Abstract
Visually guided flight control requires processing changes in the visual panorama (optic-flow) resulting from self-movement relative to stationary objects, as well as from moving objects passing through the field of view. We studied the ability of the blue-tailed damselfly, Ischnura elegans, to successfully land on a perch moving unpredictably. We tracked the insects landing on a vertical pole moved linearly 6 cm back and forth with sinusoidal changes in velocity. When the moving perch changed direction at frequencies higher than 1 Hz, the damselflies engaged in manoeuvres that typically involved sideways flight, with minimal changes in body orientation relative to the stationary environment. We show that these flight manoeuvres attempted to fix the target in the centre of the field of view when flying in any direction while keeping body rotation changes about the yaw axis to the minimum. We propose that this pursuit strategy allows the insect to obtain reliable information on self and target motion relative to the stationary environment from the translational optic-flow, while minimizing interference from the rotational optic-flow. The ability of damselflies to fly in any direction, irrespective of body orientation, underlines the superb flight control of these aerial predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The seemingly trivial approach of an insect to land on a perching spot, such as a twig, flower or reed requires delicate sensorimotor control between the visual system and the flight and leg muscles that regulate landing dynamics (Goodman 1960). When the perching point is moving relative to the environment, as in the case of landing on another animal or on a flower swaying in the wind [e.g., hawkmoths (Sprayberry and Daniel 2007), honeybees (Zhang et al. 1990)], the task of controlling landing becomes even more complex due to latencies between sensory processing and motor output as both insect and perch are moving at different speeds and directions relative to the stationary visual environment. The most challenging type of visual approach is likely aerial pursuits in which a flying insect chases another manoeuvring insect in the air [e.g., chasing conspecifics: flies (Land and Collett 1974; Collett and Land 1975), bees (van Praagh et al. 1980); chasing prey: Odonata (Tillyard 1917), robber flies (Wardill et al. 2017); or chasing a host: parasitic wasps (van Achterberg and Durán 2011)]. Approaching such moving targets requires precise sensory-motor feedback on small spatial and temporal scales to guide the manoeuvring insect to the moving target. Prey items may employ direct evasive tactics or have a natural erratic flight pattern that aids in avoiding predation [e.g., flies (Golding et al. 2001; Combes et al. 2012), butterflies (Srygley and Chai 1990; Jantzen and Eisner 2008)]. In turn, the sensory-motor system of insects adapted to aerial pursuits, such as dragonflies and damselflies, should be particularly well tuned to catching targets that move unpredictably.
Aerial interception of small moving targets (prey) is common in dragonflies (Olberg et al. 2000, 2007). Dragonfly brains possess specific ‘target-selective’ descending neurons that selectively transmit information on movement of small targets from the visual system and brain to the thoracic motor neurons that affect wing motion (Olberg et al. 2000, 2007). These seem to operate at a closed feedback loop since dragonflies have been shown to update their interception path in response to directional changes in their prey’s flight trajectory (Olberg 2012). However, more recently, Mischiati et al. (2014) and Bomphrey et al. (2016) provided evidence that the neuronal control mechanisms behind chasing behaviour are more complex than the scenarios portrayed above; namely, Mischiati et al. (2014) showed that when dragonflies adjust the bearing angle and their body orientation in reaction to changes in the speed and direction of the target, they rely on predictive internal models of the motion of their own body and head and that of the target. Such internal predictive models are well known from vertebrates such as hawks (Kane et al. 2015) and bats (Ghose et al. 2006), but had not previously been described in invertebrates. In addition, the decision whether or not to initialize take-off after a small moving target seems to follow basic rules based on the target’s projection on the retina to determine if the target size and speed matches those of a suitable prey (Lin and Leonardo 2017). While dragonflies have their flight apparatus and the nervous system adapted to aerial chases, their ability to track and land on moving perches has not been explored. Landing on a large stationary target involves a different neuronal circuitry (Borst 2014), but the task of landing on a moving perch requires tracking and responding to the movement of the perch. Are odonates good at landing on moving perches as they are at capturing small targets in the air?
The optic-flow (the change in visual information over time) perceived during movement can be divided into translational and rotational components. The translational optic-flow results from the linear motion relative to the stationary panorama and can be used for reliable measurement of the distance and speed of objects in the surroundings. Rotational optic-flow is derived from the rotational motion of the viewer relative to the stationary panorama. While it may provide information on changes in orientation of a flying insect, it does not contribute to measuring the distance and speed of objects in the visual field (Taylor and Krapp 2008). In-fact, rotational optic-flow can interrupt the process of visual information integration (Gibson 1951; Koenderink 1986; Taylor and Krapp 2008; Boeddeker and Hemmi 2010). Therefore, to extract flight control information mostly from the translational optic-flow, insects limit their rotational movements to short head or body saccades interspaced with longer periods of constant gaze direction (Collett and Land 1975; van Hateren and Schilstra 1999; Land 1999; Tammero and Dickinson 2002; Ribak et al. 2009).
The extraction of credible information from the optic-flow to aid in landing has been studied extensively by Lee (1976) who suggested that plummeting gannets, human drivers, docking hummingbirds, and pigeons use retinal image size and angular velocities to measure the instantaneous time to collision (Lee and Reddish 1981; Lee et al. 1991, 1993) as a parameter aiding landing or avoiding collision. Alternatively, Borst and Bahde (1986) and Borst (1990) suggested that spatial and temporal integration by motion detectors in the visual system is the mechanism eliciting the landing behaviour in flies. According to that theory, integration is being made between many variables, including the differences between images from both eyes and temporal integration between them. While the parameters measured may differ, in both theories the insect approaching a stationary target rely on processing temporal changes in visual information to indirectly estimate the distance to approaching objects. As the perch becomes closer to the observer, the angular projection subtended on the retina expands, and insects use this expansion rate as well as the instantaneous projected object size to estimate proximity to the perch and adjust their flight speed accordingly (Wagner 1982; Evangelista et al. 2010; van Breugel and Dickinson 2012; Baird et al. 2013).
