feeding attempts and feeding bouts compared to the control/bats exposed to the modified call. Distress calls are known to induce neural responses in the auditory cortex (Martin et al. 2017), amygdala (Gadziola et al. 2016) and hypothalamic–pituitary–adrenal (HPA) axis (Mariappan et al. 2013), and share functional similarities with a human’s fearful scream (Hechavarria et al. 2020). Neuronal circuits processing social calls may shape the receptive field selectivity, plasticity and temporal firing pattern (Naumann and Kanwal 2011; Peterson and Wenstrup 2012), possibly due to the contribution of amygdala circuits in aversive or appetitive learning (Namburi et al. 2015; Beyeler et al. 2016). The reconciliation of amygdala activity also depends on the experience, i.e., aversive sound and angry prosody (Andics et al. 2010; Leitman et al. 2010), and raises the possibility that distress call-induced activation may be linked with threatened stimuli-mediated activation of the amygdala associated with aggression or fear (Peterson and Wenstrup 2012; Michael 2019), anxiety-like disorder in humans or social communications with negative emotions (Erkin and Wagen 2007; Sengupta et al. 2018). Therefore, the individuals exposed to distress calls/PBs of distress calls showed fewer feeding attempts and bouts to the novel odour during training and testing. The call sequence of the modified call may be within the acoustic range of C. sphinx and activate different populations of neurons in the amygdala (Gadziola et al. 2012) but may not encode stressful information (Mariappan et al. 2016). Thus, bats exposed to modified calls learned to the novel odour and responded during retention.
Distress call-specific syllable sequence induces expression of c-Fos in auditory circuit
The activity-dependent expression of c-Fos is the most commonly used marker to measure neuronal activity, and c-Fos immunoreactivity is elevated in distinct brain regions paired with vocalization or receiving vocal signals compared with silent bats (Ganesh et al. 2010b; Schwart and Smotherman 2011). In this study, a higher level of c-Fos expression was noted in the NCM of bats exposed to distress/PB distress calls compared to modified calls. This can be interpreted as showing that the specific structure of the distress call/PB of the distress call might promote the induction of c-Fos in the NCM region, as in other animal models (Monbureau et al. 2015), and the expression of c-Fos has been shown to play key roles in synaptic plasticity, learning and memory (Luscher Dias et al. 2016; Mukilan et al. 2018; Kanemoto et al. 2020). Further exposure to distress calls/PBs of distress calls increased c-Fos expression compared to the modified call group. This is possibly due to the specific feature of a distress call that activates auditory cortex neurons and demonstrates experience-dependent plasticity and learning in other bat species (de Hoz et al. 2018; Hörpel and Firzlaff 2019), other animal models and humans (Kanwal and Rauschecker 2007; Behler and Uppenkamp 2020). Earlier studies in other animal models and in humans demonstrated that in the amygdala receiving auditory input (Sander and Scheich 2001), a stressful auditory signal induces the expression of c-Fos in the amygdala and develops fear memory associated with the auditory signal (Nauman and Kanwal 2011; Chaaya et al. 2019). It is well known that amygdala neurons respond to social calls selectively, and their activity is positively correlated with social interactions (Katayama et al. 2009), including fear-related signals in bats (Nauman and Kanwal 2011) and humans (Whalen et al. 2004). In other models, multimodal brain cluster analysis suggest that movement (flight, vocalization, body movement) associated motor control circuits and auditory associated motor control circuits are under general cerebral motor system. However, the activation of movement associated motor circuits did not induce IEGs expression in the hippocampus, and higher auditory brain regions (Nelson 1996; Feenders et al. 2008). Therefore, observed elevated level of c-Fos expression in NCM and amygdala is possibly due to auditory input, which activates emotional reactivity, fear learning and memory circuits embedded in the amygdala (Namburi et al. 2015; Beyeler et al. 2016) and humans (Wiethoff et al. 2009).
