Abstract
Background
Melanoma antigen gene A2 (MAGE-A2) is one of the most cancer–testis antigens overexpressed in various types of cancers. Silencing the MAGE-A2 expression inhibited the proliferation of prostate cancer (PCa) cells and increased the chemosensitivity. However, the expression pattern of MAGE-A2 in PCa tissue samples and its prognostic and therapeutic values for PCa patients is still unclear.
Methods
In this study, for the first time, the staining pattern and clinical significance of MAGE-A2 were evaluated in 166 paraffin-embedded prostate tissues, including 148 cases of PCa and 18 cases of high-grade prostatic intraepithelial neoplasia (HPIN), by immunohistochemical analysis.
Results
The simultaneous expression of both nuclear and cytoplasmic patterns of MAGE-A2 with different staining intensities was observed among studied cases. Increased expression of MAGE-A2 was significantly found in PCa tissues compared to HPIN cases (P < 0.0001). Among PCa samples, the strong staining intensity of nuclear expression was predominantly observed in comparison with cytoplasmic expression in PCa tissues (P < 0.0001). A significant and inverse correlation was found between the cytoplasmic expression of MAGE-A2 and increased Gleason score (P = 0.002). Increased cytoplasmic expression of MAGE-A2 was associated with longer biochemical recurrence-free survival (BCR-FS) and disease-free survival (DFS) of patients (P = 0.002, P = 0.001, respectively). In multivariate analysis, Gleason score and cytoplasmic expression of MAGE-A2 were independent predictors of the BCR-FS (P = 0.014; P = 0.028, respectively).
Conclusions
Taken together, cytoplasmic expression of MAGE-A2 was inversely proportional to the malignant grade and duration of recurrence of the disease in patients with PCa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is one of the most common cancers that can be diagnosed. In the United States, one in five cancers diagnosed is the PCa. It is estimated that approximately 164,690 Americans will develop PCa [1]. PCa is a serious health threat to men of advanced age, and the second leading cause of cancer-associated death among men population [2]. Androgen ablation or hormone therapy is the first line of treatment for PCa as well as benign prostatic hyperplasia (BPH). However, advanced and resistant type of PCa named castrate-resistant prostate cancer (CRPCa) is associated with metastasis and death. In this case, standard treatments, such as androgen deprivation and chemotherapy, will not cure and new strategies for the treatment of PCa are crucial [3].
Recent studies have shown that active or passive immunotherapy either alone or in combination with current treatments can be an effective therapeutic strategy for advanced PCa; thus, it is essential to identify and characterize new specific antigens that are up-regulated in PCa [4]. In addition, due to the high prevalence of the PCa, false-positive results of prostate-specific antigen (PSA) measurement, the prevalence of the disease in the elderly population, and possible side effects of treatment, there is always a need to identify new biomarkers in PCa to distinguish high-grade malignancy from low grade as well as disease progression. Subsequently, new and effective therapies can be established and developed for CRPCa [5].
Cancer/testis antigens (CTAs) are a group of tumor-associated antigens (TAA) and a unique class of heterogeneous proteins that their expressions are restricted in immune-privileged sites, such as the placenta, fetal ovary, and testes, but not in other normal tissues. However, these antigens are aberrantly overexpressed in many types of cancers, reflect DNA hypomethylation and the activation of a silenced genetic program, which ultimately leads to cancer progression and tumor immunogenicity [6]. According to chromosomal location, CTAs are classified into two groups: the CT-X antigens are on the X chromosome and non-X CT antigens on the other chromosomes [7]. The immunogenicity of CTAs in cancer has made them a suitable candidate for vaccination in many solid tumors. Among these antigens, melanoma-associated antigen (MAGE) have considered a promising TAA for cancer immunotherapy [8].
Among MAGE family, the MAGE-A sub-family are encoded by 12 homologous genes called MAGE-A1–MAGE-A12 and located on chromosome Xq28. MAGE-As have been identified in many tumor types, including non-small-cell lung carcinoma (NSCLC) [9], breast cancer [10], osteosarcoma [11], diffuse large B-cell testicular lymphoma [12], synovial sarcoma [13], gastric cancer [14], colorectal cancer [14], brain tumor [15], and PCa [7]. It has been shown that the gene expression of MAGE-A2 was up-regulated in CRPC. The silencing MAGE-A2 impaired proliferation of prostate cancer cells, while increasing the sensitivity of cells to chemotherapy drugs [7]. However, its protein expression and clinical significance are not apparent in patients with PCa.
