Abstract
Purpose
To investigate the influencing factors of acute kidney injury (AKI) following retrograde intrarenal surgery (RIRS).
Methods
The data of patients who underwent RIRS for kidney stones between January 2018 and June 2022 at two tertiary centers were retrospectively analyzed. Demographic data of patients were obtained. According to kidney disease: Improving Global Outcomes (KDIGO) criteria, those with and without AKI were divided into two groups. Preoperative, intraoperative, and postoperative predictive factors of patients were investigated between the groups. In addition, the influencing factors of AKI were examined by multivariate analysis.
Results
This study included 295 (35.7%) women and 532 (64.3%) men. The mean age was 50.03 ± 15.4 years (range 18–89), and mean stone size was 15.5 ± 6.1 mm (range 6–47). Overall, 672 of patients (81.3%) were stone-free after the initial treatment. According to KDIGO, 110 of patients (13.3%) had AKI during the postoperative period. Univariate analysis showed that stone size (P = .003), previous stone surgery (P = .010), renal malformations (P = .017), high operative time (P = < .001), high preoperative creatinine value (P = .036), intraoperative complications (P = .018), and postoperative urinary tract infection (P = .003) had significant influence on the AKI after RIRS. Multivariate analysis excluded previous stone surgery, high preoperative creatinine value, renal malformations, and intraoperative complications from the logistic regression model, whereas other factors maintained their statistically significant effect on AKI, indicating that they were independent predictors.
Conclusions
Stone size, operative time, postoperative urinary tract infection, and diabetes mellitus are significant predictors of AKI. During RIRS, urologists should consider the factors that increase the risk of AKI and evaluate the treatment outcomes based on these factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Socioeconomic status, environmental factors, genetic predisposition, and certain metabolic disorders are risk factors for kidney stones [1]. Although the risk for stone recurrence varies, it has been estimated as 50% between the 5 and 10 years [2]. Emerging evidence demonstrates that nephrolithiasis may increase the risk of chronic kidney disease [3].
Kidney stones can be treated with several modalities, including shock wave lithotripsy, retrograde intrarenal surgery (RIRS), and percutaneous nephrolithotomy (PNL). The choice of treatment depends on several factors, such as stone size, symptom severity, obstruction degree, kidney function, stone location, and urinary tract infection (UTI) status [4].
Among treatment options, RIRS has contributed greatly to active stone removal and is preferred worldwide by numerous urologist [4]. The incidence of RIRS-related complications ranges between 9 and 25%, and the majority of these complications are minor complications not requiring intervention, such as fever, infection, hematuria, and postoperative pain [5,6,7]. The rate of complications will increase gradually, especially if the improved effectiveness of RIRS over time is considered. Interestingly, the incidence of complications related to PNL, a more invasive procedure, varies between 3 and 34%. The rate of RIRS-related complications may be attributed to the gradual increase in the utilization of RIRS [8].
Relevant literature studies on RIRS focus on varying factors, including infection, urosepsis, and stone-free rates. Moreover, comparisons have involved patient-related factors (e.g., age, sex, and systemic diseases), stone-related factors (e.g., stone location and size), and intraoperative factors (e.g., operative time and intraoperative complications) [5, 9,10,11]. Increased intrarenal pressure (IRP) during RIRS plays an important role in forniceal rupture and renal parenchymal damage due to congestion; it induces pathophysiological processes in the upper urinary tract, including intrarenal or pyelovenous backflow and ureteral contractions [12, 13]. However, to our best knowledge, no detailed studies have evaluated the effect of RIRS on kidney function. In the present study, the primary aim was to investigate the impact of RIRS on kidney function along with relevant factors. The KDIGO criteria, which can be practically and easily utilized, were used to evaluate acute kidney injury (AKI) (stage 1: 1.5–1.9 times higher than the baseline, an increase of 0.3 mg/dL (26.5 mmol/L), or ˂ 0.5 mL/kg/h for 6–12 h) [14]. Meanwhile, the secondary aim of the present study was to compare the preoperative, intraoperative, and postoperative influencing factors in patients with and without AKI according to the KDIGO criteria.
Material and methods
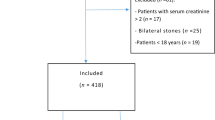
In this study, the data of 827 patients who underwent RIRS for kidney stones (295 women and 532 men with a mean age of 50.03 ± 15.4 years [range, 18–89 years]) were retrospectively analyzed. The single inclusion criterion was RIRS for kidney stones, while the exclusion criteria were age under 18 years, end-stage kidney disease, and additional surgical interventions during RIRS.