Distress call syllable sequences are specifically shifts expression of c-Foss, protein phosphatase-1 (PP-1) and p300 in amygdala, and hippocampus
Odor-associated stimuli are connected through multiple regions of the brain, and input from sensory neurons in the olfactory bulb transmits olfactory information to other brain regions, including the olfactory bulb, amygdala and hippocampus (Wilson et al. 2004; Sosulski et al. 2011). The amygdaloid complex is known to connect two sensory systems (i.e., auditory and olfactory) that are sensitive to stress, which can lead to impairments in learning and memory (Soudry et al. 2011; Kiyokawa et al. 2012). Distress calls induce neuronal activation in the amygdala, resulting in an increase in neuronal excitability that may recall the fear memory of C. sphinx associated with distress calls (Chattarji et al. 2015) or may suppress exploratory behaviour to the novel odor, possibly by inhibiting amygdala output (Colas-Zelin et al. 2012). Thus, the bats exposed to distress calls/PBs of distress calls showed fewer feeding attempts and bouts towards novel odors. However, the modified call acoustic properties are not similar to the conspecific distress call, are unfamiliar to the bats (Mariappan et al. 2016), and may not provide specific information to the receiver (Fallow et al. 2013). Supporting earlier observations, in this study, individuals exposed to the modified calls learned the novel odor and responded during retention.
The reciprocal interaction between the amygdala and the cortex/hippocampus has been known to alter behavioural metaplasticity by aversive/threatening stimuli (Schmidt et al. 2013; Saha et al. 2020). Therefore, the transcription of target genes was examined in the hippocampus. The training-induced expression of p300 was significantly lower in the hippocampus of bats exposed to distress calls/PBs of distress calls. However, p300 expression levels were elevated in control/bats exposed to modified calls after the bats were trained with novel odor, which suggests that p300 was activated by novel odour stimulus (Oliveira et al. 2011). This observation suggests that novel odor-induced stimuli play an agnostic role in p300 expression in the hippocampus and may further activate the transcription of target genes (Oliveira et al. 2011). Conversely, transcription of CREB-targeted genes is suppressed by protein phosphatases (PP1α, PP2A) and phosphorylation of CREB (Koshibu et al. 2009; Mauna et al. 2011), thereby reducing synaptic plasticity. In line with earlier reports, the level of PP1α in the hippocampus was significantly low in bats exposed to modified calls and similar to control bats, but not in bats exposed to distress calls/PBs of distress calls (Koshibu et al. 2009; Mauna et al. 2011). These results suggest that reduction of the PPs and elevated expression levels of CBP/P300 could be linked with bat learning and responses to the novel odor.
Distress call syllable-specific sequences regulates olfactory learning
Activity-dependent expression of BDNF and its tyrosine receptor kinase B1 (TrkB1) has been known to regulate synaptic plasticity, LTP and memory formation (Sakata et al. 2013; Zhong et al. 2016). In this study, the level of BDNF was significantly lower in bats exposed to distress calls/PBs of distress calls than in control bats/bats exposed to modified calls. The observed results suggest that exposure to the distress call/PB of the distress call activates the amygdala and possibly modifies amygdala activity and plasticity in other brain regions, including the hippocampus (Schmidt et al. 2013), which may alter metaplasticity. Thus, exposed bats showed few feeding attempts and bouts to novel odour during training and testing. Supporting this observation, earlier studies in other models reported that exposure to stress reduces BDNF expression in the hippocampus (Makhathini et al. 2017). Elevated levels of BDNF in the hippocampus after training with the novel odor in the control/modified call-exposed group could be correlated with a greater number of responses to the novel odor (Sakata et al. 2013; Tong et al. 2018). In addition, it has been demonstrated that elevated levels of BDNF in the hippocampus act through its high-affinity receptor TrkB1 to trigger synaptic plasticity/ long-term memory (Nasrallah et al. 2019). The expression level of TrkB1 was significantly higher in the trained control and the bats exposed to modified calls than in the distress call/PB of the distress call-exposed groups. The observed behaviours of the control/modified call-exposed groups are in line with earlier reports stating that overexpression/activation of TrkB1 in the hippocampus enhances learning and memory (Karpova et al. 2014; Nasrallah et al. 2019). Reduced levels of TrkB1 possibly disrupt synaptic plasticity; therefore, individuals exhibit very low feeding bouts to novel odors, which is similar to other animal models that have shown impaired learning and memory (Badowska-Szalewska et al. 2010; Ren et al. 2015). These results suggest that BDNF and TrkB1 expression may be tightly controlled by input from the amygdala, which may act as a master switch in learning and memory in different environmental stimuli and biological contexts.