We have conducted the present study to evaluate the pattern of MAGE-A2 expression in a large series of PCa patients and high-grade prostatic intraepithelial neoplasia (HPIN) after prostatectomy and needle sampling. In addition, we have analyzed the relationship between expression of MAGE-As and clinicopathologic outcomes.
Material and methods
A total of 166 paraffin-embedded prostate tissues, including 148 PCa (including 118 prostatectomy samples and 30 needle biopsies) and 18 HPIN samples, were obtained from Hasheminejad Kidney Center (major university-based referral Urology Hospital in Tehran, Iran) after obtaining informed consent from patients. These samples pertained to patients who underwent simple prostatectomy, radical prostatectomy, and PCa needle biopsies before receiving standard current treatments, including hormone therapy, radiotherapy, or chemotherapy. None of the surgical patients also received preoperative hormone or radiation therapy. All of these samples were diagnosed over the time period of 2006–2017 in Hasheminejad Kidney Center. Pathological reports were reviewed to collect diagnosis and other pathologic reports, including tumor type and grade (Gleason score; Gs), tumor volume, vascular invasion, lymph node involvement, the involvement of the adjacent tissues with tumor, and pathologic tumor stage (pTNM staging). Medical reports of all patients were also reviewed to collect patients’ age and serum PSA level. Stained slides with hematoxylin and eosin (H&E) were evaluated to identify the best area of paraffin-embedded tissues for preparing tissue microarray (TMA) of each specimen.
The pTNM was defined according to the AJCC/UICC TNM staging system and tumor grade as Gs system was determined based on the International Scoring System Society of Urological Pathology (ISUP) consensus [16, 17].
Regarding available PCa patients, we collected survival data on biochemical recurrence (BCR; the increase of serum PSA level to above 0.2 ng/ml with a steady increase), time of clinical symptoms, treatments received after surgery, patients who were refractory or susceptible to treatment, and cause of death from the year of surgery or primary treatments until the end of 2019. BCR was defined as the time from radical prostatectomy or primary treatment to the date of the rise in serum PSA in PCa. Disease-free survival (DFS) rates in PCa were calculated from the date of radical prostatectomy to BCR, clinical symptoms, metastasis, or PCa-related death during treatment with androgen deprivation therapy (ADT), radiotherapy, and chemotherapy. Patients’ information were fully anonymous. This research study was approved by the Iran University of Medical Sciences (IUMS) Research Ethics Committee.
Immunohistochemistry
Immunohistochemical analysis for MAGE-A2 antigen was performed on TMA (for prostatectomy blocks) and needle biopsy slides. The examination was done using a standard chain polymer-conjugated (Envision) technique. Tissue sections were cut 5-μm and mounted on charged slides (Superfrost Plus, Thermo Scientific, Germany). After deparaffinization by 60 °C temperature, slides were rehydrated by immersion in decreasing grades of ethanol. Subsequently, endogenous peroxidase activity was inhibited by immersing the tissues for 20 min in methanol containing 0.3% hydrogen peroxide (H2O2). Antigens retrieval were performed by autoclaving for 10 min in citrate buffer (pH 6). After antigen retrieval, the slides were incubated overnight with primary antibody (anti-MAGE-A2 antibody, Biorbyt, UK) with different dilutions (1/100, 1/500, 1/1000; overnight at 4 °C) to obtain the best dilution. The optimal dilution was found to be 1:500. After washing with Tris-buffered saline (TBS), tissues were incubated in the secondary antibody, EnVision™ + /HRP, DualLink Rabbit/Mouse (Dako, Denmark) for 60 min at room temperature with the addition of 3, 3′ diaminobenzidine (DAB, Dako) to achieve visualization of the antigen. Slides were counterstained with hematoxylin (Dako), dehydrated in alcohol with increasing grades of ethanol, cleared in xylene (Dako), and mounted for visualization. The negative controls, consisting of TBS instead of primary antibody and PCa tissue with a non-reactive antibody, confirmed the specificity of the staining. Testicular tissue and melanoma were considered as positive controls.