When the perch is moving, however, it is not enough to estimate the distance to it; the observer must also steer to adjust its trajectory to the change in position of the target. While quite a few studies (some are mentioned above) dealt with landing on a stationary perch, very few studies dealt with insects landing on moving ones (Zhang et al. 1990). Harder still, is the need to land on a perch that can abruptly change its direction of movement, forcing the insect to steer and change direction during the approach. Two alternative basic behavioural strategies are employed by animals for guidance to a moving target: the first strategy is known as ‘tracking’, in which the pursuer reacts to the instantaneous position of the target by steering to minimize the angle between its flight path and the bearing vector to the target (Collett and Land 1978). Examples of such tracking behaviour have been demonstrated in hoverflies (Collett and Land 1975), blowflies (Boeddeker et al. 2003), ground beetles (Gilbert 1997) and teleost fish (Lanchester and Mark 1975), to name just a few. The second guidance strategy is known as ‘parallel navigation’, which involves the prediction of the future position of the moving target (from target velocity) and steering to meet it at that predicted location. The ability to predict the future trajectory of the target, and intercept it has a simple guidance law: in parallel navigation, the insect simply flies closer to the target while steering to keep the target at a constant bearing angle (Olberg et al. 2007). Unlike in tracking, this fixed bearing angle does not align the flight direction to the current position of the target. Rather, it guides the insect in the direction of the future meeting point between its trajectory and that of the moving target.
During predatory attacks dragonflies use parallel navigation to intercept the prey (Olberg et al. 2000; Olberg 2012) as well as tracking to decide whether to chase it (Lin and Leonardo 2017). Given that different neuronal pathways are used for predatory attacks and landing, we were intrigued by the question how Odonates use sensory-motor feedback to approach perches that move unpredictably. If present, internal models might suggest some inherent ability to translate the dynamics of perch motion to predict where the perch will move next. This may be possible for simple ballistic trajectories but seems unlikely when the perch changes speed and direction abruptly (e.g., a swinging reed). Similarly, applying a parallel navigation strategy while approaching the unpredictably moving perch would mean that the dragonfly would need to constantly update the future contact point with the perch (Ghose et al. 2006). Hence, a possible solution to dealing with unpredictable perch movements is to use tracking or fixate (Reichardt and Wenking 1969) on the perch, since this allows steering to a moving target based only on its current position, without the need to predict its future position. However, tracking suffers from latencies between sensory processing and motor response. If the perch is accelerating or changing direction, by the time the position data are processed and the muscle react, the information is outdated. Hence, it is currently unclear which insects can land on perches moving unpredictably and how do they manage to do it?
Here we examine how damselflies approach such moving perches. Members of the Odonata order exhibit superb flight manoeuvrability (Wakeling and Ellington 1997; Gravish et al. 2015; Bomphrey et al. 2016). There has been substantial research into the ability of dragonflies to track and intercept their prey (Olberg 2012; Mischiati et al. 2014; Lin and Leonardo 2017) but their landing capabilities and behaviour have not been studied. The other members of Odonata, damselflies (Zygoptera), have rarely been studied in terms of either target pursuit or landing. Damselflies are aerial predators as well, but they have two distinct anatomical and physiological differences from dragonflies. First, their two compound eyes do not meet at the dorsal–frontal side of the head to form the specialized fovea that dragonflies use to track moving targets (Tillyard 1917; Olberg et al. 2007). Second, although both dragonflies and damselflies can flap their two pairs of wings in and out of phase (Pfau 1991), damselflies display a higher flexibility in their flapping kinematics (Wakeling and Ellington 1997) and some damselflies can, to some extent, alter the kinematics of the contralateral wings within the same pair (Pfau 1991; Grabow and Rüppell 1995; Kassner et al. 2016). This increased plasticity in flapping kinematics could be associated with improved flight manoeuvrability. Damselflies are usually found in the dense vegetation of ponds, thus requiring flight in a much more cluttered and complex 3D environment (Tillyard 1917; Silsby 2001) compared to dragonflies, which spend most of their flight time over open water. These anatomical and ecological differences make damselflies an intriguing, yet unexplored, research model for studying visual tracking and landing in a flying insect.
We tested the ability of the blue-tailed damselfly (Ischnura elegans) to land on a moving perch that constantly changes its speed and direction, thus moving in an unpredictable manner. We hypothesized that if damselflies employ a parallel navigation strategy to steer during the landing approach they will constantly update their interception path during the approach to the manoeuvring perch. We further hypothesized that their ability to correct their flight path successfully during the approach would decrease as the perch will move faster and change direction at higher frequencies, increasing the demand on the sensory-motor response. This higher demand might either require a change in interception strategy or define a limit to the target capture ability. To test these hypotheses, we filmed damselflies landing on moving perches using high-speed video cameras and analysed the approach strategy compared to approaching a stationary perch.
Materials and methods
Insects
Adult blue–tailed damselflies (I. elegans) were collected from field sites in northern and central Israel. All insects were brought to the laboratory and tested on the same day of capture. We only used females in the experiments since males have proven to be less cooperative when placed inside the flight arena (described next). A similar inter-sex behavioural difference was noted previously in dragonflies (Olberg et al. 2007). Insects were individually introduced into the flight arena one at a time.
Experimental setting
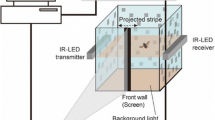
The flight arena (Fig. 1a) comprised a rectangular box (0.39 × 0.59 × 0.49 m, H × L × W). The inner walls were white and coated with transparent cellulose acetate sheets that provided a slippery surface to prevent the insects from landing on them. A checkered pattern (spatial wavelength of 7 cm) provided visual contrast on the walls (Tammero and Dickinson 2002; van Breugel and Dickinson 2012; Baird et al. 2013). The transparent glass ceiling of the arena prevented the insects from escaping while enabling illumination and filming of the inside of the arena from above.
To stimulate landing behaviour, we used the natural tendency of the insect to land on elevated perches. A vertical wooden pole (length 0.3 m, diameter 2.5 mm), positioned in the middle of the flight arena, served as the only elevated perching point. The pole was mounted on a chart recorder (Kipp & Zonen, BD41 dual channel chart recorder) located beneath the floor of the arena. By connecting the chart recorder to a function generator (AFG-2000 series, Good Will instruments Co., Ltd.) we were able to move the pole in prescribed kinematics. When released, the insects flew to the pole and perched on it. When disturbed they typically flew inside the arena for several seconds and then returned to the pole. When the pole was moved, the insects readily manoeuvred to chase and land on it. To encourage the insects to fly when resting on the pole or ground we used gentle touch with the tip of a second wooden pole.