Conclusion
Linking with other studies, distress calls are functionally similar to a human’s fearful screams, and the specific structure of the distress call activates the NCM and the amygdala of the receiver. The acoustic structure of the distress call/PB of the distress call may activate neuronal circuits involved in aggressive, fearful and affiliative behaviour embedded in the amygdala, which significantly induces c-Fos expression compared to bats exposed to modified calls. Interestingly, the reciprocal interaction between the amygdala and the cortex/hippocampus could be differentially regulated by the distress call/PB of the distress call/modified call, which further selectively drives the expression of PP1, CBP/P300 and BDNF and its receptor TrkB1 following training to the novel odor. Therefore, the exposure of C. sphinx to distress calls/PBs of distress calls influences conspecific olfactory learning and memory. Furthermore, this model can be used to understand the fear/threatened stimuli related to social communication-induced learning and memory disorders (Fig. 9).
Abbreviations
- ALP:
-
Alkaline phosphatase
- bAFM:
-
Broadband arched frequency modulation
- BCIP:
-
5-Bromo-4-chloro-3-indolylphosphate disodium
- BDNF:
-
Brain-derived neurotrophic factor
- CREB-1:
-
Cyclic AMP response element binding protein-1
- ERK-1/2:
-
Extracellular-signal-regulated kinase-1/2
- GAPDH:
-
Glyceraldehydes-3-phosphate dehydrogenase
- IEG:
-
Immediate early gene
- LTP:
-
Long-term potentiation
- nAFM:
-
Narrow band arched frequency modulation
- NBT:
-
Nitro-blue tetrazoliumchloride
- NCM:
-
Caudomedial neostriatum
- PBs:
-
Playbacks
- PKA:
-
Protein kinase A
- PP-1:
-
Protein phosphatase-1
- TrkB1:
-
Tyrosine kinase B1
References
Andics A, McQueen JM, Petersson KM, Gál V, Rudas G, Vidnyánszky Z (2010) Neural mechanisms for voice recognition. Neuroimage 52:1528–1540. https://doi.org/10.1016/j.neuroimage.2010.05.048
Badowska-Szalewska E, Spodnik E, Klejbor I, Morys J (2010) Effects of chronic forced swim stress on hippocampal brain-derived neutrophic factor (BDNF) and its receptor (TrkB) immunoreactive cells in juvenile and aged rats. Acta Neurobiol Exp (wars) 70:370–381
Behler O, Uppenkamp S (2020) Activation in human auditory cortex in relation to the loudness and unpleasantness of low-frequency and infrasound stimuli. PLoS ONE 15(2):e0229088. https://doi.org/10.1371/journal.pone.0229088
Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, Luck R, Wildes CP, Tye KM (2016) Divergent routing of positive and negative information from the amygdale during memory retrieval. Neuron 90:348–361. https://doi.org/10.1016/j.neuron.2016.03.004
Bohn KM, Schmidt-French B, Ma ST, Pollak GD (2008) Syllable acoustics, temporal patterns, and call composition vary with behavioural context in Mexican free-tailed bats. J Acoust Soc Am 124:1838–1848. https://doi.org/10.1121/1.2953314
Budenz T, Heib S, Kusch J (2009) Functions of bat social calls: the influence of local abundance, interspecific interactions and season on the production of pipistrelle (Pipistrellus pipistrellus) type D social calls. Acta Chiropterol 11:173–182. https://doi.org/10.3161/150811009X465794
Carter G, Schoeppler D, Manthey M, Knornschild M, Denzinger A (2015) Distress calls of a fast- flying bat (Molossus molossus) provoke inspection flights but not cooperative mobbing. PLoS ONE 10(9):e0136146. https://doi.org/10.1371/journal.pone.0136146