TMA construction
Tissue microarray blocks were prepared as described previously [18, 19]. For each specimen, H&E slides were used to identify and mark out targeted areas of tumor and HPIN prostatectomy tissues by an experienced pathologist (M.A). From each corresponding paraffin-embedded tissue, three representative tissue areas were selected and microarray samples were punched with a diameter of 0.6 mm from selected areas of primary block and precisely transferred into a secondary paraffin block using Tissue Arrayer Minicore (ALPHELYS, Plaisir, France). TMA blocks were prepared in three copies for each sample; the mean scoring of three cores was considered as the final score.
Evaluation of immunostaining
The staining intensity and percentage of positive tissue cells were evaluated using a semi-quantitative scoring system. A professional pathologist (M.A), without previous knowledge of pathological and clinical data of patients, evaluated immunohistochemistry-stained samples by optical microscope.
Scoring system
Staining intensity was evaluated as negative or zero (no expression), 1+ (weak), 2+ (moderate), and 3+ (strong). Percentage of MAGE-A2 positive cells was scored as 0–100%. The final score was calculated by H-score (histochemical score) for each case by multiplying the staining intensity and the percentage of positive cells, and a final score of 0–300 was obtained [20]. The mean of H-scores (for nuclear expression: 282 and cytoplasmic expression: 252) was considered as the cut-off point to stratify the samples as low or high expression of MAGE-A2 in PCa.
Statistical analysis
All data were analyzed using the SPSS statistical software package version 25 (SPSS, Chicago, IL, USA). The comparison of MAGE-A2 expression in PCa and HPIN was analyzed using Mann–Whitney test and only PCa group using One-Sample Kolmogorov–Smirnov test. Receiver operating characteristic (ROC) curve analysis was performed to determine the sensitivity, specificity, and differentiation of MAGE-A2 expression between PCa and HPIN groups. Pearson’s χ2, Spearman’s R tests, and One-way ANOVA were performed to analyze the significance of the correlation between MAGE-A2 expression and clinicopathological parameters. Kaplan–Meier method (with log-rank test) was used for survival analysis. Cox regression analysis was performed for significant of MAGE-A2 expression, pathological and clinical parameters as independent factors. Multivariate analysis was used based on the results of univariate analysis. P value of less than 0.05 was considered as significant. Charts were drawn using Prism ™ version 8.3.0 software (Graph Pad Inc., San Diego, CA, USA) Graphs.
Results
Study population
Overall, the mean age of the study population was 67 years (range 49–90). Mean age of PCa patients was 66 years (range 49–90 years) and HPIN 65 years (range 49–79). One hundred and nineteen PCa cases had available tumor volume information. Based on the cut-off mean point, tumor volume percentages were classified into two groups: values of smaller than 33 (70 cases, 58.8%) and values of greater than 33 (49 cases, 41.2%). Preoperative or pre-treatment serum PSA levels were grouped as < 4, 4–10, and > 10 ng/ml (range 2.8–352, mean 21.7 ng/ml) [20]. Of 80 PCa cases for whom the PSA data were available, 2 (2.5%) had a PSA of < 4 ng/ml, 42 (52.5%) PSA 4–10 ng/ml, and 36 (45%) PSA > 10 ng/ml. Of 148 cases of PCa, 54 (36.5%) showed Gs of 6, 63 (42.6%) Gs of 7, 17 (11.5%) Gs of 8, 9 (6.1%) Gs of 9, and 5 (3.4%) Gs of 10. Of 103 cases for whom pTNM staging data were available, 61 (59.2%) of cases were in stage pT2, 41 (39.8%) of cases pT3, and 1 (1%) of case pT4.
Pathological and clinical data of patients and tumor characteristics are shown in Table 1. Adjacent tissue involvement, including the spread of the tumor out of prostate tissue to the seminal vesicles and vasa deferentia, bladder neck, and lymph nodes, was determined. Involvement of perineural, vascular invasion, and surgical margins are also shown in Table 2.
