Abstract
Many insects possess smooth adhesive pads on their legs, which adhere by thin films of a two-phasic secretion. To understand the function of such fluid-based adhesive systems, we simultaneously measured adhesion, friction and contact area in single pads of stick insects (Carausius morosus). Shear stress was largely independent of normal force and increased with velocity, seemingly consistent with the viscosity-effect of a continuous fluid film. However, measurements of the remaining force 2 min after a sliding movement show that adhesive pads can sustain considerable static friction. Repeated sliding movements and multiple consecutive pull-offs to deplete adhesive secretion showed that on a smooth surface, friction and adhesion strongly increased with decreasing amount of fluid. In contrast, pull-off forces significantly decreased on a rough substrate. Thus, the secretion does not generally increase attachment but does so only on rough substrates, where it helps to maximize contact area. When slides were repeated at one position so that secretion could accumulate, sliding shear stress decreased but static friction remained clearly present. This suggests that static friction which is biologically important to prevent sliding is based on non-Newtonian properties of the adhesive emulsion rather than on a direct contact between the cuticle and the substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many insects are capable of running upside down on smooth surfaces, carrying loads and withstanding pull-off and shear forces equivalent to more than 100 times their own body weight (Gorb 2001; Federle et al. 2002). The detailed mechanisms of how this impressive performance is achieved and of how insects master the conflicting tasks of running and of making stable adhesive contacts are still not sufficiently understood.
Adhesive organs of insects are either “smooth” pads characterized by a specialized, soft cuticle (e.g., in ants and bees, cockroaches and grasshoppers), or they are “hairy”, i.e., densely covered with flexible adhesive setae, e.g., in flies, beetles and earwigs (Beutel and Gorb 2001). In the tarsal pads of both groups, adhesion is mediated by small volumes of fluid secreted into the contact zone (flies: Walker et al. 1985; bugs: Edwards and Tarkanian 1970; beetles: Ishii 1987). Recent findings show that insect pad secretion is an emulsion consisting of a hydrophilic and a hydrophobic phase (Gorb 2001; Federle et al. 2002; Vötsch et al. 2002). However, the detailed function of the two-phasic nature of the fluid is still unknown.
The presence of a liquid film sandwiched between the pad cuticle and the surface has given rise to models of “wet adhesion”, which explain the insects’ sticking ability by the capillarity and viscosity of the fluid film (Dewitz 1884; Stork 1980; Walker 1993; Walker et al. 1985; Lees and Hardie 1988; Langer et al. 2004). Experimental support for the adhesion-enhancing role of insect foot pad secretion was reported in two studies which investigated the effect of removing the fluid (Edwards and Tarkanian 1970; Dixon et al. 1990). A loss of adhesive function was observed after treating the pads with organic solvents (in Rhodnius prolixus: Edwards and Tarkanian 1970) or silica gel (in Aphis fabae: Dixon et al. 1990). However, both treatments may have dehydrated the soft pad cuticle, which by itself can lead to reduced adhesion (Jiao et al. 2000).
A variety of physical models predict the forces due to the capillarity and viscosity of a fluid film between two solid adherends for different geometries, e.g., two parallel flats, sphere on flat, flexible tape on flat (Israelachvili 1992; Francis and Horn 2001; Bhushan 2003; Piau et al. 2005). For all geometries, adhesive forces are predicted to be inversely proportional to the amount of fluid present in the contact zone (i.e., to fluid film thickness). Similarly, the shear resistance of a fluid film decreases with its thickness. Thus, the physical models predict that insects should actually maximize forces by secreting less fluid. Obviously, it is still unclear whether insect “adhesive” secretion increases or reduces pad attachment forces. What is the function of this fluid?
The simple wet adhesion model is particularly challenged by the insects’ capacity to sustain friction forces (Federle et al. 2002, 2004). A continuous Newtonian fluid film between the pad cuticle and the surface would lubricate the pad contact zone so that pads should always readily slide. However, the capacity to generate static and dynamic shear forces is essential for insect locomotion and maneuverability. By measuring the capacity of ants to sustain shear forces on a smooth turntable, we have recently shown that friction forces are indeed inconsistent with the assumption of a continuous Newtonian fluid film and concluded that a direct interaction of the pad with the surface is involved (Federle et al. 2002, 2004). However, the detailed nature of this interaction has remained unclear. Here we investigate the biomechanics of smooth adhesive pads by focusing on the friction of single pretarsal pads in stick insects. Our work differs from previous studies on insect shear forces (Gorb and Scherge 2000; Federle et al. 2004); in that we tested sliding for large amplitudes of movement and directly measured shear stress (i.e., friction force per unit contact area) under different experimental conditions. With these experiments, we address the following questions: (1) Can insect adhesive pads generate static friction? (2) How is the friction of adhesive pads influenced by load (normal force)? (3) How does shear stress depend on sliding velocity? (4) How does the fluid secretion influence adhesive and frictional forces?