Chaaya N, Jacques A, Belmer A, Richard DJ, Bartlett SE, Battle AR, Johnson LR (2019) Localization of contextual and context removed auditory fear memory within the basolateral amygdala complex. Neuroscience 398:231–251. https://doi.org/10.1016/j.neuroscience.2018.12.004
Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM (2015) Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci 18:1364–1375. https://doi.org/10.1038/nn.4115
Colas-Zelin D, Light KR, Kolata S, Wass C, Denman-Brice A, Rios C, Szalk K, Matzel LD (2012) The imposition of, but not the propensity for, social subordination impairs exploratory behaviors and general cognitive abilities. Behav Brain Res 232:294–305. https://doi.org/10.1016/j.bbr.2012.04.017
de Deus JL, Amorim MR, Ribeiro AB, Barcellos-Filho PCG, Ceballos CC, Branco LGS, Cunha AOS, Leão RM (2020) Loss of brain-derived neurotrophic factor mediates inhibition of hippocampal long-term potentiation by high-intensity sound. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-020-00881-8
de Hoz L, Gierej D, Lioudyno V, Jaworski J, Blazejczyk M, Cruces-Solís H, Beroun A, Lebitko T, Nikolaev T, Knapska E, Nelken I, Kaczmarek L (2018) Blocking c-Fos expression reveals the role of auditory cortex plasticity in sound frequency discrimination learning. Cereb Cortex 28:1645–1655. https://doi.org/10.1093/cercor/bhx060
Elangovan V, Priya EYS, Marimuthu G (2006) Olfactory discrimination ability of the short nosed fruit bat Cynopterus sphinx. Acta Chiropterol 8:247–253. https://doi.org/10.3161/1733-5329
Erkin A, Wager TD (2007) Funtional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder and specific phobia. Am J Psychiatry 164:1476–1488. https://doi.org/10.1176/appi.ajp.2007.07030504
Fallow PM, Pitcher BJ, Magrath RD (2013) Alarming features: birds use specific acoustic properties to identify heterospecific alarm calls. Proc Biol Sci 280:20122539. https://doi.org/10.1098/rspb.2012.2539
Feenders G, Liedvogel M, Rivas M, Zapka M, Horita H, Hara E, Wada K, Mouritsen H, Jarvis ED (2008) Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS ONE 3(3):e1768. https://doi.org/10.1371/journal.pone.0001768
Fenton MB (2003) Eavesdropping on the echolocation and social calls of bats. Mamm Rev 33:193–204
Gadziola MA, Grimsley JMS, Faure PA, Wenstrup JJ (2012) Social vocalizations of big brown bats vary with behavioural context. PLoS ONE 7(9):e44550. https://doi.org/10.1371/journal.pone.0044550
Gadziola MA, Shanbhag SJ, Wenstrup JJ (2016) Two distinct representations of social vocalizations in the basolateral amygdala. J Neurophysiol 115:868–886. https://doi.org/10.1152/jn.00953.2015
Ganesh A, Bogdanowicz W, Haupt M, Marimuthu G, Rajan KE (2010a) Role of olfactory bulb serotonin in olfactory learning in the greater short-nosed fruit bat, Cynopterus sphinx (Chiroptera: Pteropodidae). Brain Res 1352:108–117. https://doi.org/10.1016/j.brainres.2010.06.058
Ganesh A, Raghuram H, Nathan PT, Marimuthu G, Rajan KE (2010b) Distress call-induced gene expression in the brain of the Indian short-nosed fruit bat, Cynopterus sphinx. J Comp Physiol A 196:155–164. https://doi.org/10.1007/s00359-009-0502-z
Hechavarría JC, Jerome Beetz M, García-Rosales F, Kössl M (2020) Bats distress vocalizations carry fast amplitude modulations that could represent an acoustic correlate of roughness. Sci Rep. https://doi.org/10.1038/s41598-020-64323-7