Expression of MAGE-A2 in PCa and HPIN
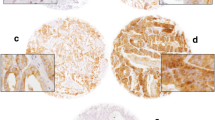
The immunohistochemical analysis was performed to evaluate the expression of MAGE-A2 in prostate tissues. The level of expression was assessed and analyzed by the overall H-score. The MAGE-A2 protein was predominantly expressed in both cytoplasm and nucleus of epithelial cells with the same pattern in tissues of the PCa and HPIN (Fig. 1). The cytoplasmic pattern had a variety of staining intensities in epithelial cells of PCa. No negative staining was detected in this series of tissues except in one case of nuclear expression of PCa tissues. Of 148 PCa cases stained for MAGE-A2, weak, moderate, and strong intensities for cytoplasmic expression were observed in 5 (3.4%), 51 (34.5%), and 92 (62.2%) of cases, respectively. For nuclear expression, negative, weak, moderate, and strong intensities were 1 (0.7%), 0 (0), 5 (3.4%), and 142 (95.9%) of PCa cases, respectively. Of 18 HPIN samples, weak, moderate, and strong staining for cytoplasmic expression were detected in 10 (55.6%), 7 (38.9%), and 1 (5.6%), respectively. For nuclear expression, weak, moderate, and strong intensities were 1(5.6%), 13 (72.2%), and 4 (22.2%) of HPIN cases, respectively.
Staining pattern of MAGE-A2 expression in cores (original magnification × 100; a1 and d1) and needle biopsies (original magnification × 400; b and c). a1 and a2 Strong staining for both nuclear and cytoplasmic expressions with 100% positive cells (PCa tissue with low grade, Gs 3 + 4), b moderate staining for cytoplasm expression with 100% positive cells and strong intensity for nuclear expression with 100% positive cells (PCa tissue with high grade, Gs 8), c moderate staining for cytoplasm expression with 100% positive cells and strong intensity for nuclear expression with 100% positive cells (PCa tissue with high grade, Gs 10), d1 and d2 weak staining for cytoplasmic expression with 100% positive cells and moderate staining for nuclear expression of HPIN tissue with 100% positive cells. e Simultaneous expression of the non-tumor part (orange arrow) and the tumor part (red arrow) in a tissue section. The non-tumor part is less color intensity than the tumor part (original magnification × 400), f staining of PCa tissue without primary antibody (original magnification × 400, negative control). g staining of PCa tissue with a non-reactive antibody (original magnification × 400, anti-CD11b antibody, negative control), h and i MAGE-A2 expressions in testis tissue and melanoma, respectively (original magnification × 400, positive controls). a2 and d2 are a1 and d1 Images with higher magnification (× 400), respectively
The majority of PCa cases were stained strong for both cytoplasmic and nuclear expressions (62.2% and 95.9%, respectively), whereas HPIN samples mostly showed weak staining for cytoplasmic expression and moderate staining for nuclear expression (55.6% and 72.2%, respectively). The mean intensity of both cytoplasmic and nuclear MAGE-A2 expressions were higher among PCa samples (mean (SD) = 2.58 (0.55) and 2.94 (0.3), respectively) compared to HPIN (mean(SD) = 1.5 (0.61) and 2.16 (0.51), respectively). The mean of H-score for cytoplasmic and nuclear expressions were 252 and 287 in PCa cases and 150 and 212 in the HPIN group, respectively.
A significant difference was found between staining intensity of nuclear and cytoplasm expressions as well as H-score of nuclear and cytoplasm expressions in PCa group (One-Sample Kolmogorov–Smirnov test; for all: P < 0.0001) (Fig. 2a). The Mann–Whitney test was performed to compare differences between the expression of MAGE-A2 in PCa and HPIN. A significant difference was observed in the staining intensity and H-score for both cytoplasmic and nuclear expressions of MAGE-A2 between PCa and HPIN (for all; P < 0.0001) (Fig. 2b). Similarly, ROC curve analysis showed an AUC of 0.653 for nuclear expression vs cytoplasmic expression in terms of H-score in PCa group (Fig. 2c), the AUC of 0.869 for nuclear expression of PCa vs nuclear expression of HPIN (Fig. 2d), and the AUC of 0.861 for cytoplasmic expression of PCa vs cytoplasmic expression of HPIN (Fig. 2e). These data indicated that there were the difference and discrimination for nuclear and cytoplasmic expressions of MAGE-A2 in PCa as well as the nuclear and cytoplasmic expressions of MAGE-A2 between PCa and HPIN.