All procedures were performed using either a 7.5 Fr (Flex-X®, Karl Storz, Tuttlingen, Germany) or an 8.4 Fr (Olympus America Inc., Center Valley, PA, USA) flexible ureteroscope, a 9.5 Fr single-use flexible ureteroscope (Uscope 3022®, Zhuhai Pusen Medical Technology Co., Ltd., Zhuhai, China), or a 9.3 Fr Innovex single-use flexible ureteroscope (Anqing Medical, Shanghai, China).
During RIRS, the patients were placed in the lithotomy position and underwent active dilatation using an 8-French (Fr) semi-rigid ureteroscope (Karl Storz, Tuttlingen, Germany). After placement of the safety guidewire, the usage of UAS was decided according to the surgeon's preference or the thickness of the scope. If the UAS was placed, a 9.5–11 Fr or 10–12 Fr or 12–14 Fr (35 cm) access sheath (Cook Medical, Bloomington, IN, USA) was used in appropriate cases. Under scopic guidance, the stones were reached and fragmented under direct visualization. In cases of ureteral pathologies that would hinder the passing of the scope, a JJ stent was placed, and the surgery was postponed to 2 weeks later.
The stones were fragmented using a holmium: YAG laser (Dornier MedTech, Wessling, Germany) until the fragments were small enough to pass out spontaneously. A JJ stent was placed at the end of the surgery in cases of increased stone size, edema, or bleeding. Fragments sized above 4 mm were considered as residual stones. The residual stones were evaluated by X-ray of the kidney, ureter, and bladder and USG at 2–4 weeks later at the outpatient clinic. The modified Satava and Clavien classification systems were utilized for evaluating the intraoperative and postoperative complications of RIRS. On abdominopelvic CT, we measured for single stones as the largest diameter of the stone, and we defined the sum of long axes in the case of multiple stones.
Patient demographic and clinical data included age, presence of systemic diseases or anatomic malformations, stone location, stone size, HU, preoperative BUN level, eGFR, urine analysis findings, and urine culture values. Preoperative data consisted of the operative time, JJ stent insertion, stone removal in one or more sessions, and presence of residual stones. Along with the preoperative and intraoperative data, any postoperative complications were also evaluated. The patients were divided into two groups according to the presence of AKI postoperatively: Group 1 consisted of patients with AKI, while Group 2 consisted of those without AKI. Data were compared between the groups, and the influencing factors of AKI were determined according to the KDIGO criteria.
Statistical analysis
The Statistical Package for Social Sciences (SPSS®, IBM in Armonk, New York, USA) was used for the analysis of the data obtained from Group1 and Group 2. Chi-square test and independent T-test were used for comparison of the groups subsequent to the assessment of the data whether it is fit for normal distribution. Logistic regression analysis was performed to evaluate the factors affecting AKI. Data, which considered as statistically significant in the univariate analysis and considered to be clinically effective were included to multivariate analysis. 95% CI and P < 0.05 were the thresholds for statistical significance.
Results
This study included 827 patients who underwent RIRS for kidney stones. Of them, 295 (35.7%) were women, and 532 (64.3%) were men. The mean patient age was 50.03 ± 15.4 years (range 18–89 years). The mean stone size was 15.5 ± 6.1 mm (range 6–47 mm), and approximately 44.3% of the stones were lower calyx stones. The mean operative time was 51.6 ± 12.4 min (range 30–105 min). The mean preoperative and postoperative creatinine levels were 0.88 ± 0.26 and 0.94 ± 0.37, respectively. The SFR was 81.3% (Table 1). Approximately 13.1% and 16.6% developed intraoperative and postoperative complications, respectively (Supplementary Table). According to the KDIGO criteria, 110 (13.3%) patients had AKI postoperatively.
Preoperative factors
The mean age and sex in Groups 1 and 2 were similar (age: t-test, P = 0.74; sex: Chi-square test, P = 0.638). The mean stone size was larger in Group 1 (17.4 ± 6.1 mm) than in Group 2 (15.3 ± 6.1 mm) (t-test, P = 0.003). Conversely, the number of stones (single or multiple) was similar between the groups (Chi-square test, P = 0.639).