The perch was moved back and forth, at varying speeds, defined by a sinusoidal voltage signal. The oscillatory harmonic movement pattern ensured that the position, speed and acceleration of the perch were all changing with time, thus making it impossible for the damselflies to use one of these instantaneous measurements to predict the future position of the perch. However, the same perch motion repeated itself in each cycle within a trial. Preliminary experiments revealed no improvements in damselflies landing as trials progressed. Hence, we ruled out the option that the insects can learn and respond to the repetitive movement pattern. We designed the signals to move the perch at a constant amplitude of 6 cm (~ 4 wing lengths) and varied the frequencies between trials to 1, 2 and 2.5 Hz (average speed of 0.12, 0.24 or 0.3 ms, respectively). In another experiment, we designed the signal to move the perch at a larger amplitude (15 cm) with a frequency of 1 Hz (average speed of 0.3 ms−1). Finally, a control experiment was carried out with the perch kept motionless, representing the approach of damselflies to landing on stationary perch.
The frequencies used in the experiments were chosen based on preliminary trials that had revealed more than 50% of failure to land when the perch was moved at frequencies higher than 2.5 Hz. For simplicity, the experiments are referred to hereafter as ‘stationary’, ‘1’, ‘2’ and ‘2.5’ (with the numbers denoting target frequency) in the 6 cm amplitude experiments. The experiment of 15 cm movement at 1 Hz is referred to hereafter as 1′. Note that in experiments 2.5 and 1′ the perch moved at the same average speed although the distance travelled and frequency were different. Hence the two experiments enabled us to determine which is the more difficult to land on: a perch that changes direction at higher frequency or one that moves a greater distance in each cycle.
Research design
Individuals were introduced into the flight arena (one at a time) for about an hour prior to the first trial. We verified prior to the trials that the damselflies were voluntarily flying around the arena and landing on the stationary perch. The frequency of the perch’s movement in each trial was determined randomly to prevent learning or fatigue from affecting variation between experiments. Each trial ended when the insect had landed on the perch. A damselfly that failed to land on the moving perch was replaced with another individual. Experiments ‘stationary’, 1, 2 and 2.5 were repeated with the same individuals. Experiment 1′ was performed with a different set of individuals.
High-speed video recording and data analysis
All trials were filmed at 1000 frames per second simultaneously by three high-speed video cameras (Fastcam SA3, Photron Inc., Fig. 1b). The cameras were spatially calibrated (Hedrick 2008) and temporally synchronized to allow the extraction of 3D positions of the insect and target in each film frame. In total, 289 trials were filmed. From these, we first discarded all trials in which the insect seemed to collide with the perch rather than manoeuvring and landing on it voluntarily. Voluntary landings were defined from films that clearly showed a landing response [i.e., extension of legs towards the target prior to landing (Wagner 1982; Borst and Bahde 1986; Baird et al. 2015)]. This criterion left 83 films showing the insect approaching the perch (supplementary Table 1). These comprised 17, 16, 24 and 26 films from 15 different individuals for the stationary, 1, 2 and 2.5 experiments, respectively. An additional 14 films from 6 different individuals were analysed from the 1′ experiment.
The exact height on the pole that the damselflies were aiming for during the approach was unknown. Since the perch (vertical pole) was perpendicular to the ground (i.e., aligned with the z-axis, Fig. 1a) we treated the data as two-dimensional and, unless noted otherwise, analysed the approach in the horizontal (XY) plane.
The initiation of approach toward the perch was identified from a distinct head turn towards the perch, followed by steering toward it. From that point we digitized, in each movie frame, three points (Fig. 2): the joint between head and thorax (point P1), the joint between thorax and abdomen (point P2) and a point on the target (point P3, the point that the damselfly landed on). Next, the digitized position data were filtered with a Butterworth low-pass filter (cut-offs frequency = 50 Hz) to remove digitization noise. Based on the three digitized points, three instantaneous vectors were defined in each film frame: b (body) was defined as the vector connecting points P2 and P1, t (target = perch direction) was defined as the vector connecting points P1 and P3, and v (flight direction) was defined as the instantaneous horizontal velocity of the insect. The latter was numerically derived from the change in position of P1, in the XY plane, with time, as described in Rayner and Aldridge (1985). The three vectors were used to define two instantaneous angles (‘v2b’ and ‘b2t’) in the XY plane (Fig. 2b). These were defined between the flight direction and the longitudinal body axis (v2b) and between the longitudinal body axis and direction to the perch (b2t). The change in b (Δb) during each trial was found by subtracting the initial body azimuth angle b(t=0) from the instantaneous body azimuth angle b(t) at each video frame.
The head remained fixed relative to the body during most of the time within a trial. However, distinct head saccades were occasionally observed, and their timing and frequency of occurrence were noted during the analysis of the films.
Statistical analysis
All statistical analyses were performed using SPSS statistics (IBM, version 21). Since the same individuals were tested in the stationary, 1, 2 and 2.5 experiments (supplementary Table 1), we accounted for repeated measurement in the statistical design. Six different individuals were used in experiment 1′ (n = 6). In cases in which one individual performed more than once in an experiment we first averaged all trials by the same individual per experiment and then carried out statistical tests using the averages. Non-parametric tests were used when normality and homogeneity of variance assumptions were unsupported by the data. Data in box plots are shown using ranges, medians and 2nd and 3rd quartiles rather than averages to reflect the asymmetric data distribution within every experiment.
Results
When first introduced into the flight arena, the damselflies seemed to be attracted to the perch almost instantly, readily attempting to land on it voluntarily.
Flight trajectories
When approaching a stationary perch, the insects either turned and flew directly towards it (6 out of 20 trials, e.g., Fig. 3a and supplementary Film 1) or flew sideways to bring the target into the centre of field of view (b2t = 0) and then flew towards the target (14 out of 20 trials, e.g., Fig. 3b and supplementary Film 2). In general, flights towards the perch moving at 1 Hz did not differ from flights to stationary perches (see below). When the perch was moving at frequencies higher than 1 Hz, the trajectories of the damselflies became more complex, showing a distinct ‘zigzag’ pattern (e.g., Fig. 3c, supplementary Film 3, 4).
Examples of approach to the perch. The damselfly head (circles) and body (b, black thick lines) are depicted every 10 ms during the approach. Also shown are the instantaneous vectors t (thin grey lines). a Direct approach to a stationary perch. b Indirect approach to a stationary perch. c Approach to a perch changing direction at 2 Hz
Angle between the body’s long axis and the perch (b2t)
The distribution of b2t changed between the different experiments (Fig. 4). The mean absolute angular position of the perch relative to the body tended to increase with the increase in moving perch frequency [Repeated measures ANOVA (RMANOVA), p < 0.001, n = 15, Fig. 4f]. Post hoc paired sample t tests (with Bonferroni correction for six comparisons, α = 0.0083) revealed that in the stationary perch experiment the mean absolute b2t was lower than in exp. 2 (p = 0.001) and in exp. 2.5 (p < 0.001). In addition, the mean absolute b2t in exp. 1 was lower than b2t in exp. 2.5 (p = 0.006). There was no statistical difference in mean absolute b2t between exp. 1′ and exp. 2.5 (Independent samples t test, p = 0.111).