Different expressions of MAGE-A2 in PCa and HPIN groups with immunohistochemical analysis. a Nuclear and cytoplasmic expressions of MAGE-A2 among PCa samples (One-Sample Kolmogorov–Smirnov test). b nuclear and cytoplasmic expressions of MAGE-A2 among PCa and HPIN groups (Mann–Whitney test). c ROC curve analysis represents nuclear expression vs cytoplasmic expression of MAGE-A2 in PCa (AUC = 0.653, 95% CI 0.59–0.71), d nuclear expression of MAGE-A2 in PCa vs HPIN (AUC = 0.869, 95% CI 0.75–0.97) and e cytoplasmic expression of MAGE-A2 in PCa vs HPIN (AUC = 0.861, 95% CI 0.76–0.95)
Association of MAGE-A2 expression in terms of H-score with clinicopathological parameters
Pearson’s χ2 analysis showed a statistical significant inverse correlation between cytoplasmic expression of MAGE-A2 in terms of H-score (cut-off = 252) with Gs (P = 0.002), indicating that the decreased level of MAGE-A2 cytoplasmic expression was more often found in higher Gs PCa tissues (Fig. 3a). To compare differences between the cytoplasmic expression of MAGE-A2 within the Gs subgroups (Gs 6–10), we used the Tukey HSD post hoc comparisons, indicating a highly significant difference between Gs 6 with Gs 9 and 10 (P = 0.036, P = 0.014, respectively) as well as Gs 7 with Gs 10 (P = 0.027; Fig. 3a). Similarly, further analysis based on the subcategories of the Gs 6 and 7 (3 + 4) as low grades and 7 (4 + 3) – 10 as high grades showed a significant inverse correlation between cytoplasmic expression of MAGE-A2 with Gs (P = 0.001), demonstrating that the decreased level of MAGE-A2 was more found in higher Gs (Fig. 3c). No significant correlation was found between Gs and categorized Gs (low and high grades) with nuclear expression of MAGE-A2 (P = 0.554, P = 1, respectively; Fig. 3b and c). In addition, a significant correlation was found between the nuclear MAGE-A2 expression and the pTNM system (P = 0.009).
Correlation between Gs and MAGE-A2 expression. a Immunohistochemical analysis of cytoplasmic MAGE-A2 expression with Gs, b nuclear MAGE-A2 expression with Gs (One-way ANOVA). c Immunohistochemical analyses of nuclear and cytoplasmic MAGE-A2 expressions with Gs classified as low and high grades (Pearson's Chi-square test). C cytoplasmic expression, Gs gleason score, N nuclear expression
The nuclear and cytoplasmic expressions of MAGE-A2 were not correlated with other tumor characteristics, including lymph node involvement, vascular invasion, or tumor invasion to surrounding tissues (Table 2).
Prognostic significance of MAGE-A2 expression in terms of H-score in PCa
Kaplan–Meier analysis (with log-rank test) was used to investigate the association between MAGE-A2 expression with BCR-free survival (BCR-FS) and DFS. Based on the mean H-score, nuclear and cytoplasmic expressions of MAGE-A2 were divided into high and low expressions. BCR and DFS information were obtained from the time of surgery or primary treatment until the end of 2019 (for patients whose information were accessible; N = 57). The mean postoperative follow-up period was 70 months (SD = 4.89), median 57 months, and the range was 5–153 months. Table 3 shows the main characteristics of patients included for survival analysis.
There was a significant association between cytoplasmic expression of MAGE-A2 with BCR-FS and DFS of patients (P = 0.002, P = 0.001, respectively) (Fig. 4a, b). The mean BCR-FS rates for patients with low and high MAGE-A2 cytoplasmic expressions were 72 (SD = 10) and 127 (SD = 9) months, respectively. The mean DFS rates for patients with low and high MAGE-A2 cytoplasmic expressions were 69 (SD = 10) and 123 (SD = 9.2) months, respectively.
Survival analysis for MAGE-A2 expression in PCa patients. a Kaplan–Meier curves of cytoplasmic MAGE-A2 expression for BCR-FS (P = 0.002), b cytoplasmic MAGE-A2 expression for DFS (P = 0.001), c nuclear MAGE-A2 expression for BCR-FS (P = 0.133), and d nuclear MAGE-A2 expression for DFS (P = 0.168). BCR-FS biochemical recurrence-free survival, DFS disease-free survival
There was no significant association between nuclear expression of MAGE-A2 with BCR-FS and DFS of patients (P = 0.133, P = 0.168, respectively) (Fig. 4c, d). The mean BCR-FS rates for patients with low and high MAGE-A2 nuclear expressions were 73.3 (SD = 16.93) and 104.44 (SD = 8.78) months, respectively. The mean DFS rates for patients with low and high MAGE-A2 nuclear expressions were 73.3 (SD = 16.93) and 99.21 (SD = 8.63) months, respectively.