Hörmann D, Tschapka M, Rose A, Knörnschild M (2020) Distress calls of nectarivorous bats (Galssophaga soricina) encode individual and species identity. Bioacostics 30(3):253–271. https://doi.org/10.1080/09524622.2020.1720815
Hörpel SG, Firzlaff U (2019) Processing of fast amplitude modulations in bat auditory cortex matches communication call-specific sound features. J Neurophysiol 121:1501–1512. https://doi.org/10.1152/jn.00748.2018
Huang X, Kanwal JS, Jiang T, Long Z, Luo B, Yue X, Gu Y, Feng J (2015) Situational and age-dependent decision making during life threatening distress in Myotis macrodactylus. PLoS ONE 10(7):e0132817. https://doi.org/10.1371/journal.pone.0132817
Kalin NH, Takahashi LK, Chen FL (1994) Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res 656:182–186
Kanemoto M, Nakamura T, Sasahara M, Ichijo H (2020) Stress-related neuronal clusters in sublenticular extended amygdala of basal forebrain show individual differences of positions. Front Neural Circ 28(14):29. https://doi.org/10.3389/fncir.2020.00029
Kanwal JS, Rauschecker JP (2007) Auditory cortex of bats and primates: managing species-specific calls for social communication. Front Biosci 12:4621–4640. https://doi.org/10.2741/2413
Karpova NN, Lindholm JS, Kulesskaya N, Onishchenko N, Vahter M, Popova D, Ceccatelli S, Castrén E (2014) TrkB overexpression in mice buffers against memory deficits and depression-like behavior but not all anxiety- and stress-related symptoms induced by developmental exposure to methyl mercury. Front Behav Neurosci 8:315. https://doi.org/10.3389/fnbeh.2014.00315
Katayama T, Jodo E, Suzuki Y, Hoshino KY, Takeuchi S, Kayama Y (2009) Phencyclidine affects firing activity of basolateral amygdala neurons related to social behaviour in rats. Neuroscience 159:335–343. https://doi.org/10.1016/j.neuroscience.2009.01.002
Kiyokawa Y, Wakabayashi Y, Takeuchi Y, Mori Y (2012) The neural pathway underlying social buffering of conditioned fear responses in male rats. Eur J Neurosci 36:3429–3437. https://doi.org/10.1111/j.1460-9568.2012.08257.x
Knörnschild M, Feifel M, Kalko EKV (2013) Mother–offspring recognition in the bat Carollia perspicillata. Anim Behav 86:941–948. https://doi.org/10.1016/j.anbehav.2013.08.011
Knörnschild M, Feifel M, Kalko EKV (2014) Male courtship displays and vocal communication in the polygynous bat Carollia perspicillata. Behaviour 151:781–798. https://doi.org/10.1163/1568539X-00003171
Koshibu K, Gräff J, Beullens M, Heitz FD, Berchtold D, Russig H, Farinelli M, Bollen M, Mansuy IM (2009) Protein phosphatase 1 regulates the histone code for long-term memory. J Neurosci 29:13079–13089. https://doi.org/10.1523/JNEUROSCI.3610-09.2009
Leitman DI, Wolf DH, Ragland JD, Laukka P, Loughead J, Valdez JN, Javitt DC, Turetsky BI, Gur RC (2010) “It’s not what you say, but how you say it”: a reciprocal temporo-frontal network for affective prosody. Front Hum Neurosci 26(4):19. https://doi.org/10.3389/fnhum.2010.00019
Lima SL, Keefe JMO (2013) Do predators influence the behaviour of bats? Biol Rev 88:626–644. https://doi.org/10.1111/brv.12021
Luo B, Jiang T, Liu Y, Wang J, Lin A, Wei X, Feng J (2013) Brevity is prevalent in bat short-range communication. J Comp Physiol A 199:325–333. https://doi.org/10.1007/s00359-013-0793-y