Distribution of b2t angles in the stationary experiment (a) and experiments 1, 2, 2.5 and 1′ (b–e, respectively). Each shows the frequency of occurrence (colour code) of b2t angles (horizontal axes) for each of the insects (vertical axes). f Median b2t in the different experiments. Upper and lower error bars represent the maximum and minimum observed and the boxes’ lower and upper boundaries represent the first and third quartiles. Horizontal lines within the boxes are the median. Capital letters a–c denote significance levels by paired samples t test (post hoc to RMANOVA) after Bonferroni correction. Differences between experiment 2.5 and 1′ are not statistically significant (NS)
Change in body orientation (azimuth)
Changes in b2t can result from the movement of the perch relative to the body, as well as changes in orientation of the body in 3D space. Hence, next we examined the contribution of the two components to the observed change in b2t.
The direction of the damselflies’ longitudinal body axis remained relatively fixed as the insect manoeuvred during the approach (Fig. 5). The median change in body orientation as the insect zig-zagged towards the perch was approximately 20°, with no significant differences in body azimuth change among the different experiments (Friedman’s test, p = 0.392). Figure 6 provides examples of a typical ‘chase’ after the perch, showing that compared to the direction of t that changed constantly (primarily due to motion of the perch sideways) the direction of b showed only moderate, saccadic changes.
Change in the azimuth angle of b (\(\varDelta\)b) in the different experiments. a–e The stationary experiment and experiments, 1, 2, 2.5 and 1′, respectively. Each shows the frequency of occurrence (colour code) of \(\varDelta\)b angles (horizontal axes) for each of the insects (vertical axes). f Median \(\varDelta\)b in the different experiments (boxes and error bars representation are the same as in Fig. 4). No significant differences were found among the various experiments
Examples of changes in angle b2t and direction of b and t in time during a chase. Left vertical axes represent the angles and right vertical axes represent the perch’s position along the line of motion. Each asterisk indicates an event of a head saccade. a Stationary experiment—all components remain relatively constant. b Experiment 1. c Experiment 2. d, e Experiments 2.5 and 1′, respectively. Note that the periodic changes in b are modest compared to changes in t and position of the perch. In c–e head saccades are visibly associated with changes in the direction of vector b. f the definition of b2t and colour code in a–e
Head saccades
Gaze shifts performed by discrete rapid head rotations (saccades) were not observed during the approach to the stationary perch or in exp. 1. They did occur, at low frequency, in all other experiments (Fig. 7). The instantaneous b2t angle in the film frame, showing initiation of head movement, was on average 37° ± 8.1°, 43° ± 21.1° and 41° ± 8.6° in the 2, 2.5 and 1′ experiments, respectively (data from n = 6 individuals in each experiment). The angles did not differ between exp. 2 and exp. 2.5 (Paired samples t test, n = 6, p = 0.353), nor between exp. 2.5 and 1′ (independent samples t test, n = 6, p = 0.823, Fig. 7a). The number of saccades per second did vary between exps. 1′ and 2.5, with damselflies making more saccades per second in the 1′ experiment (independent samples t test, n = 6, p = 0.009, Fig. 7b).
Saccades during pursuit in experiments 2, 2.5 and 1′ (n = 6). a Absolute b2t angle at which the damselflies made a saccade. No significant differences were found among all experiments (boxplot definitions are as in Fig. 4). b Mean number of saccades per second in experiments 2, 2.5 and 1′. Saccade frequency was significantly higher in experiment 1′ than in experiments 2 and 2.5. Whiskers denote SE
Angle between flight direction and body axis (v2b)
The insects adjusted their flight direction to keep the moving perch close to the centre of the visual field. As the perch moved faster and at higher frequencies, the mean angle of flight direction relative to the body (v2b) increased (RMANOVA, p < 0.001, n = 15, Fig. 8f) reaching 90° in some cases (Fig. 9c-e). Post hoc paired samples t tests (with Bonferroni correction, α = 0.0083) revealed that the direction in which the damselflies flew with respect to the direction of the longitudinal body axis did not significantly differ between the stationary exp. and exp. 1 (p = 0.062). The angle v2b in the stationary exp. and exp. 1 was smaller from the angle in exp. 2 and 2.5 (stationary vs. exp. 2: p = 0.001; exp. 1 vs. exp. 2: p = 0.008) but the angle did not significantly differ between exp. 2.5 and 1′ (independent samples t test, p = 0.1, Fig. 8f). Damselflies flew sideways (60° < v2b < 120° consistently for at least 150 ms) in an average speed of 0.19 ± 0.04 ms−1 (n = 9 damselflies) and 0.25 ± 0.04 ms−1 (n = 5 damselflies) in exps. 2.5 and 1′, respectively.
Change in v2b in the different experiments. a–e are experiments stationary, 1, 2, 2.5 and 1′, respectively. Each shows the frequency of occurrence (colour code) of v2b angles (horizontal axes) for each of the insects (vertical axes). f Median v2b in the different experiments (box and error bar representations are as in Fig. 4). The v2b angle increased with the increase in target frequency
Examples of changes in angle v2b (purple) and direction of b (blue) and v (green) in time during a chase (axes definitions are as in Fig. 6). Each asterisk indicates an event of a head saccade. a Stationary experiment—b remains relatively constant while v changes (see exemplary trajectory in Fig. 3b). b Experiment 1. c–e Experiments 2, 2.5 and 1′, respectively. f The definition of v2b and colour code in a–e
The angle between flight direction and body axis versus the angle between body’s long axis and the perch
Since both the mean b2t and v2b varied between experiments, and changes in b2t were mostly due to motion of the perch, we examined the association between b2t and v2b. The b2t angle was significantly correlated with v2b only in exp. 2 (Spearman rank coefficient r = 0.671, p < 0.001, Fig. 10c). Next, we examined within all the experiments how the frequency of the moving perch and b2t affect v2b. For this, we tested the data from all experiments in a General Linear Model (GLM) with b2t serving as a covariate. Both the frequency of the moving perch (p = 0.005) and the b2t angle (p = 0.008) were found to be significant in affecting the v2b angle (Fig. 10f). Data from all experiments were best fitted by a power relationship (y = 8.426 × 0.619, R2 = 0.489, p < 0.001) denoting an increase in sideways flight (v2b) with increase in b2t (the angle between the longitudinal axis of the body and direction to the perch).