Forty (36.4%) patients in Group 1 and 130 (18.1%) patients in Group 2 had diabetes mellitus (Chi-square test, P < 0.001). Meanwhile, the number of patients with hypertension and chronic kidney disease was similar between the groups (Chi-square test, P = 0.349 and P = 0.184, respectively.
The proportion of patients previously treated for stones was higher in Group 1 (52.7%) than in Group 2 (38.8%) (Chi-square test, P = 0.011). Similarly, the proportion of patients with renal anomalies was higher in Group 1 (12.7%) than in Group 2 (5.6%) (Chi-square test, P = 0.020).
The rate of the presence of a JJ stent preoperatively was similar between the groups (30 patients in Group 1 and 199 patients in Group 2; P = 1.000). Preoperative UTI was present in 144 (20.1%) patients in Group 1 and 35 (31.8%) patients in Group 2. The rate of preoperative UTI was higher in Group 1 than in Group 2 (Chi-square test, P = 0.013). The mean preoperative creatinine levels in Groups 1 and 2 were 0.98 ± 0.4 and 0.86 ± 0.2, respectively, indicating a higher level in Group 1 (t-test, P = 0.007).
Intraoperative factors
The mean operative time was 75.3 ± 16.4 min in Group 1 and 55.2 ± 13.9 min in Group 2, indicating a longer operative time in Group 1 (t-test, P < 0.001).
The rate of access sheath usage was similar between the groups (Chi-square test, P = 0.087). In addition, the rates of disposable or reusable flexible ureterorenoscope use and postoperative stenting did not differ between them (P = 0.452 and P = 0.147, respectively) (Table 1).
Postoperative factors
The SFR was 80% (n = 88) in Group 1 and 81.5% (n = 584) in Group 2, indicating a similar rate between them (Chi-square test, P = 0.607). However, the hospitalization time was longer in Group 1 than in Group 2 (Mann–Whitney U test, P < 0.001).
Postoperative complications occurred in 34 (34.4%) patients in Group 1 and in 103 (14.4%) patients in Group 2. The postoperative complication rate was higher in Group 1 than in the Group 2 (Chi-square test, P < 0.001) (Table 1).
Influencing factors of AKI
Herein, the influencing factors of AKI were evaluated. In the univariate analysis, eight factors were significantly associated with an increased rate of AKI according to the KDIGO criteria (Table 2). However, in the multivariate analysis, diabetes mellitus (odds ratio = 3.8, P = 0.008), postoperative UTI (odds ratio = 5.5, P = 0.018), long operative time (odds ratio = 2.8, P = 0.042), and stone size (odds ratio = 3.1, P = 0.02) were associated with an increased rate of AKI (Table 2).
Discussion
Awareness of the factors influencing kidney function after RIRS is of utmost importance among both clinicians and patients. To our best knowledge, the present study is the first to evaluate specific influencing factors of kidney function following RIRS affecting up to 14% of the patients. According to the KDIGO criteria, the independent variables impacting kidney function were postoperative UTI, stone size, operative time, and diabetes mellitus. During treatment planning for patients with these factors, utmost care must be taken. Recognizing risk factors is important in assisting urologists with preoperative risk stratification to consequently provide individualized treatment recommendations and inform postoperative surveillance regimens. Moreover, early identification and appropriate management of high-risk patients could reduce postoperative morbidity and limit health care utilization. With these last two aspects, the influencing factors of kidney function after RIRS require further investigation.
RIRS is an effective and safe method, especially in the fragmentation of moderately sized kidney stones, with high SFRs. The SFR of this surgery was 81.3% in our study and 69.7–97% in previous literature [15,16,17]. Hence, the use of RIRS is expanding over time. The effectiveness of RIRS has been evaluated and compared with that of other methods considering various factors, including old age, kidney anomaly, lower calyceal stone, and large stone size. Similar SFRs have been observed, although the complication rates were either similar or at an acceptable level [10, 15,16,17].
During RIRS, kidney function is affected more than expected. The most important problem in RIRS is the inability to precisely evaluate increases in the IRP and the effect of current infection on the kidneys. In animal and human studies, pathological changes begin in the kidneys when the IRP exceeds 40 cm H2O [18, 19]. High pressures can also induce renal oxidative damage and secondary loss of kidney function due to insufficient venous flow and compression of micro vessels. Renal venous outflow obstruction is more detrimental to the kidneys than arterial obstruction owing to the presence of ischemia or reperfusion injury [20]. Moreover, in the acute phase of renal pressure elevation, the renal tubules show marked vacuolization and degeneration along with histological signs of metaplasia and pericalyceal vasculitis. These findings can occur even 4–6 weeks after the procedure [19, 21]. Similarly, in pressure-associated forniceal rupture, pyelosinous backflow has been associated with perirenal pseudocyst, retroperitoneal edema, fibrolipomatosis, perinephritic abscess, and perirenal hemorrhage [19]. In the present study, the increases in the intervention time and stone size were considered to prolong this period, thus markedly affecting kidney function.