Materials and methods
Study animals
Adult stick insects (Carausius morosus; body mass: 727 ± 145 mg; mean ± SD) were taken from a laboratory colony. To measure adhesive and frictional forces of pretarsal pads (“arolia”), stick insects were enclosed, by taking advantage of their typical stick-like camouflage position, in a hollow square metal tube so that either front or hind legs protruded from the end. Precise control of normal forces as well as pull-off and sliding movements required the adhesive pad to be largely immobilized. This was done by attaching the dorsal side of the pretarsus to a piece of firm solder wire by applying melted paraffin wax. To prevent the claws from touching the glass plate and contributing to the measured forces, the claw tips were clipped. The choice of front and hind leg was randomized; since neither forces nor shear stress differed significantly (Wilcoxon rank sum tests: friction: N = 35, P > 0.05, adhesion: N = 10, P > 0.05), data from both legs were pooled in all experiments. After each experiment the animals were weighed to the nearest milligram.
General setup
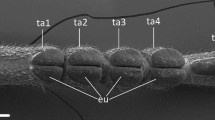
An overview of the experimental setup is shown in Fig. 1a. Forces were measured using a bending beam equipped with 350 Ω foil strain gauges (1-LY13-3/350, Vichay). A glass coverslip (12 mm × 12 mm × 0.1 mm) was attached to the distal end of the bending beam. The insect foot was brought into contact with the bottom side of the glass coverslip. The adhesive contact area was measured from above under reflected light using a stereo microscope equipped with a coaxial illuminator (Wild M3C, Leica). This method yields high contrast images of the pad contact zone showing it as a dark area on a bright background (Federle et al. 2002) as illustrated in Fig. 1. Contact areas were recorded at ten frames per second using an externally triggered Redlake PCI 1000 B/W high speed video camera mounted on a stereo microscope. Video analysis was performed offline with custom-made software using MATLAB (The Mathworks, USA). Force input channels were amplified (ME-Meßsysteme, Henningsdorf, Germany) with an I/O board (PCI-6035E, National Instruments, USA) with a sampling frequency of 100 Hz.
a Experimental setup for measuring adhesion and shear stress of insect adhesive pads. The arolium is brought into contact with a glass surface attached to a 2D bending beam force transducer for measuring friction and adhesion (i.e., normal forces). The bending beam is moved by a computer-controlled XYZ-translation stage. Forces in the normal direction can be adjusted via a feedback mechanism. Contact area is imaged from above using reflected light (see text for further details). b Scanning electron microscopy image of a stick insect pretarsus
To perform controlled movements, the bending beam was mounted on a computer-controlled 3-D DC positioning stage (M-126PD, C-843, Physik Instrumente, Germany). Motor movements, video trigger and force recording were synchronized by custom-made software using LabVIEW (National Instruments, USA). This software also enabled a normal force feedback mechanism which was used during all friction experiments (feedback frequency: 10 Hz).
Two types of force transducers were used: (1) Pull-off experiments were performed using a 1-D transducer consisting of two parallel bending beams (20 mm × 5 mm × 0.1 mm; 6 mm distance between the beams) equipped with foil strain gauges in a full bridge configuration, and a strutting at the distal end to which the glass coverslip was attached. Depending on the lever arm, the spring constant varied between 5 and 50 Nm−1; resonance frequency was 71 Hz. (2) To measure friction and adhesion forces, a 2-D bending beam was used (30 mm × 5 mm × 0.1 mm). To achieve an exact 90° angle between the two axes of the transducer, the bending beam was folded three times. Half bridges of foil strain gauges were mounted on both sections of the bending beam for the measurement of normal forces and friction (Fig. 1a). All experiments were conducted at temperatures between 21° and 28°C.
Friction force measurements
To measure friction, we performed sliding movements of stick insect arolia on the glass plate of the transducer. In all experiments, the transducer was moved in the distal direction, corresponding to a pull of the leg toward the body. As in adhesive pads of other insects, contact area and friction forces are maximal in this direction (bushcrickets: Gorb and Scherge 2000; flies: Niederegger et al. 2002; ants: Federle et al. 2001; Federle and Endlein 2004). Due to the flexibility of the pad (even in the immobilized condition, see above), relatively large movement amplitudes (4–10 mm) were required to ensure that pads did not remain in stationary contact and friction reached a plateau when pads were sliding steadily (see Fig. 5a). During the slides, normal force was kept constant by using a force feedback loop. Shear stress was calculated as the ratio of friction force to the simultaneously measured contact area.