Relationship between b2t and v2b in the stationary experiment and experiments 1, 2, 2.5 and 1′ (a–e, respectively). Each symbol denotes the average of one insect. A significant correlation was only found between b2t and v2b in experiment 2 (c). f Data from all experiments show a significant correlation between both above-noted angles. Each colour represents a different experiment (see legend)
Change in altitude in the moment before landing
While our analysis was limited to steering in the two-dimensional (horizontal) plane, we did measure the approach in the vertical plane to examine if damselflies prefer to approach for a landing from below or from above. We found that the damselflies kept their altitude fairly constant during the last 300 ms (approximately ten flapping cycles) prior to landing. Some approaches lasted less than 300 ms. In these cases, we measured the altitude change during the entire approach. Positive values of altitude change correspond to the damselfly gaining altitude during the approach. We found no distinct preference for approaching the landing point from below (supplementary Fig. 1). The mean change in altitude was 0.4 ± 0.8, 0.2 ± 0.7, 0.2 ± 0.8, 0.3 ± 0.4 cm in the stationary exp. and exps. 1, 2 and 2.5, respectively. There were no significant differences in altitude gain between the experiments (RMANOVA, n = 15, p = 0.84). There were also no differences in altitude adjustment between exp. 2.5 and 1′ (0.3 ± 0.4 cm, n = 15 and 0.2 ± 1.1 cm, n = 6, respectively, independent samples t test, p = 0.69).
Discussion
Our damselflies responded to the moving perch almost instantly and seemed more attracted to the perch when it was moving compared to when it was stationary. This observation adds to previous studies on dragonflies (Olberg 1981; Olberg et al. 2005; Nordström et al. 2011; Combes et al. 2013) reporting voluntary chasing after small moving targets. A similar attraction to moving targets was also reported in blowflies (Boeddeker et al. 2003), houseflies (Land and Collett 1974), hawkmoths (Sprayberry and Daniel 2007) and honeybees (Zhang et al. 1990). Hence the motivation to chase moving objects seems ubiquitous in flying insects, and at least in our damselflies includes chasing large objects for perching.
To approach the moving perch, our tested damselflies constantly updated their flight trajectory, based on movement of the perch as perceived from their instantaneous location. Although the perch moved repeatedly, replicating the motion pattern in every cycle, there was no evidence of the damselflies improving their landing success or anticipating perch direction change as the experiment proceeded. Thus, we consider the movement pattern of the perch, with time-varying velocity and acceleration, to appear unpredictable to our insects. Nevertheless, to eliminate the options of learning and chronological biases we changed the order of the experiments randomly within each individual.
Changes in the body-perch and body-flight direction angles
Within each back and forth movement cycle, the perch constantly changed velocity and acceleration with time. This resulted in changes in the flight trajectory of the insect which attempted to bring the perch into the centre of the field of view (b2t = 0°) and keep it there. This was executed with minimal changes in body orientation (azimuth angle of b) relative to the surrounding panorama. Consequently, flight direction changed independently of the orientation of the insect. When the perch moved faster and changed direction more frequently it tended to deviate from the centre of the field of view of the insect (b2t ≠ 0°). In response, the insect either (1) flew sideways in the direction of the perch’s movement, or (2) performed a head saccade when the angle to the perch (b2t) deviated approximately ± 40° (Fig. 7a) from zero. Thus, the basic guidance law, as evident from our experiments, is relatively simple. The insect steers according to the current angular position of the perch, and changes flight direction to minimize the angle b2t. However, steering to change flight direction is performed with minimal changes in body orientation: i.e., minimization of b2t is achieved by changing the flight direction (vector v) rather than changing the orientation of the long axis of the body (b). As a result, v2b is the main time-varying parameter. The association between b2t and v2b in the different experiments suggests that the more the perch deviates from the centre of the field of view of the damselfly, the higher the likelihood of sideways flight. This is true up to when the damselfly reaches a point at which it flies sideways, i.e., a further increase in v2b would cause it to fly backwards (Fig. 9c–e). After correcting flight direction to regain b2t = 0° the damselflies moved towards the perch with the vectors v, b and t aligned.
Steering strategies in response to perch movement
In the classical description of object fixation and animal chases, ‘tracking’ typically refers to steering to minimize the angle between flight direction (v) and the current target direction (t). This resembles the landing strategy observed here. However, in most previous studies flight direction is assumed to be coupled with body orientation so that steering typically involved torques and rotating the body (b) to face the new flight direction; i.e., a condition in which both v2b and b2t are minimized throughout the entire chase. This was not the case here, except for the final section of the approach, after b2t had reached 0°.
Some insects, such as dragonflies (Olberg et al. 2000; Olberg 2012; Mischiati et al. 2014) and hoverflies (Collett and Land 1978), are known to use parallel navigation when chasing other insects in the air. In the classical description of parallel navigation, the intercepting insect steers to keep the target at a fixed direction in its field of view (90° > b2t > 0° and constant). This was not the case seen in the landing response of our damselflies. Rather, it appears that damselflies both keep their body direction constant, together with keeping the perch at the centre of their field of view by constantly changing their flight path.
Hence, when landing on a stationary or an unpredictably moving perch our damselflies took a somewhat intermediate approach: they fixed their body orientation in space while moving sideways to move the position of the perch on their retina to the centre of their field of view. Consequently, the approach to a stationary perch was not necessarily via the shortest path (Fig. 3b). Both the approach to a stationary perch and the approach towards a moving perch involved some flying sideways, with the damselflies more prone to fly sideways (i.e., v2b angle was closer to 90°, Fig. 8f) when the frequency of the perch’s direction change, increased.
Side-slip flight during the approach
Damselflies appear to be able to completely separate between their heading and flight direction, so that flight trajectory adjustments to minimize the angle between the longitudinal axis and line of sight to the perch in the horizontal plane can be performed without changing body yaw. Sideways flight has been reported previously in dragonflies as a by-product of a fast yaw turn (Alexander 1986). It has also been noted in other insects, such as hawkmoths (Sprayberry and Daniel 2007; Greeter and Hedrick 2016), honey bees (Zhang et al. 1990) and flies (Land and Collett 1974; van Breugel and Dickinson 2012) in connection to low flight speed or as a result of inertial force caused by turning. In our study, sideways flight seems to be a part of the strategy of approaching moving perches. Not only did the damselflies exhibit sideway flight while chasing a perch moving at high-speed, but they also did so more frequently when the perch changed direction at higher frequencies.