These results showed that BCR-FS and DFS rates were significantly longer for patients whose tumors were classified as the high expression for cytoplasmic MAGE-A2 expression, as compared with patients whose tumors were classified as the low expression for cytoplasmic MAGE-A2.
Significant parameters affecting DFS in the univariate analysis included cytoplasmic expression of MAGE-A2, Gs stratified 6, 7 (3 + 4) as low grades vs 7 (4 + 3) – 10 as high grades, and TNM stage (T2 vs T3). The parameters in BSR-FS included cytoplasmic expression of MAGE-A2 and Gs. In multivariate analysis, GS (low grades vs high grades) and cytoplasmic expression of MAGE-A2 were significantly related to BCR-DF (P = 0.014; P = 0.028, respectively). Based on the results of this study, Gs expression and cytoplasmic expression of MAGE-A2 were independent factors of poor prognosis in PCa (Table 4).
Discussion
Cancer–testis antigens, such as MAGE family, considerate as target antigens for promising immunotherapy due to the high immunogenicity and low expression of these antigens in normal tissue. In addition, the MAGE family has attracted a lot of considerations as biomarkers in various types of cancers. For example, the MAGE-A2 expression level was overexpressed in glioma tissues and a high level of MAGE-A2 expression associated with poor overall survival in patients with glioma [21]. The MAGE-A gene expression in peripheral blood was related to poor prognosis in patients with lung cancer [22]. The increased MAGE-A9 expression level was also associated with poor prognosis for survival in patients with esophageal squamous cell carcinoma [23]. The MAGE family has more than 40 human proteins. Their expression is restricted to the testis and placenta and is aberrantly overexpressed in cancer where they can be immunogenic [24]. Major studies have been devoted to the evaluation of the MAGE-A11 as androgen receptor coregulator in PCa [25, 26]. However, limited studies have looked at other components of the family in PCa. Due to the different expression and function of this family in various cancers, it is therefore necessary to evaluate separately components of these family in patients with cancer. In the current study, we investigated the MAGE-A2 protein expression in a well-characterized series of prostate tissues consisting of PCa and HPIN using EnVision method (highly sensitive for staining tissue). The staining pattern of MAGE-A2 expression was analyzed with the pathological and clinical features of the patients with PCa. Survival analysis was also performed to investigate the significance of the MAGE-A2 as a potential prognostic predictor.
Suyama et al. [7] showed that MAGE-A2 is predominantly expressed in CRPCa using genome microarray. However, the expression pattern of MAGE-A2 in PCa tissue specimens and its prognostic and therapeutic significance for PCa patients is still unclear. In the present study, epithelial cells of PCa and HPIN tissues were simultaneously stained as both cytoplasmic and nuclear pattern. All of our examined prostate tissues were stained with a variety of intensities for cytoplasmic expression. HPIN is a condition where neoplastic growth of epithelial cells occurs within preexisting benign prostatic acini or ducts [27]. The level of MAGE-A2 expression was significantly higher in PCa compared to HPIN, indicating over-expression of MAGE-A2 in PCa tissues with wide variety of malignant grades. Strong intensity of staining was observed for both cytoplasmic and nuclear MAGE-A2 expressions in PCa. Although no association was found between nuclear expression of MAGE-A2 with Gs, recurrence, survival data, and other clinicopathological parameters, the mean intensity of staining and H-score in nuclear expression were much higher than those in cytoplasmic expression (96% vs 62.2%, 287 vs 252, respectively). The main activity of MAGE-A2 is in the nucleus and functionally related to the p53 tumor suppressor gene. MAGE-A2 inhibits the association between the p53 and its binding sites on chromatin. This protein also interacted with epigenetic controllers, such as histone deacetylase 3, as well as inhibits p53-dependent apoptosis in response to chemotherapy and increases cellular resistance [28]. It is suggested that the strong nuclear expression of MAGE-A2 in comparison with cytoplasmic expression indicates its translocation into the nucleus and increased activity in epithelial cells of PCa tissue.