“Little” versus “accumulated” secretion
To examine the influence of the amount of pad secretion between the arolium and the substrate during proximal sliding movements, two different conditions were compared: (1) “Accumulated secretion”: All slides were performed from the same starting point resulting in the attachment pad leaving behind more and more fluid on the surface. (2) “Little secretion”: Every repetition was performed on a “new” position, where the glass plate was still clean. Ten stick insects were tested under both conditions with seven repetitions. Sliding velocity was 500 μms−1 covering an amplitude of 10 mm; normal force was set to 1 mN. Humidity was kept above 80% using a humidifier (see below) to reduce possible effects due to evaporation of the hydrophilic part of the two-phasic secretion (Federle et al. 2002). The maximum friction force of each slide and its corresponding contact area in the video recording were used for further analysis.
Humidity effect
Observations using interference reflection microscopy have shown that the hydrophilic component of the secretion is volatile (Federle et al. 2002). To investigate the effect of fluid evaporation on friction forces, we performed a series of trials to compare “accumulated” secretion forces under high (> 80%) and low (< 30%) humidity. Paired high–low humidity experiments were conducted with less than 2 min time in between both conditions. Humidity was increased by an ultrasonic humidifier (Honeywell, BH-860 E) and enclosing the setup with transparent plastic foil. Five consecutive slides were performed with 500 μms−1 and 10 mm amplitude. The first and the last slides of each series were evaluated as “little” and “accumulated” secretion, respectively. We used the maximum friction force of each slide and its corresponding contact area. Eleven pads from three animals were tested once under both humidities (N = 11).
Normal force
We tested the influence of normal force on sliding friction by performing 4 mm sliding movements under four different feedback-controlled loads: 2, 1, 0.5 and − 0.1 mN. As insect adhesive pads are viscoelastic (Gorb et al. 2000), there is a strong loading–unloading hysteresis and for a given force, contact areas are smaller during loading than during unloading. To be able to include a negative load in this experiment, and to make conditions comparable with each other, all pads were preloaded with 2 mN for 1 s before being set to the desired normal force by unloading. The effect of normal forces was tested both for “little” and “accumulated” secretion. The maximum friction force of each slide and corresponding contact area were used for further analysis. Ten pads were tested twice under randomized orders of applied normal forces and pooled resulting in N = 20.
Velocity dependence
To examine the influence of different velocities four consecutive slides were performed with each slide conducted at a different velocity. The experiment was performed for “accumulated” secretion (see above). We used velocities of 20, 50, 100 and 250 μms−1. The order of velocities was randomized. Due to limitations of the recording time and the size of the glass plate used, different amplitudes had to be used for the four conditions (v 20,50,100: 4 mm, v 250: 10 mm). To make it possible to compare friction forces between the different velocities, forces were measured both after (1) an equal time of sliding (16 s) and (2) after an equal sliding distance (4 mm). Both types of data were used separately for further analysis.
Remaining friction
After a sliding movement (80% humidity, velocity 20 μms−1), the motor was stopped and the pad left in contact. We measured the remaining friction force of the pad 2 min after the end of the sliding movement.
Adhesion force: consecutive pull-offs
To investigate the effect of pad fluid on adhesive forces, we performed a series of nine consecutive pull-offs (to deplete secretion) from smooth glass and from a rough surface (Aluminium oxide polishing paper, Ultra Tec, USA, roughness average R a = 0.5 μm). Pull-offs for each pad were performed perpendicular to the surface, alternately from the smooth and the rough substrate (N = 10). Approach and detach velocity was 500 μms−1. After the pad had been brought into contact, a short proximal movement was performed (100 μms−1) followed by 2 s pause with the normal force set to 1 mN to ensure good contact between the arolium and the substrate. To accelerate the depletion of pad fluid, the pad was brought into contact with laboratory filter paper before each pull-off for 2 s with a normal force of 1 mN.