Previous studies on hawkmoths (Sprayberry and Daniel 2007; Greeter and Hedrick 2016) and honey bees (Zhang et al. 1990) used an artificial stimulus that moved sideways to study the tracking behaviour of those insects. It was found that the hawkmoth Manduca sexta could fly sideways to track artificial flowers that were moved at an average speed of 0.06 ms−1 (Sprayberry and Daniel 2007). The lateral movement of M. sexta was analysed by Greeter and Hedrick (2016) and it was found that they do so by rolling their body, thus tilting their net force vector to have a sideways component. Honeybees could track a landing spot that was moved sideways at a maximum speed of approximately 0.3 ms−1 (Zhang et al. 1990). In that study, it was shown that bees also use translational manoeuvres, i.e., flying sideways, in addition to rotating about the yaw axis when getting closer than ca. 15 cm from the target. A study on robber flies (Wardill et al. 2017) found that, in the final part of their interception course, some flies flew backwards to keep a small moving target at an anterior point on their visual field while flying in a trajectory that was tangential to that of the prey. Hence, reports on sideways and even backwards flight in other insects seem to be associated with the insect tracking a moving object.
Possible reasons for the observed side-slip flight
Our present results clearly show that damselflies manoeuvre and fly sideways to land on a moving perch with minimal changes in body yaw. However, it is not clear why they do so as oppose to simply turning towards their desired landing spot and manoeuvring towards it. This question is particularly intriguing because damselflies often employed the same strategy when flying to stationary perch. Why do they not fly to the stationary perch by the shortest route? First, we ruled out the option that the complex trajectories were inherent behavioural movement patterns aimed at achieving motion camouflage while tracking moving objects. In motion camouflage, the pursuer moves relative to the target in a way that would make it appear as a stationary object from the target’s perspective (Srinivasan and Davey 1995; Mizutani et al. 2003). Using this approach, a damselfly that tracks its prey or conspecific may minimize the risk of being discovered. This behavioural strategy has been observed in hoverflies (Collett and Land 1975) and dragonflies (Mizutani et al. 2003) with respect to chasing conspecifics. In order for the damselflies to camouflage their motion from the moving perch, their instantaneous t vectors (t(t), connecting their head and the perch) should intersect at a small area so that their image would remain stationary on the target’s retina (Srinivasan and Davey 1995). We therefore examined whether subsequent instantaneous range vectors intersected at a location no greater than 0.5 m from the target (in the direction of the pursuer). If they indeed intersected, we measured whether the next intersection point was located no more than 3 mm (in any direction) from the current intersection point. Our data revealed no elements of motion camouflage in exp. 1. In the remaining exps. we found that 5 out of 28 trials (17.8%) in exp. 2, 3 out of 31 trials in exp. 2.5 (9.6%) and 5 out of 14 trials (35.7%) in exp. 1′ had brief periods in the approach that according to the criteria above, could be qualified as motion camouflage. These brief segments of the pursuit were nonetheless very short and seemed to be the exception rather than the normal behaviour. Hence, we do not consider motion camouflage to be the cause of the observed behaviour.
Another option is that keeping body yaw constant improves controllability during landing by locking one parameter to limit the number of degrees of freedom needed to be controlled. However, this idea does not explain why sideways translation is more easily controlled compared to body yaw. We propose that favouring translation over rotation can be explained as an attempt to visually estimate distances from the perch and walls while minimizing rotational optic-flow from the surrounding panorama. By avoiding yaw turns, the damselfly can see the perch as well as the stationary elements of the visual panorama and acquire information both on self-movement relative to the environment and on the movement of the perch. This notion is demonstrated in Fig. 11 as two hypothetical examples of a damselfly moving next to a wall with a visual pattern. When the damselfly is moving sideways without changing the orientation of the body (Fig. 11a), the different landmarks on the wall and perch provide a pattern of retinal angular velocities (Fig. 11c) that can be used to estimate the distance to each landmark as well as the motion of the insect relative to the wall. If the damselfly instead rotates its body to follow the perch (Fig. 11b), all landmarks on the background will have the same angular velocities (Fig. 11d). In both cases the insect keeps the perch at the centre of its visual field while it moves. Keeping the perch at the centre of the visual field provides a simple steering law by which to control flight trajectory. It also facilitates estimating the speed and distance from the perch based on its expansion on the retina. Finally, linear motion (Fig. 11a) would facilitate observing the perch movement relative to the background, thus helping to separate the speed and distance of the perch from the panorama in the background. Therefore, side-slip flight employed to derive optic-flow from pure translation while approaching a moving perch can help the insect to navigate inside a cluttered environment with stationary objects, while minimizing interference from rotational optic-flow.
Sideways pursuit (a) vs. yaw rotation pursuit (b). Black silhouettes denote the initial position of the damselfly. Blue silhouettes denote damselfly position after movement. Note that in both cases the target is fixed in the centre of the field of view and moves at the same magnitude. c Angular velocity of the background perceived by the damselfly. Every line denotes a different arbitrary point on the background seen by the damselfly while flying sideways. The angular velocity of the background on the retina is higher in the middle of the field of view compared to the periphery. As the damselfly rotates (b), the angular velocity of the background remains constant and all points appear to have the same angular velocity
Head saccade during the approach
Insects can acquire new objects that enter their field of view or slip from a desired location on the field of view by making gaze shifts using fast head or body saccades (Collett and Land 1975). Such fast orientation changes enable them to fix the object within a certain anatomical region of the eye, the fovea. Head saccades avoid the need to change body orientation and minimize the visual input derived from rotational optic-flow by shortening the duration of image blur during gaze shifts (Collett and Land 1975; van Hateren and Schilstra 1999; Land 1999; Tammero and Dickinson 2002; Ribak et al. 2009). Our damselflies made more head saccades in exp. 1′ than in any other experiment. In that experiment the perch moved a greater distance per cycle, but the average speed of the perch was the same as in exp. 2.5 and it changed direction less frequently. Damselflies extended their legs to land on the perch when they were about 4 cm from it (mean = 3.9 ± 0.61 cm for all exps. except exp. 1′; 4.1 ± 1.86 cm in exp. 1′). From these distances, the amplitude of the target’s motion (6 and 15 cm) results in a subtended angle of 73° and 124°, respectively. We found that damselflies performed head saccades when the perch reached ~ 40° from the centre of their field of view. Hence, it seems that objects that move greater distances in a cycle (exp. 1′) posed a bigger challenge for the sensory-motor system, resulting in a higher likelihood of the perch reaching a higher b2t angle and thus leading to the damselflies making more frequent head saccades.