Luscher Dias T, Fernandes Golino H, Mourade Oliveria VE, Dutra Moraes MF, Schenatto Pereira G (2016) c-Fos expression predicts long-term social memory retrieval in mice. Behav Brain Res 313:260–271. https://doi.org/10.1016/j.bbr.2016.07.030
Ma J, Nauman RT, Kanwal JS (2010) Fear conditioned discrimination of frequency modulated sweeps within species-specific calls of mustached bats. PLoS 5(5):e10579. https://doi.org/10.1371/journal.pone.0010579
Maddox SA, Watts CS, Schafe GE (2013) p300/CBP histone acetyltransferase activity is required for newly acquired and reactivated fear memories in the lateral amygdala. Learn Mem 20:109–119. https://doi.org/10.1101/lm.029157.112
Makhathini KB, Abboussi O, Stein DJ, Mabandla MV, Daniels WMU (2017) Repetitive stress leads to impaired cognitive function that is associated with DNA hypomethylation, reduced BDNF and a dysregulated HPA axis. Int J Dev Neurosci 60:63–69. https://doi.org/10.1016/j.ijdevneu.2017.04.004
Mariappan S, Bogdanowicz W, Marimuthu G, Rajan KE (2013) Distress calls of the greater short-nosed fruit bat Cynopterus sphinx activate hypothalamic-pituitary-adrenal (HPA) axis in conspecifics. J Comp Physiol A 199:775–783. https://doi.org/10.1007/s00359-013-0838-2
Mariappan S, Bogdanowicz W, Raghuram H, Marimuthu G, Rajan KE (2016) Structure of distress call: implication for specificity and activation of dopaminergic system. J Comp Physiol A 202:55–65. https://doi.org/10.1007/s00359-015-1053-0
Martin LM, García-Rosales F, Beetz MJ, Hechavarría JC (2017) Processing of temporally patterned sounds in the auditory cortex of Seba’s short-tailed bat, Carollia perspicillata. Eur J Neurosci 46:2365–2379. https://doi.org/10.1111/ejn.13702
Mauna JC, Miyamae T, Pulli B, Thiels E (2011) Protein phosphatases 1 and 2A are both required for long-term depression and associated dephosphorylation of cAMP response element binding protein in hippocampal area CA1 in vivo. Hippocampus 21:1093–1104. https://doi.org/10.1002/hipo.20823
Michael (2019) Spectral call features provide information about the aggression level of greater mouse-eared bats (Myotis myotis) during agonistic interactions. https://doi.org/10.1080/09524622.2017.1359798
Minatohara K, Akiyoshi M, Okuno H (2016) Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front Mol Neurosci 8:78. https://doi.org/10.3389/fnmol.2015.00078
Monbureau M, Barker JM, Leboucher G, Balthazart J (2015) Male song quality modulates c-Fos expression in the auditory forebrain of the female canary. Physiol Behav 147:7–15. https://doi.org/10.1016/j.physbeh.2015.04.005
Mukilan M, Bogdanowicz W, Marimuthu G, Rajan KE (2018) Odour discrimination learning in the Indian greater short-nosed fruit bat (Cynopterus sphinx): differential expression of Egr-1, C-fos and PP-1 in the olfactory bulb, amygdala and hippocampus. J Exp Biol 221(Pt 12):jeb175364. https://doi.org/10.1242/jeb.175364
Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, Wickersham IR, Gray JM, Tye KM (2015) A circuit mechanism for differentiating positive and negative associations. Nature 520:675–678. https://doi.org/10.1038/nature14366
Nasrallah P, Haidar EA, Stephan JS, El Hayek L, Karnib N, Khalifeh M, Barmo N, Jabre V, Houbeika R, Ghanem A, Nasser J, Zeeni N, Bassil M, Sleiman SF (2019) Branched-chain amino acids mediate resilience to chronic social defeat stress by activating BDNF/TRKB signaling. Neurobiol Stress 11:100170. https://doi.org/10.1016/j.ynstr.2019.100170