Statistics
All data were tested for the presence of normal distribution (Shapiro–Wilk test) and homogeneity of variances (Bartlett’s test). If both conditions were met, parametric tests were performed, otherwise their non-parametric equivalents. For comparisons between multiple groups analyses of variance (parametric) or Kruskal–Wallis tests (non-parametric) were used. Page’s non-parametric L test (Page 1963) was used to test for monotonic changes between groups (e.g., consecutive pull-offs). The indices of the L value (L m,n) denote “conditions” (m) and “number of samples” (n). Graphical representation in the form of boxplots comply with Chambers et al. (1983); outliers are not displayed. Data values given within the text are either medians in case of not normally distributed data or means with standard deviation in case of normally distributed data. All statistical analyses were done using R (R Development Core Team 2005).
Results
Friction force measurements
“Little” versus “accumulated” secretion
To evaluate the friction characteristics of adhesive pads (arolia) in stick insects (C. morosus), we performed long distance sliding movements of front and hind leg pads on glass. We found that friction and shear stress steadily increased in the course of each slide and only began to reach a plateau after approx. 15 s (corresponding to 7.5 mm distance covered, Fig. 2a). Friction force and shear stress were highly reproducible between consecutive slides when they were performed at new positions of the glass plate (“little secretion”, Fig. 2a). However, when the consecutive sliding movements were repeated at the same position, forces decreased from trial to trial in a highly regular pattern (“accumulated secretion”, Fig. 2b). Shear stress in the last slide amounted to only 32% of the first slide (medians: first slide 101 kPa, last slide 38 kPa). For “accumulated secretion”, the decrease of shear stress was highly significant (Fig. 2d; Page’s L test: L 9,10 = 1,037, P < 0.001), whereas no change was found for “little secretion” (Fig. 2c; ANOVA for the effect of slide number and individual pad; effect of slide number: F 1,7 = 0.284, P > 0.1).
Shear stress measurement in single adhesive pads of Carausius morosus. a Friction force in experiment consisting of seven consecutive long distance slides performed on a clean smooth glass plate (“little secretion”), b same as a, but consecutive slides performed at the same position (“accumulated secretion”); c, d shear stress data, pooled results from ten pads. Grayscales indicate identical slide numbers in all plots
Humidity effect
We could not confirm any effects of humidity on pad friction (Fig. 3). Friction forces and shear stress did not differ significantly between low (< 30%) and high (> 80%) humidity (Wilcoxon signed rank tests: P > 0.4 for both force and shear stress with “little” and “accumulated” secretion; N = 11).
Normal force
Both for “little” and “accumulated” secretion, normal forces had a significant influence on friction force and contact area (Fig. 4a, b, ANOVA; “little secretion”: Force: F 1,19 = 8.05, P < 0.01, Area: F 1,19 = 45.8, P < 0.001; “accumulated secretion”: Force F 1,19 = 74.9, P < 0.001, Area: F 1,19 = 106.9, P < 0.001). However, normal forces had no effect on shear stress for “little secretion” (Fig. 4c, ANOVA: F 1,19 = 0.09, P > 0.05). Only for “accumulated secretion” was shear stress dependent on normal force (Fig. 4c, ANOVA: F 1,19 = 15.4, P < 0.001), but it explained less variation of friction than pad contact area. Thus, the increase of friction with normal force is mainly based on an increase of contact area (at least when no secretion has accumulated).
Velocity dependence
Friction forces and corresponding shear stress increased significantly with velocity in the presence of secretion (“accumulated secretion”). This increase was highly significant no matter if force was measured after a constant time of 16 s (Fig. 5b) or after a constant amplitude of 4 mm (Page’s L tests: L 4,14 > 291 and P < 0.001 for friction and shear stress). To estimate the relation between shear stress and velocity, linear regressions were performed for each individual pad (constant amplitude). Intercepts were positive in 100% of all pads for “accumulated” (N = 14) secretion. Using the medians of the slopes and intercepts, the relationship between velocity v and shear stress τ can be written as: τ = 31.4 + 0.2· v (τ in kPa, v in μms−1).
Remaining friction
As the friction forces at the onset of sliding were velocity-dependent (Fig. 5a) and thus questionable as evidence in favor of static friction, we tested for the presence of static friction by measuring the remaining friction force after the end of a sliding movement (Fig. 6). Even 2 min after the movement had ended, we still measured a considerable friction under both conditions (median “little”: 3.36 mN, N = 88; median “accumulated”: 2.33 mN, N = 7). Shear stress was smaller for “accumulated” secretion (Wilcoxon rank sum test: W = 83, P < 0.01, median “little”: 64 kPa, median “accumulated”: 19 kPa).