There are different opinions regarding the use of UAS, and the relationship between ureterorenoscope diameter and complications. IRP is reduced by selecting larger UAS and a small ureteroscope, thus may reducing infection [22, 23]. On the contrary, another prospective study stated that the use of UAS in the treatment of large kidney stones was ineffective in reducing complications [24]. In the systematic review by EAU Section of Urolithiasis, Chugh S et al. stated the use of UAS was 25.8% patients and ranged across studies [5]. In our study, the use of UAS was 36.9%, and mostly in reusable URS. The reason for this was the smaller diameter of UAS. Similar to our study, the decrease in the use of UAS in recent studies may be due to the avoidance of complications related to UAS, surgical experience and the increase in single-use URS. In the absence of randomized data, the true impact of UAS on surgical outcome remains unclear [25]. In our study, there were diameter differences in URS types. In the current literature, there was no difference between disposable and reusable ureterorenoscopies in terms of SFR, operative time, urosepsis, infection, complications, and hospitalization time, even in those with diameter differences [26, 27]. The results of our study were similar.
Another important factor affecting kidney function is infection. IRP elevation and infection affect each other and increase the risk of sepsis to approximately 8.1%. An increased stone load and associated prolonged operative time also increase the risk of bacteremia and sepsis [28]. In the meta-analysis by Sun et al. comparing 14 studies, a positive preoperative urine culture was the most important risk factor, followed by female sex, diabetes mellitus, stent placement, and prolonged operative time [9]. Age, cumulative stone diameter, and renal failure were not potential risk factors. In the meta-analysis by Chugh et al. [5], the risk of urinary infection and sepsis after ureteroscopy was higher in patients with a high Charlson comorbidity index, elderly patients, female patients, patients with long-term permanent ureteral stents before the procedure, and patients with a neurogenic bladder and high body mass index. Ma and colleagues performed a similar review where they identified female sex, preoperative stent placement, diabetes mellitus, positive preoperative urine culture, and longer procedure time as the key determinants of the postoperative urosepsis risk [29]. In these three studies, the use of rigid and flexible ureteroscopes was evaluated simultaneously. However, the disease risk is increased with the use of a flexible ureteroscope per se. If these outcomes are compared with those in our study, the risk factors would be similar. While the risk increases fivefold in the presence of postoperative UTI, the risk is close by fourfold in the presence of diabetes mellitus and increases threefold with an increased stone size.
Studies investigating the effect of RIRS on kidney function are limited, and no reports have evaluated the adverse effects after surgery on solitary kidneys. Lai et al.[30] evaluated RIRS in 60 patients with solitary kidneys and observed a positive change in the creatinine level after 1 month; they also found that only nine patients had grade 1 and 2 complications. Guisti et al.[31] could not determine a significant difference in the serum creatinine levels of their 29 patients on the first and third postoperative months despite the presence of a minor increase. Although the number of patients in the above-mentioned studies is small, their findings are valuable. In study by Hoarau et al. [32], which was analyzed 163 patients with renal stones treated by RIRS, renal function deterioration occurred in 4.9% of the patients and renal function improvement occurred in 14.1% of the patients. In their study, preoperative chronic kidney disease and multiple procedures have affected kidney function at a mean follow-up of ten months in the univariate analysis; however, they did not find a significant factor in the multivariate analysis [32]. On the contrary, the effect of PNL on kidney function has been investigated more widely. PNL yielded small parenchymal scars and reduced focal function, especially in the entrance area. Approximately 5.6% of patients have been reported to have impaired kidney functions [33,34,35]. Furthermore, kidney function was reported to be more affected in the presence of staghorn stone, solitary kidney, hypertension, urinary diversion, neurogenic bladder, and recurrent stone disease [33,34,35]. Considering previous and the present findings, patient-related risk factors are the key determinants of kidney function in both PNL and RIRS. History of nephrolithiasis is the important predictor for risk of chronic kidney disease [36]. In most of the studies related to the RIRS in recent years, the primary outcome is the efficacy of RIRS (SFR) and the secondary outcome on safety of RIRS. On safety, especially the most common major complications (urosepsis, modified Clavien score) were compared. Theoretically, it is obvious that RIRS will cause kidney damage through its direct mechanical effect and indirect effects (IRP, inflammation, pericalyceal vasculitis) on the kidney. However, the short- and long-term effects of RIRS on renal tissue has not been comprehensively studied. Our aim was to provide information about the results on the short-term effects on renal tissue of the RIRS by comparing several factors.
Kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin are the leading novel biomarkers used efficiently in the diagnosis of AKI. The levels of these biomarkers increase especially in the early period of nephrotoxic and ischemic renal damage [37]. Several publications show that their levels increase during ureteroscopy and in the early postoperative period [38]. However, the most important limitations of these biomarkers are that they are not used routinely, do not have a cut-off value, and can differ between diagnostic tests. In the present study, the KDIGO criteria were preferred, as they are a practical and easily applicable parameter compared with serum creatine level changes before and after surgery. Our patients had low KDIGO stages; however, our data on the effect of RIRS on kidney function are valuable.
The limitations of the current study include the inherent limitation of a retrospective design, inclusion of heterogeneous groups, comparison of multiple factors, short-term evaluation of the BUN level, and lack of a long-term evaluation. In addition, other limitation was that we could not evaluate or regulate factors such as UAS use, URS diameter, laser duration, fragmentation type, laser energy power more appropriately and in detail.
Conclusion
Stone size, operative time, postoperative UTI, and diabetes mellitus are significant predictors of AKI. During RIRS, urologists should consider the factors that increase the risk of AKI and evaluate the treatment outcomes based on these factors.
Data availability
Data are available on request.
References
Trinchieri A (2008) Epidemiology of urolithiasis: an update. Clin Cases Miner Bone Metab 5:101–106
Rule AD, Lieske JC, Li X, MeltonKrambeck LJAE, Bergstralh EJ (2014) The ROKS nomogram for predicting a second symptomatic stone episode. J Am Soc Nephrol 25:2878–2886. https://doi.org/10.1681/asn.2013091011
Zhe M, Hang Z (2017) Nephrolithiasis as a risk factor of chronic kidney disease: a meta-analysis of cohort studies with 4,770,691 participants. Urolithiasis 45:441–448. https://doi.org/10.1007/s00240-016-0938-x
Geraghty RM, Jones P, Somani BK (2017) Worldwide trends of urinary stone disease treatment over the last two decades: a systematic review. J Endourol 31:547–556. https://doi.org/10.1089/end.2016.0895
Chugh S, Pietropaolo A, Montanari E, Sarica K, Somani BK (2020) Predictors of urinary infections and urosepsis after ureteroscopy for stone disease: a systematic review from EAU section of urolithiasis (EULIS). Curr Urol Rep 21:16. https://doi.org/10.1007/s11934-020-0969-2
Perez Castro E, Osther PJ, Jinga V, Razvi H, Stravodimos KG, Parikh K, Kural AR et al (2014) Differences in ureteroscopic stone treatment and outcomes for distal, mid-, proximal, or multiple ureteral locations: the Clinical Research Office of the Endourological Society ureteroscopy global study. Eur Urol 66:102–109. https://doi.org/10.1016/j.eururo.2014.01.011
Somani BK, Giusti G, Sun Y, Osther PJ, Frank M, De Sio M, Turna B et al (2017) Complications associated with ureterorenoscopy (URS) related to treatment of urolithiasis: the clinical research office of endourological society URS global study. World J Urol 35:675–681. https://doi.org/10.1007/s00345-016-1909-0
Wollin DA, Preminger GM (2018) Percutaneous nephrolithotomy: complications and how to deal with them. Urolithiasis 46:87–97. https://doi.org/10.1007/s00240-017-1022-x
Sun J, Xu J, OuYang J (2020) Risk factors of infectious complications following ureteroscopy: a systematic review and meta-analysis. Urol Int 104:113–124. https://doi.org/10.1159/000504326
de la Rosette J, Denstedt J, Geavlete P, Keeley F, Matsuda T, Pearle M, Preminger G et al (2014) The clinical research office of the endourological society ureteroscopy global study: indications, complications, and outcomes in 11,885 patients. J Endourol 28:131–139. https://doi.org/10.1089/end.2013.0436