The fovea of dragonflies is anatomically associated with the area where the two compound eyes meet on the head and it is focused on a dorsal area of the visual field (Sherk 1978). Studies have shown that during predatory attacks dragonflies approach their prey from below (Corbet 1962; Olberg et al. 2005, 2007; Mischiati et al. 2014; Lin and Leonardo 2017). The compound eyes of damselflies are completely separated. Hence, it is not clear if these insects have a similar fovea. In our experiments, the mean change in altitude in the last ten wing beats indicated on a mean altitude gain of 0.3 ± 0.09 cm (mean of all the different experiments). However, there did not seem to be a very distinct preference for approaching the landing point from below (supplementary Fig. 1). The difference in approach behaviour between damselflies and dragonflies may result from the differences in eye anatomy or from the method of tracking a perch as opposed to a small target (prey). It would be interesting to correlate the differences in eye anatomy between damselflies and dragonflies with their landing behaviour.
Foraging in the natural habitat of damselflies (within the vegetation of wetlands) probably requires not only superb manoeuvring skills, but also complex visual information processing to allow collision free flight and tracking predators and prey within a complex (cluttered) and sometimes dynamic (moving) 3D visual environment. By keeping their orientation relative to the visual panorama constant while trying to land on a moving perch, damselflies may be attempting to alleviate the task of extracting visual information from their environment. The acrobatic ability to land on a perch oscillating at 2.5 Hz, and amplitudes > 18 folds the body width, underline the superb flight control and manoeuvrability of these remarkable insects.
References
Alexander DE (1986) Wind tunnel studies of turns by flying dragonflies. J Exp Biol 122:81–98
Baird E, Boeddeker N, Ibbotson MR, Srinivasan MV (2013) A universal strategy for visually guided landing. Proc Natl Acad Sci 110:18686–18691
Baird E, Fernandez DC, Wcislo WT, Warrant EJ (2015) Flight control and landing precision in the nocturnal bee Megalopta is robust to large changes in light intensity. Front Physiol 6:1–7
Boeddeker N, Hemmi JM (2010) Visual gaze control during peering flight manoeuvres in honeybees. Proc R Soc B Biol Sci 277:1209–1217
Boeddeker N, Kern R, Egelhaaf M (2003) Chasing a dummy target: smooth pursuit and velocity control in male blowflies. Proc Biol Sci 270:393–399
Bomphrey RJ, Nakata T, Henningsson P, Lin H-T (2016) Flight of the dragonflies and damselflies. Philos Trans R Soc B Biol Sci 371:20150389
Borst A (1990) How do flies land. Biosciences 40:292–299
Borst A (2014) Fly visual course control: behaviour, algorithms and circuits. Nat Rev Neurosci 15:590–599
Borst A, Bahde S (1986) What kind of movement detector is triggering the landing response of the housefly? Biol Cybern 55:59–69
Collett TS, Land MF (1975) Visual control of flight behaviour in the hoverfly Syritta pipiens L. J Comp Physiol A 99:1–66
Collett TS, Land MF (1978) How hoverflies compute interception courses. J Comp Physiol 125:191–204
Combes SA, Rundle DE, Iwasaki JM, Crall JD (2012) Linking biomechanics and ecology through predator-prey interactions: flight performance of dragonflies and their prey. J Exp Biol 215:903–913
Combes SA, Salcedo MK, Pandit MM, Iwasaki JM (2013) Capture success and efficiency of dragonflies pursuing different types of prey. Integr Comp Biol 53:787–798
Corbet PS (1962) A biology of dragonflies, 1st edn. H. F. & G. Witherby Ltd, Burley
Evangelista C, Kraft P, Dacke M et al (2010) The moment before touchdown: landing manoeuvres of the honeybee Apis mellifera. J Exp Biol 213:262–270
Ghose PK, Horiuchi TK, Krishnaprasad PS, Moss CF (2006) Echolocating bats use a nearly time-optimal strategy to intercept prey. PLoS Biol 4:e108
Gibson JJ (1951) The perception of the visual World. Am J Psychol 64:440
Gilbert C (1997) Visual control of cursorial prey pursuit by tiger beetles (Cicindelidae). J Comp Physiol A 181:217–230
Golding YC, Ennos ARR, Edmunds M (2001) Similarity in flight behaviour between the honeybee Apis mellifera (Hymenoptera: Apidae) and its presumed mimic, the dronefly Eristalis tenax (Diptera: Syrphidae). J Exp Biol 204:139–145
Goodman LJ (1960) The landing responses of insects. J Exp Biol 37:854–878
Grabow K, Rüppell G (1995) Wing loading in relation to size and flight characteristics of european Odonata. Odonatologica 24:175–186
Gravish N, Peters JM, Combes SA, Wood RJ (2015) Collective flow enhancement by tandem flapping wings. Phys Rev Lett 115:188101
Greeter JSM, Hedrick TL (2016) Direct lateral maneuvers in hawkmoths. Biol Open 5:72–82
Hedrick TL (2008) Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir Biomim 3:34001
Jantzen B, Eisner T (2008) Hindwings are unnecessary for flight but essential for execution of normal evasive flight in Lepidoptera. Proc Natl Acad Sci USA 105:16636–16640
Kane SA, Fulton AH, Rosenthal LJ (2015) When hawks attack: animal-borne video studies of goshawk pursuit and prey-evasion strategies. J Exp Biol 218:212–222
Kassner Z, Dafni E, Ribak G (2016) Kinematic compensation for wing loss in flying damselflies. J Insect Physiol 85:1–9
Koenderink JJ (1986) Optic flow. Vision Res 26:161–179
Lanchester BS, Mark RF (1975) Pursuit and prediction in the tracking of moving food by a teleost fish (Acanthaluteres spilomelanurus). J Exp Biol 63:627–645
Land MF (1999) Motion and vision: why animals move their eyes. J Comp Physiol A 185:341–352
Land MF, Collett TS (1974) Chasing behaviour of houseflies (Fannia canicularis). J Comp Physiol 89:331–357
Lee DN (1976) A theory of visual control of braking based on information about time-to-collision. Perception 5:437–459
Lee DN, Reddish PE (1981) Plummeting gannets: a paradigm of ecological optics. Nature 293:293–294
Lee DN, Reddish PE, Rand DT (1991) Aerial docking by hummingbirds. Naturwissenschaften 78:526–527