Adhesion force: consecutive pull-offs
Similar to the build-up of force seen during each long distance sliding experiment (Fig. 2), forces significantly increased when consecutive pull-offs were performed at “new” positions of the smooth glass substrate (Page’s L test: L 9,10 = 2,647, P < 0.05; white boxes in Fig. 7). On the rough substrate, however, a different effect was found. Here, pull-off forces did not increase but even significantly decreased (Page’s L test: L 9,10 = 2,450, P < 0.01; gray boxes in Fig. 7).
Discussion
Role of pad secretion for attachment to smooth and rough substrates
Our study on the biomechanics of smooth adhesive organs in stick insects demonstrates the role of the tarsal fluid secretion for adhesion and friction. Previous studies on insects suggested that the pad fluid generally enhances adhesion (Edwards and Tarkanian 1970; Dixon et al. 1990). However, our data show that both friction and adhesion of insect pads on smooth glass are greater when less secretion is present. This behavior is consistent with physical models of fluid-based adhesion and lubrication (Israelachvili 1992; Francis and Horn 2001; Bhushan 2003; Piau et al. 2005). A thinner fluid film between the pad and the surface not only results in more strongly curved menisci and thus more negative Laplace pressures but it will also increase forces due to viscosity. If adhesion and friction are enhanced for smaller amounts of pad secretion, however, the question arises as to why insects don’t conserve energy resources and simply secrete less or even no fluid.
Our findings indicate that perhaps the most important function of adhesive secretion is to provide sufficient attachment to rough substrates. When the pads were depleted from secretion in the course of multiple consecutive pull-offs on a rough substrate, adhesive forces decreased. A similar effect was found in toe pads of tree frogs, where attachment to very rough surfaces was improved by wetting the surface with a stream of water (Barnes et al. 2002). The influence of fluid films on the adhesion between two solids has been studied in a classical paper by McFarlane and Tabor (1950). They found that increasing the surface roughness of glass by abrasion resulted in strongly reduced adhesion due to a loss of contact area, which was partly restored by applying water films or high humidity. Whether the substrate cavities can be filled out by the fluid depends on the relation between the height of the surface peaks and the thickness of the fluid layer (Fig. 8). Maximum adhesion will be reached when there is just enough fluid to fill out the substrate cavities, in which case the fluid layer thickness is in the same magnitude as the surface roughness amplitude (Bhushan 2003; Persson et al. 2005).
Schematic diagram illustrating the adhesion-enhancing role of insect pad secretion on a rough substrate. Due to the high surface roughness, the pad cannot completely fill out the substrate cavities. When there is only little secretion (a), only small menisci form at the tips of surface asperities and the real area of contact is small. If there is more fluid present in the contact zone (b, “accumulated secretion”), the small menisci merge and create a larger contact area, which gives rise to greater effective adhesion
The effect of surface roughness can not only be compensated by a fluid film but also if one of the adherends is very soft so that it can adapt to the surface profile (Fuller and Tabor 1975). Smooth adhesive pads of insects are indeed extremely soft and deformable (Gorb et al. 2000). In other animals, e.g., flies, beetles, spiders and lizards, compliance is achieved by a “hairy” design of adhesive pads (Stork 1983). However, if adhesive contacts are not extremely fine as in the “dry” adhesive systems of spiders and lizards, the surface roughness to which even a very soft smooth pad or a larger adhesive seta can make complete contact is limited (Fuller and Tabor 1975; Persson 2002). Thus, insect pad secretion can enhance adhesion on very rough surfaces where a dry pad would make only partial contact (Fig. 8a). Most of the substrates that insects encounter in nature are in fact not perfectly smooth but characterized by some degree of surface roughness. In the course of evolution, adhesive pads may have been optimized for better attachment to these “real” substrates rather than for superior contact to smooth surfaces.
Role of pad secretion for friction forces
The second important result emerging from this study concerns the presence of static friction and the role of the fluid secretion for static and dynamic forces. Even though shear forces of insect pads have often been measured (Stork 1980; Gorb and Scherge 2000; Gorb et al. 2001; Betz 2002; Federle et al. 2004; Gorb and Gorb 2004), the presence of a static component of friction has never been directly demonstrated. Our results confirm our previous conclusion (Federle et al. 2004) that despite the presence of a liquid in the contact zone, smooth adhesive pads generate static friction. In contrast to the classical concept of static friction between two solids, the forces at the onset of sliding did not always show a peak and were velocity dependent (Fig. 5a). This indicates that the transition from rest to sliding is associated with a dynamic, rate-dependent process, which may involve the release of the contact zone by peeling. To demonstrate that pads can indeed sustain a “static” shear stress, we measured the friction force for 2 min after a sliding movement had stopped. The remaining shear stress was considerable both for “little” and “accumulated” secretion.