Naumann RT, Kanwal JS (2011) Basolateral amygdala responds robustly to social calls: spiking characteristics of single unit activity. J Neurophysiol 105:2389–2404. https://doi.org/10.1152/jn.00580.2010
Nelson RJ (1996) Interactions between motor commands and somatic perception in sensorimotor cortex. Curr Opin Neurobiol 6:801–810. https://doi.org/10.1016/s0959-4388(96)80031-6
Oliveira AM, Estévez MA, Hawk JD, Grimes S, Brindle PK, Abel T (2011) Subregion-specific p300 conditional knock-out mice exhibit long-term memory impairments. Learn Mem 18:161–169. https://doi.org/10.1101/lm.1939811
Peng S, Zhang Y, Zhang J, Wang H, Ren B (2010) ERK in learning and memory: a review of recent research. Int J Mol Sci 11:222–232. https://doi.org/10.3390/ijms11010222
Peterson DC, Wenstrup JJ (2012) Selectivity and persistent firing responses to social vocalizations in the basolateral amygdala. Neuroscience 217:154–171. https://doi.org/10.1016/j.neuroscience.2012.04.069
Pfalzer G, Kusch J (2003) Structure and variability of bat social calls: implications for specificity and individual recognition. J Zool Lond 261:21–33. https://doi.org/10.1017/S0952836903003935
Rajan KE, Marimuthu G (1999) Postnatal growth and age estimation in the Indian false vampire bat Megaderma lyra. J Zoo (london) 248:529–534. https://doi.org/10.1111/j.1469-7998.1999.tb01052.x
Ratcliffe JM, ter Hofstede HM (2005) Roosts as information centers: social learning and food preferences of bats. Biol Lett 1:72–74. https://doi.org/10.1098/rsbl.2004.0252
Ren Q, Ma M, Yang C, Zhang JC, Yao W, Hashimoto K (2015) BDNF-TrkB signalingin the nucleus accumbens shell of mice has key role in methamphetamine withdrawal symptoms. Transl Psychiatry 5:e666. https://doi.org/10.1038/tp.2015.157
Russ JM, Jones G, Racey PA (2005) Responses of soprano pipistrelles, Pipistrellus pygmaeus, to their experimentally modified distress calls. Anim Behav 70:397–404. https://doi.org/10.1016/j.anbehav.2004.11.006
Saha R, Kriebel M, Anunu R, Volkmer H, Richter-Levin G (2020) Intra-amygdala metaplasticity modulation of fear extinction learning. Eur J Neurosci. https://doi.org/10.1111/ejn.15080
Sakata K, Martinowich K, Woo NH, Schloesser RJ, Jimenez DV, Ji Y, Shen L, Lu B (2013) Role of activity-dependent BDNF expression in hippocampal-prefrontal cortical regulation of behavioral perseverance. Proc Natl Acad Sci USA 110:15103–15108. https://doi.org/10.1073/pnas.1222872110
Sander K, Scheich H (2001) Auditory perception of laughing and crying activates human amygdala regardless of attentional state. Brain Res Cogn Brain Res 12:181–198. https://doi.org/10.1016/s0926-6410(01)00045-3
Schmidt MV, Abraham WC, Maroun M, Stork O, Richter-Levin G (2013) Stress-induced metaplasticity: from synapses to behavior. Neuroscience 250:112–120. https://doi.org/10.1016/j.neuroscience.2013.06.059
Schwartz CP, Smotherman MS (2011) Mapping vocalization-related immediate early gene expression in echolocating bats. Behav Brain Res 224:358–368. https://doi.org/10.1016/j.bbr.2011.06.023
Sengupta A, Yau JOY, Jean-Richard-Dit-Bressel P, Liu Y, Millan EZ, Power JM, McNally GP (2018) Basolateral amygdala neurons maintain aversive emotional salience. J Neurosci 38:3001–3012. https://doi.org/10.1523/JNEUROSCI.2460-17.2017