On the other hand, the various intensities of staining observed in cytoplasmic MAGE-A2 expression were significantly correlated with Gs and recurrence in PCa cases. Statistical analysis showed that among the PCa samples, the level of cytoplasmic expression of MAGE-A2 decreased from low-grade to high-grade Gs. Coordinating with these data, the BCR-FS and DFS were longer in patients with cytoplasmic high expression of the MAGE-A2, indicating its possible value to apply as a candidate for tumor good prognosis and activity role of this marker in the nucleus of PCa cells. Additionally, Gs and cytoplasmic expression of MAGE-A2 were independent prognostic indicators for BCR-FS in patients with PCa. These findings suggest a significant effect between increased expression of cytoplasmic MAGE-A2 and good prognosis of the disease. In addition, it can interfere that the relationship of the low cytoplasmic expression of MAGE-A2 with the shortening of recurrence and increasing the intensity of nuclear staining compared to the expression of cytoplasm indicates the high activity of this marker in the nucleus of PCa tissue cells. We also analyzed MAGE-A2 expression with spread of the tumor to the areas around the prostate gland; no significant association was found between the MAGE-A2 expression and the invasion of tumor toward the surrounding regions of the prostate. As an explanation, data regarding the spread of the tumor into areas surrounding the prostate tissue for all patients were not available and reduced the sample size for statistical analysis.
In recent years, immunotherapy has attracted a lot of considerations in patients with PCa. Sipuleucel-T is an autologous T cell-mediated immunotherapy that targets the prostatic acid phosphatase antigen. It was approved by the Food and Drug Administration (FDA) for metastatic CRPC who are asymptomatic or minimally symptomatic [29]. However, as studies have shown, it did not prolong progression-free survival and led to a rapid increase in PSA level [30]. To promote immune responses against tumor cells and ultimately prolonged survival of patients is that to employ tumor immunogenic antigens for active immunotherapy. The relatively limited expression of MAGE antigens and their immunogenicity has propelled researches into utilizing them as targets for vaccine immunotherapies. We found that MAGE-A2 is expressed in both the cytoplasm and nucleus of PCa cells and mostly with a high intensity of staining in the nucleus, so it is promising interest for vaccinating PCa cases to activate specific CD8 + T cells (cytotoxic T lymphocyte; CTL) against tumor cells expressed MAGE-A2. In line with this study, we are evaluating the immunogenicity of the MAGE-A2 through dendritic autologous cells in patients with advanced PCa (data not published) to reveal the significance of MAGE-A2 expression for immunotherapy in patients with mCRPC.
References
Rawla P (2019) Epidemiology of prostate cancer. World J Oncol 10(2):63
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun M (2009) Cancer statistics. Ca Cancer J Clin. 59(4):225–249
Mohsenzadegan M, Seif F, Farajollahi MM, Khoshmirsafa M (2018) Anti-oxidants as chemopreventive agents in prostate cancer: a gap between preclinical and clinical studies. Recent Pat Anti-Cancer Drug Discovery 13(2):224–239
Kalantari E, Abolhasani M, Roudi R, Farajollahi MM, Farhad S, Madjd Z et al (2019) Co-expression of TLR-9 and MMP-13 is associated with the degree of tumour differentiation in prostate cancer. Int J Exp Pathol 100(2):123–132
Kretschmer A, Tilki D (2017) Biomarkers in prostate cancer–current clinical utility and future perspectives. Crit Rev Oncol/Hematol 120:180–193
Simpson AJ, Caballero OL, Jungbluth A, Chen Y-T, Old LJ (2005) Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5(8):615–625
Suyama T, Shiraishi T, Zeng Y, Yu W, Parekh N, Vessella RL et al (2010) Expression of cancer/testis antigens in prostate cancer is associated with disease progression. Prostate 70(16):1778–1787
Kruit W, Suciu S, Dreno B, Mortier L, Robert C, Chiarion-Sileni V et al (2013) Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol 31(19):2413–2420
Chen X, Wang L, Liu J, Huang L, Yang L, Gao Q et al (2017) Expression and prognostic relevance of MAGE-A3 and MAGE-C2 in non-small cell lung cancer. Oncol Lett 13(3):1609–1618
Wong P, Yeoh C, Ahmad A, Chelala C, Gillett C, Speirs V et al (2014) Identification of MAGEA antigens as causal players in the development of tamoxifen-resistant breast cancer. Oncogene 33(37):4579–4588