Kang SK, Cho KS, Kang DH, Jung HD, Kwon JK, Lee JY (2017) Systematic review and meta-analysis to compare success rates of retrograde intrarenal surgery versus percutaneous nephrolithotomy for renal stones >2 cm: an update. Medicine 96:e9119. https://doi.org/10.1097/md.0000000000009119
Osther PJS (2018) Risks of flexible ureterorenoscopy: pathophysiology and prevention. Urolithiasis 46:59–67. https://doi.org/10.1007/s00240-017-1018-6
Tokas T, Herrmann TRW, Skolarikos A, Nagele U (2019) Pressure matters: intrarenal pressures during normal and pathological conditions, and impact of increased values to renal physiology. World J Urol 37:125–131. https://doi.org/10.1007/s00345-018-2378-4
Kellum JA, Lameire N (2013) Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 17:204. https://doi.org/10.1186/cc11454
De S, Autorino R, Kim FJ, Zargar H, Laydner H, Balsamo R, Torricelli FC et al (2015) Percutaneous nephrolithotomy versus retrograde intrarenal surgery: a systematic review and meta-analysis. Eur Urol 67:125–137. https://doi.org/10.1016/j.eururo.2014.07.003
Bozzini G, Verze P, Arcaniolo D, Dal Piaz O, Buffi NM, Guazzoni G, Provenzano M et al (2017) A prospective randomized comparison among SWL, PCNL and RIRS for lower calyceal stones less than 2 cm: a multicenter experience: a better understanding on the treatment options for lower pole stones. World J Urol 35:1967–1975. https://doi.org/10.1007/s00345-017-2084-7
De Coninck V, Keller EX, Somani B, Giusti G, Proietti S, Rodriguez-Socarras M, Rodríguez-Monsalve M et al (2020) Complications of ureteroscopy: a complete overview. World J Urol 38:2147–2166. https://doi.org/10.1007/s00345-019-03012-1
Kukreja RA, Desai MR, Sabnis RB, Patel SH (2002) Fluid absorption during percutaneous nephrolithotomy: does it matter? J Endourol 16:221–224. https://doi.org/10.1089/089277902753752160
Jung HU, Frimodt-Møller PC, Osther PJ, Mortensen J (2006) Pharmacological effect on pyeloureteric dynamics with a clinical perspective: a review of the literature. Urol Res 34:341–350. https://doi.org/10.1007/s00240-006-0069-x
Li X, Liu M, Bedja D, Thoburn C, Gabrielson K, Racusen L, Rabb H (2012) Acute renal venous obstruction is more detrimental to the kidney than arterial occlusion: implication for murine models of acute kidney injury. Am J Physiol Renal Physiol 302:F519-525. https://doi.org/10.1152/ajprenal.00011.2011
Schwalb DM, Eshghi M, Davidian M, Franco I (1993) Morphological and physiological changes in the urinary tract associated with ureteral dilation and ureteropyeloscopy: an experimental study. J Urol 149:1576–1585. https://doi.org/10.1016/s0022-5347(17)36456-x
MacCraith E, Yap LC, Elamin M, Patterson K, Brady CM, Hennessey DB (2021) Evaluation of the impact of ureteroscope, access sheath, and irrigation system selection on intrarenal pressures in a porcine kidney model. J Endourol 35:512–517. https://doi.org/10.1089/end.2020.0838
Traxer O, Wendt-Nordahl G, Sodha H, Rassweiler J, Meretyk S, Tefekli A, Coz F et al (2015) Differences in renal stone treatment and outcomes for patients treated either with or without the support of a ureteral access sheath: The Clinical Research Office of the Endourological Society Ureteroscopy Global Study. World J Urol 33:2137–2144. https://doi.org/10.1007/s00345-015-1582-8
Geraghty RM, Ishii H, Somani BK (2016) Outcomes of flexible ureteroscopy and laser fragmentation for treatment of large renal stones with and without the use of ureteral access sheaths: Results from a university hospital with a review of literature. Scand J Urol 50:216–219. https://doi.org/10.3109/21681805.2015.1121407
De Coninck V, Keller EX, Rodríguez-Monsalve M, Audouin M, Doizi S, Traxer O (2018) Systematic review of ureteral access sheaths: facts and myths. BJU Int 122:959–969. https://doi.org/10.1111/bju.14389
Meng C, Peng L, Li J, Li Y, Li J, Wu J (2021) Comparison Between Single-Use Flexible Ureteroscope and Reusable Flexible Ureteroscope for Upper Urinary Calculi: A Systematic Review and Meta-Analysis. Front Surg 8:691170. https://doi.org/10.3389/fsurg.2021.691170