Lee DN, Davies MNO, Green PR, Weel D (1993) Visual control of velocity of approach by pigeons when landing. J Exp Biol 180:85–104
Lin H-T, Leonardo A (2017) Heuristic rules underlying dragonfly prey selection and interception. Curr Biol 27:1124–1137
Mischiati M, Lin H-T, Herold P et al (2014) Internal models direct dragonfly interception steering. Nature 517:333–338
Mizutani A, Chahl JS, Srinivasan MV (2003) Motion camouflage in dragonflies. Nature 423:604–605
Nordström K, Bolzon DM, O’Carroll DC (2011) Spatial facilitation by a high-performance dragonfly target-detecting neuron. Biol Lett 7:588–592
Olberg RM (1981) Object- and self- movement in the ventral nerve cord of the dragonfly. J Comp Physiol 334:327–334
Olberg RM (2012) Visual control of prey-capture flight in dragonflies. Curr Opin Neurobiol 22:267–271
Olberg RM, Worthington AH, Venator KR (2000) Prey pursuit and interception in dragonflies. J Comp Physiol A 186:155–162
Olberg RM, Worthington a H, Fox JL et al (2005) Prey size selection and distance estimation in foraging adult dragonflies. J Comp Physiol A 191:791–797
Olberg RM, Seaman RC, Coats MI, Henry AF (2007) Eye movements and target fixation during dragonfly prey-interception flights. J Comp Physiol A 193:685–693
Pfau HK (1991) Contributions of functional morphology to the phylogenetic systematics of Odonata. Adv Odonatol 5:109–141
Rayner JMV, Aldridge HDJN. (1985) Three-dimensional reconstruction of animal flight paths and the turning flight of microchiropteran bats. J Exp Biol 118:247–265
Reichardt W, Wenking H (1969) Optical detection and fixation of objects by fixed flying flies. Naturwissenschaften 56:424–424
Ribak G, Egge AR, Swallow JG (2009) Saccadic head rotations during walking in the stalk-eyed fly (Cyrtodiopsis dalmanni). Proc R Soc B Biol Sci 276:1643–1649
Sherk TE (1978) Development of the compound eyes of dragonflies (Odonata). III. Adult compound eyes. J Exp Zool 203:61–79
Silsby J (2001) Dragonflies of the world. Cambridge University Press, Cambridge
Sprayberry JDH, Daniel TL (2007) Flower tracking in hawkmoths: behavior and energetics. J Exp Biol 210:37–45
Srinivasan MV, Davey M (1995) Strategies for active camouflage of motion. Proc R Soc B Biol Sci 259:19–25
Srygley RB, Chai P (1990) Flight morphology of Neotropieal butterflies: palatability and distribution of mass to the thorax and abdomen. Oecologia 84:491–499
Tammero LF, Dickinson MH (2002) The influence of visual landscape on the free flight behavior of the fruit fly Drosophila melanogaster. J Exp Biol 205:327–343
Taylor GK, Krapp HG (2008) Sensory systems and flight stability: what do insects measure and why? Adv Insect Physiol 34:232–316
Tillyard RJ (1917) The biology of dragonflies (Odonata or Paraneuroptera). Cambridge university press, Cambridge
van Breugel F, Dickinson MH (2012) The visual control of landing and obstacle avoidance in the fruit fly Drosophila melanogaster. J Exp Biol 215:1783–1798
van Achterberg K, Durán JM (2011) Oviposition behaviour of four ant parasitoids (Hymenoptera, Braconidae, Euphorinae, Neoneurini and Ichneumonidae, Hybrizontinae), with the description of three new European species. Zookeys 125:59–106
van Hateren JH, Schilstra C (1999) Blowfly flight and optic flow. II. Head movements during flight. J Exp Biol 202 11:1491–1500
van Praagh JP, Ribi W, Wehrhahn C, Wittmann D (1980) Drone bees fixate the queen with the dorsal frontal part of their compound eyes. J Comp Physiol 136:263–266
Wagner H (1982) Flow-field variables trigger landing in flies. Nature 297:147–148
Wakeling JM, Ellington CP (1997) Dragonfly flight. II. Velocities, accelerations and kinematics of flapping flight. J Exp Biol 200:557–582
Wardill TJ, Fabian ST, Pettigrew AC et al (2017) A novel interception strategy in a miniature robber fly with extreme visual acuity. Curr Biol 27:854–859
Zhang SW, Xiang W, Zili LIU, Srinivasan MV (1990) Visual tracking of moving targets by freely flying honeybees. Vis Neurosci 4:379–386
Acknowledgements
We thank Zohar Yanai, Liron Goren and Michael Blecher for assistance with identifying damselflies. We thank Frida Matana Ben-Ami, Yoav Gothilf and Maor Knafo for providing live food for feeding the damselflies and Shira Holand for aiding us with the damselflies’ figures. We also thank the staff of the Meier I. Segals Garden for Zoological Research for logistical support. All experiments with animals were carried out in accord with the laws of Israel, and the guidelines outlined by Tel Aviv University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Number of trials for each individual in each experiment (TIF 921 KB)
Supplementary Figure 1
Frequency of occurrence of altitude change during the last 300 ms of the approach in the various experiments. Positive and negative altitude change values indicate on an approach from below and from above the final landing point, respectively (TIF 660 KB)
Supplementary Film 1 Stationary experiment. Straight flight towards the target. The trajectory an orientation of the insect is depicted in 3D in Fig. 3a of the main text (WMV 8049 KB)
Supplementary Film 2 Stationary experiment. The damselfly flies sideways without altering its body orientation towards the stationary target. The trajectory an orientation of the insect is depicted in 3D in Fig. 3b of the main text (WMV 2853 KB)
Supplementary Film 3 Experiment 2. The damselfly flies sideways while minimizing body orientation changes. The trajectory an orientation of the insect is depicted in 3D in Fig. 3c of the main text (WMV 11260 KB)
Supplementary Film 4 A damselfly landing on the moving perch moving at 2 Hz shown at actual speed (WMV 2853 KB)
Rights and permissions
About this article
Cite this article
Kassner, Z., Ribak, G. Role of side-slip flight in target pursuit: blue-tailed damselflies (Ischnura elegans) avoid body rotation while approaching a moving perch. J Comp Physiol A 204, 561–577 (2018). https://doi.org/10.1007/s00359-018-1261-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-018-1261-5