Shafie NJ, Rahman NA, Sah SA, Rosely NF, Sufian M (2014) Feeding behaviour of Cynopterus sphinx (Pteropodidae) under captive conditions. Trop Life Sci Res 25:53–59
Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR (2011) Distinct representations of olfactory information in different cortical centres. Nature 472(7342):213–216. https://doi.org/10.1038/nature09868
Soudry Y, Lemogne C, Malinvaud D, Consoli SM, Bonfils P (2011) Olfactory system and emotion: common substrates. Eur Ann Otorhinolaryngol Head Neck Dis 128:18–23. https://doi.org/10.1016/j.anorl.2010.09.007
Tong MT, Kim TP, Cleland TA (2018) Kinase activity in the olfactory bulb is required for odor memory consolidation. Learn Mem 25:198–205. https://doi.org/10.1101/lm.046615.117
Tressler J, Schwartz C, Wellman P, Hughes S, Smotherman M (2011) Regulation of bat echolocation pulse acoustics by striatal dopamine. J Exp Biol 214:3238–3247. https://doi.org/10.1242/jeb.058149
Walter MH, Schnitzler HU (2019) Spectral call features provide information about the aggression level of greater mouse-eared bats (Myotis myotis) during agonistic interactions. Bioacoustics 28:1–25. https://doi.org/10.1080/09524622.1359798
Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, Johnstone T (2004) Human amygdala responsivity to masked fearful eye whites. Science 306(5704):2061. https://doi.org/10.1126/science.1103617
Wiethoff S, Wildgruber D, Grodd W, Ethofer T (2009) Response and habituation of the amygdala during processing of emotional prosody. NeuroReport 20:1356–1360. https://doi.org/10.1097/WNR.0b013e328330eb83
Wilson DA, Best AR, Sullivan RM (2004) Plasticity in the olfactory system: lessons for the neurobiology of memory. Neuroscientist 10:513–524. https://doi.org/10.1177/1073858404267048
Wright GS, Chiu C, Xian W, Wilkinson GS, Moss CF (2014) Social calls predict foraging success in big brown bats. Curr Biol 24:885–889. https://doi.org/10.1016/j.cub.2014.02.058
Zhong L, Luo F, Zhao W, Feng Y, Wu L, Lin J, Liu T, Wang S, You X, Zhang W (2016) Propofol exposure during late stages of pregnancy impairs learning and memory in rat offspring via the BDNF-TrkB signalling pathway. J Cell Mol Med 20:1920–1931. https://doi.org/10.1111/jcmm.12884
Acknowledgements
KER thank the anonymous reviewer for their suggestions that improved this manuscript. This Project is financially supported by Tamil Nadu State Council for Higher Education (TANSCHE) and Rashtriya Uchchatar Shiksha Abhiyan (RUSA) 2.0-Biological Sciences. The Department of Animal Science is supported by Department of Science and Technology (DST)-Fund for Improvement of S&T Infrastructure (FIST) and DST-Promotion of University Research and Scientific Excellence (PURSE). Experimental protocol used in this study was approved by Bharathidasan University Wild Animal Ethics Committee (03/AS/BUWAE/2008), which was in compliance with the laws in India. Experiments were designed to minimize the number of animals used.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajan, K.E. Olfactory learning and memory in the greater short-nosed fruit bat Cynopterus sphinx: the influence of conspecifics distress calls. J Comp Physiol A 207, 667–679 (2021). https://doi.org/10.1007/s00359-021-01505-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-021-01505-2