The friction of smooth adhesive pads has been reported to increase with normal load (Gorb and Scherge 2000; Gorb et al. 2002). Even though our friction measurements are much larger than the values reported by Gorb and Scherge (2000), (taken with only 10 μm amplitude and probably with “accumulated” secretion), our findings confirm this effect and demonstrate that it is mainly based on changes in adhesive contact area. In contrast, shear stress itself was relatively insensitive to variations of normal force at least when no secretion had accumulated. This behavior is consistent with the view that friction depends on the “real” area of contact (Bowden and Tabor 1950). Scaling of friction with contact area has been found mainly in situations of close contact where friction is dominated by adhesion (Homola et al. 1990) such as the sliding friction of rubber on glass (Barquins and Roberts 1986). Thus, in order to be able to sustain larger friction forces, insects need to maximize their pad contact area. Under natural conditions, this is only rarely achieved by increasing the load on foot pads. On the contrary, the pad contact area of ants was found to increase with a stronger pull away from the surface (Federle and Endlein 2004). Insects take advantage of direction-dependent pad designs and active and passive unfolding mechanisms, and can increase contact area by pulling their legs toward the body (Federle et al. 2001; Niederegger et al. 2002).
Several possible mechanisms might explain the insects’ ability to sustain static shear forces. First, the meniscus of the fluid film between pad and substrate may be deformed when the pad is displaced horizontally. Due to the tendency of the contact angle to return to equilibrium, there will be a retentive force. However, a quantitative estimate analogous to our previous analysis (Federle et al. 2004) shows that the maximum possible shear stress due to surface tension amounts to only τ ≅ 0.5 kPa and is therefore two orders of magnitude smaller than the measured static shear stress. This confirms that the contribution of surface tension forces cannot explain the observed static friction.
Second, the adhesive pad cuticle could make direct contacts to the substrate. This could be related to the direct contact of surface asperities with the cuticle across the adhesive liquid film, if surface roughness is greater than the fluid film thickness (Roberts 1971). Using interference reflection microscopy, we estimated the thickness of the adhesive liquid film in C. morosus and Oecophylla smaragdina ants to range between 90 and 160 nm near the edge of the pad contact zone (Federle et al. 2002). Secretion films of up to 50 nm thickness get deposited at the trailing edge of sliding pads (Federle et al. 2002, and unpublished results). Even though we have not quantified the deposited fluid volume during the “accumulated secretion” experiments in this study, the visible trails of deposited secretion indicated that pads slid on secretion films of considerable thickness. Moreover, the fact that shear stress was sensitive to normal force only in the “accumulated” but not in the “little secretion” condition confirms that the fluid films were very thick (Fig. 4). As the surface roughness of glass is probably much smaller than the thickness of the fluid film, at least in the “accumulated” condition, it is unlikely that the penetration of asperities can explain static friction.
Alternatively, “dry” contacts could form by dewetting of a metastable, “triboactive” liquid film (Brochard-Wyart and de Gennes 1994; Martin and Brochard-Wyart 1998; Martin et al. 2002). We have not observed any evidence for such a process using interference reflection microscopy (Federle et al. 2002). Moreover, the wetting properties of the fluid suggest that dewetting is unlikely to occur. The stability of the thin fluid film between the pad cuticle and the surface depends on the sign of the spreading coefficient S = γGC − (γGF + γFC), where γGC, γGF and γFC are the glass/cuticle, glass/fluid and fluid/cuticle interfacial tensions, respectively (Martin et al. 2001). The fluid film is stable if S is positive (Martin and Brochard-Wyart 1998). As insect adhesive secretion completely wets the pad cuticle and forms only small contact angles with glass (Federle et al. 2002; Vötsch et al. 2002), both γGF and γFC are probably small and the film may be stable.
Third, it is possible that the pad secretion has non-Newtonian, shear-thinning properties, which could involve a solid-like behavior for small shear stresses. In fact, such a “yield stress” is a characteristic feature of emulsions, especially if the volume fraction of the disperse phase is large (Barnes 1994; Tadros 1994). The rate-dependence of shear stress in insect adhesive pads as observed in this work and in our previous study on ants (Federle et al. 2004) could be fully explained by the rheological behavior of an emulsion. In many emulsions, shear stress is an approximately linear function of shear rate, with a positive intercept corresponding to the yield stress. The relationship between velocity and shear stress in insect pads also shows a positive intercept (Federle et al. 2004 and this study).