Davis NF, Quinlan MR, Browne C, Bhatt NR, Manecksha RP, D’Arcy FT, Lawrentschuk N et al (2018) Single-use flexible ureteropyeloscopy: a systematic review. World J Urol 36:529–536. https://doi.org/10.1007/s00345-017-2131-4
Zhong W, Leto G, Wang L, Zeng G (2015) Systemic inflammatory response syndrome after flexible ureteroscopic lithotripsy: a study of risk factors. J Endourol 29:25–28. https://doi.org/10.1089/end.2014.0409
Ma YC, Jian ZY, Yuan C, Li H, Wang KJ (2020) Risk factors of infectious complications after ureteroscopy: a systematic review and meta-analysis based on adjusted effect estimate. Surg Infect (Larchmt) 21:811–822. https://doi.org/10.1089/sur.2020.013
Lai D, Chen M, He Y, Li X, Wan S (2018) Safety and efficacy of retrograde intrarenal surgery for the treatment of renal stone in solitary kidney patients. Ren Fail 40:390–394. https://doi.org/10.1080/0886022x.2018.1487861
Giusti G, Proietti S, Cindolo L, Peschechera R, Sortino G, Berardinelli F, Taverna G (2015) Is retrograde intrarenal surgery a viable treatment option for renal stones in patients with solitary kidney? World J Urol 33:309–314. https://doi.org/10.1007/s00345-014-1305-6
Hoarau N, Martin F, Lebdai S, Chautard D, Culty T, Azzouzi AR, Bigot P (2015) Impact of retrograde flexible ureteroscopy and intracorporeal lithotripsy on kidney functional outcomes. Int Braz J Urol 41:920–926. https://doi.org/10.1590/s1677-5538.Ibju.2014.0402
Kurien A, Baishya R, Mishra S, Ganpule A, Muthu V, Sabnis R, Desai M (2009) The impact of percutaneous nephrolithotomy in patients with chronic kidney disease. J Endourol 23:1403–1407. https://doi.org/10.1089/end.2009.0339
Moskovitz B, Halachmi S, Sopov V, Burbara J, Horev N, Groshar D, Nativ O (2006) Effect of percutaneous nephrolithotripsy on renal function: assessment with quantitative SPECT of (99m)Tc-DMSA renal scintigraphy. J Endourol 20:102–106. https://doi.org/10.1089/end.2006.20.102
Nouralizadeh A, Sichani MM, Kashi AH (2011) Impacts of percutaneous nephrolithotomy on the estimated glomerular filtration rate during the first few days after surgery. Urol Res 39:129–133. https://doi.org/10.1007/s00240-010-0310-5
Keddis MT, Rule AD (2013) Nephrolithiasis and loss of kidney function. Curr Opin Nephrol Hypertens 22:390–396. https://doi.org/10.1097/MNH.0b013e32836214b9
Devarajan P (2011) Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr 23:194–200. https://doi.org/10.1097/MOP.0b013e328343f4dd
Göger YE, Özkent MS, Topçu C, Atıcı A, Sönmez MG, Balasar M, Gürbilek M (2022) Can urinary KIM-1 and NGAL predict management endoscopic surgery in acute unilateral obstructive stone disease? Results from a prospective cohort study. Urol Int 106:446–454. https://doi.org/10.1159/000517883
Acknowledgements
None
Funding
The authors declare that they have no financial competing interests exist.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest disclosure
The authors declare that they have no competing interests exist.
Ethics of approval statement
The institutional human research ethics committee approved the protocol 2021/2993 (Necmettin Erbakan University, Meram School of Medicine, Ethics Committee). The analysis and data collection were performed in compliance with the Declaration of Helsinki.
Patient consent statement
An information leaflet was given, and informed consent was obtained for all patients.
The clinical trial registration number
This present study does not require a clinical trial registration, due to the retrospective nature of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Göger, Y.E., Özkent, M.S., Kılınç, M.T. et al. Influencing factors of acute kidney injury following retrograde intrarenal surgery. World J Urol 41, 857–864 (2023). https://doi.org/10.1007/s00345-023-04301-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04301-6