Zou C, Shen J, Tang Q, Yang Z, Yin J, Li Z et al (2012) Cancer-testis antigens expressed in osteosarcoma identified by gene microarray correlate with a poor patient prognosis. Cancer 118(7):1845–1855
Hudolin T, Kastelan Z, Ilic I, Levarda-Hudolin K, Basic-Jukic N, Rieken M et al (2013) Immunohistochemical analysis of the expression of MAGE-A and NY-ESO-1 cancer/testis antigens in diffuse large B-cell testicular lymphoma. J Trans Med 11(1):123
Kakimoto T, Matsumine A, Kageyama S, Asanuma K, Matsubara T, Nakamura T et al (2019) Immunohistochemical expression and clinicopathological assessment of the cancer testis antigens NY-ESO-1 and MAGE-A4 in high-grade soft-tissue sarcoma. Oncol Lett 17(4):3937–3943
Lee T-B, Lim S-C, Moon Y-S, Choi C-H (2013) Melanoma antigen gene family A as a molecular marker of gastric and colorectal cancers. Oncol Rep 30(1):234–238
Lee M-H, Son E-I, Kim E, Kim I-S, Yim M-B, Kim S-P (2008) Expression of cancer-testis genes in brain tumors. J Korean Neurosurg Soc 43(4):190
Cheng L, Montironi R, Bostwick DG, Lopez-Beltran A, Berney DM (2012) Staging of prostate cancer. Histopathology 60(1):87–117
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA (2016) The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 40(2):244–252
Kononen J, Bubendorf L, Kallionimeni A, Bärlund M, Schraml P, Leighton S et al (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4(7):844–847
Zanjani LS, Madjd Z, Abolhasani M, Rasti A, Shariftabrizi A, Mehrazma M et al (2019) Human telomerase reverse transcriptase protein expression predicts tumour aggressiveness and survival in patients with clear cell renal cell carcinoma. Pathology 51(1):21–31
Mohsenzadegan M, Madjd Z, Asgari M, Abolhasani M, Shekarabi M, Taeb J et al (2013) Reduced expression of NGEP is associated with high-grade prostate cancers: a tissue microarray analysis. Cancer Immunol Immunother 62(10):1609–1618
Meng Q, Luo G, Liu B, Sun Y, Yan Z (2018) Melanoma-associated antigen A2 is overexpressed in glioma and associated with poor prognosis in glioma patients. Neoplasma 65(4):604–609
Gu L, Sang M, Yin D, Liu F, Wu Y, Liu S et al (2018) MAGE-A gene expression in peripheral blood serves as a poor prognostic marker for patients with lung cancer. Thoracic Cancer 9(4):431–438
Qi Y, Cao KX, Xing FC, Zhang CY, Huang Q, Wu K et al (2017) High expression of MAGE-A9 is associated with unfavorable survival in esophageal squamous cell carcinoma. Oncol Lett 14(3):3415–3420
Weon JL, Potts PR (2015) The MAGE protein family and cancer. Curr Opin Cell Biol 37:1–8
Su S, Parris AB, Grossman G, Mohler JL, Wang Z, Wilson EM (2017) Up-regulation of follistatin-like 1 by the androgen receptor and melanoma antigen-A11 in prostate cancer. Prostate 77(5):505–516
Karpf AR, Bai S, James SR, Mohler JL, Wilson EM (2009) Increased expression of androgen receptor coregulator MAGE-11 in prostate cancer by DNA hypomethylation and cyclic AMP. Mol Cancer Res 7(4):523–535
Brawer MK (2005) Prostatic intraepithelial neoplasia: an overview. Rev Urol 7(Suppl 3):S11
Ujiie H, Kato T, Lee D, Hu H-P, Fujino K, Kaji M et al (2017) Overexpression of MAGEA2 has a prognostic significance and is a potential therapeutic target for patients with lung cancer. Int J Oncol 50(6):2154–2170
Handy CE, Antonarakis ES (2018) Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Fut Oncol 14(10):907–917
Holl EK, McNamara MA, Healy P, Anand M, Concepcion RS, Breland CD et al (2019) Prolonged PSA stabilization and overall survival following sipuleucel-T monotherapy in metastatic castration-resistant prostate cancer patients. Prostate Cancer Prostatic Dis 22(4):588–592
Acknowledgements
This study was conducted as a research project and supported by a grant from Iran University of Medical Sciences (Grant Number #31-33720).
Funding
The funding source is a college institute and has sponsored the project financially and approved.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institution at which this study was conducted.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khalvandi, A., Abolhasani, M., Madjd, Z. et al. Reduced cytoplasmic expression of MAGE-A2 predicts tumor aggressiveness and survival: an immunohistochemical analysis. World J Urol 39, 1831–1843 (2021). https://doi.org/10.1007/s00345-020-03395-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03395-6