Our findings provide evidence in favor of the “emulsion” mechanism. Static friction was still clearly present even when secretion had accumulated. Based on the above considerations, neither meniscus deformation nor direct contacts between the cuticle and the surface are plausible explanations. By virtue of its yield stress, even a relatively thick and continuous film of adhesive emulsion could sustain static friction forces. Further work is required to clarify in detail how the composition of this emulsion is related to its rheological properties and to its adhesive and frictional performance.
We assume that using a thixotropic emulsion as an adhesive fluid is an advantageous strategy because it conveys the benefits of wet adhesion and particularly the superior performance on rough substrates without sacrificing the ability to withstand shear forces. The combination of both factors, sufficient contact to rough substrates and resistance against sliding, might be an essential prerequisite for the insects’ capability to maneuver on plant surfaces.
References
Barnes H (1994) Rheology of emulsions—a review. Colloids Surf A 91:89–95
Barnes J, Smith J, Oines C, Mundl R (2002) Bionics and wet grip. Tire Technol Int 12/2002:56–60
Barquins M, Roberts A (1986) Rubber-friction variation with rate and temperature—some new observations. J Phys D Appl Phys 19:547–563
Betz O (2002) Performance and adaptive value of tarsal morphology in rove beetles of the genus Stenus (Coleoptera, Staphylinidae). J Exp Biol 205:1097–1113
Beutel RG, Gorb SN (2001) Ultrastructure of attachment specializations of hexapods, (Arthropoda): evolutionary patterns inferred from a revised ordinal phylogeny. J Zool Syst Evol Res 39:177–207
Bhushan B (2003) Adhesion and stiction: Mechanisms, measurement techniques, and methods for reduction. J Vac Sci Technol B 21:2262–2296. DOI 10.1116/1.1627336
Bowden F, Tabor D (1950) The friction and lubrication of solids. Oxford University Press, Oxford
Brochard-Wyart F, de Gennes PG (1994) Dewetting of a water film between a solid and a rubber. J Phys Condens Matter 6:A9–A12
Chambers JM, Cleveland WS, Kleiner B, Tukey PA (1983) Graphical methods for data analysis. Wadsworth & Brooks/Cole., Pacific Grove
Dewitz H (1884) Über die Fortbewegung der Thiere an senkrechten Flächen vermittels eines Secretes. Pflügers Arch Ges Physiol 33:440–481
Dixon A, Croghan P, Gowing R (1990) The mechanism by which aphids adhere to smooth surfaces. J Exp Biol 152:243–253
Edwards J, Tarkanian M (1970) The adhesive pads of Heteroptera: a re-examination. Proc R Entom Soc Lond A 45:1–5
Federle W, Endlein T (2004) Locomotion and adhesion: dynamic control of adhesive surface contact in ants. Arthr Struct Dev 33:67–75
Federle W, Brainerd E, McMahon T, Hölldobler B (2001) Biomechanics of the movable pretarsal adhesive organ in ants and bees. Proc Natl Acad Sci USA 98:6215–6220
Federle W, Riehle M, Curtis A, Full R (2002) An integrative study of insect adhesion: mechanics and wet adhesion of pretarsal pads in ants. Integr Comp Biol 42:1100–1106
Federle W, Baumgartner W, Hölldobler B (2004) Biomechanics of ant adhesive pads: frictional forces are rate- and temperature-dependent. J Exp Biol 207:67–74
Francis B, Horn R (2001) Apparatus-specific analysis of fluid adhesion measurements. J Appl Phys 89:4167–4174. DOI 10.1063/1.1351057
Fuller KNG, Tabor D (1975) The effect of surface roughness on the adhesion of elastic solids. Proc R Soc Lond A 345:327–342
Gorb S (2001) Attachment devices of insect cuticle. Kluwer, Dordrecht
Gorb S, Gorb E (2004) Ontogenesis of the attachment ability in the bug Coreus marginatus (Heteroptera, Insecta). J Exp Biol 207:2917–2924
Gorb S, Scherge M (2000) Biological microtribology: anisotropy in frictional forces of orthopteran attachment pads reflects the ultrastructure of a highly deformable material. Proc R Soc Lond B 267:1239–1244
Gorb S, Jiao Y, Scherge M (2000) Ultrastructural architecture and mechanical properties of attachment pads in Tettigonia viridissima (Orthoptera Tettigoniidae). J Comp Physiol A 186:821–831
Gorb S, Gorb E, Kastner V (2001) Scale effects on the attachment pads and friction forces in syrphid flies. J Exp Biol 204:1421–1431
Gorb S, Beutel R, Gorb E, Jiao Y, Kastner V, Niederegger S, Popov V, Scherge M, Schwarz U, Vötsch W (2002) Structural design and biomechanics of friction-based releasable attachment devices in insects. Integr Comp Biol 42:1127–1139
Homola A, Israelachvili J, McGuiggan P, Gee M (1990) Fundamental experimental studies in tribology: the transition from “interfacial” friction of undamaged molecularly smooth surfaces to “normal” friction with wear. Wear 136:65–83
Ishii S (1987) Adhesion of a leaf-feeding ladybird Epilachna vigintioctomaculata (Coleoptera, Coccinellidae) on a vertically smooth surface. Appl Entom Zool 22:222–228
Israelachvili J (1992) Intermolecular and surface forces. Academic, London
Jiao Y, Gorb S, Scherge M (2000) Adhesion measured on the attachment pads of Tettigonia viridissima (Orthoptera, Insecta). J Exp Biol 203:1887–1895
Langer MG, Ruppersberg JP, Gorb S (2004) Adhesion forces measured at the level of a terminal plate of the fly’s seta. Proc R Soc Lond B 271:2209–15. DOI 10.1098/rspb.2004.2850
Lees A, Hardie J (1988) The organs of adhesion in the aphid Megoura viciae. J Exp Biol 136:209–228
Martin P, Brochard-Wyart F (1998) Dewetting at soft interfaces. Phys Rev Lett 80:3296–3299
Martin A, Buguin A, Brochard-Wyart F (2001) Dewetting nucleation centers at soft interfaces. Langmuir 17:6553–6559
Martin A, Buguin A, Brochard-Wyart F (2002) “Cerenkov” dewetting at soft interfaces. Europhys Lett 57:604–610
McFarlane JS, Tabor D (1950) Adhesion of solids and the effect of surface films. Proc R Soc Lond A 202:224–243
Niederegger S, Gorb S, Jiao Y (2002) Contact behaviour of tenent setae in attachment pads of the blowfly Calliphora vicina (Diptera, Calliphoridae). J Comp Physiol A 187:961–970
Page EB (1963) Ordered hypotheses for multiple treatments: a significance test for linear ranks. J Am Stat Assoc 58:216–230
Persson B (2002) Adhesion between an elastic body and a randomly rough hard surface. Eur Phys J E 8:385–401. DOI 10.1140/epje/i2002-10025-1
Persson B, Albohr O, Tartaglino U, Volokitin A, Tosatti E (2005) On the nature of surface roughness with application to contact mechanics, sealing, rubber friction and adhesion. J Phys Condens Matter 17:R1–R62
Piau JM, Ravilly G, Verdier C (2005) Peeling of polydimethylsiloxane adhesives at low velocities: cohesive failure. J Polym Sci B Polym Phys 43:145–157
R Development Core Team (2005) R: A language and environment for statistic computing. R Foundation for Statistical Computing, Vienna, Austria
Roberts A (1971) The shear of thin liquid films. J Phys D Appl Phys 4:433–440
Stork N (1980) Experimental analysis of adhesion of Chrysolina polita (Chrysomelidae: Coleoptera) on a variety of surfaces. J Exp Biol 88:91–107
Stork N (1983) A comparison of the adhesive setae on the feet of lizards and arthropods. J Nat Hist 17:829–835
Tadros T (1994) Fundamental principles of emulsion rheology and their applications. Colloids Surf A 91:39–55
Vötsch W, Nicholson G, Müller R, Stierhof Y, Gorb S, Schwarz U (2002) Chemical composition of the attachment pad secretion of the locust Locusta migratoria. Insect Biochem Mol Biol 32:1605–1613
Walker G (1993) Adhesion to smooth surfaces by insects—a review. Int J Adhesion Adhesives 13:3–7
Walker G, Yue A, Ratcliffe J (1985) The adhesive organ of the blowfly, Calliphora vomitoria: a functional approach (Diptera: Calliphoridae). J Zool Lond 205:297–307
Acknowledgments
We wish to thank Andreas Eckart for helping in the development of motor control programs in LabVIEW. This study was financially supported by research grants of the Deutsche Forschungsgemeinschaft (SFB 567 “Mechanisms of interspecific interactions of organisms” and Emmy-Noether grant FE 547/1-3 to WF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drechsler, P., Federle, W. Biomechanics of smooth adhesive pads in insects: influence of tarsal secretion on attachment performance. J Comp Physiol A 192, 1213–1222 (2006). https://doi.org/10.1007/s00359-006-0150-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-006-0150-5