Abstract
New gene expressed in prostate (NGEP) is a newly diagnosed prostate-specific gene that is expressed only in normal prostate and prostate cancer cells. Discovery of tissue-specific markers may promote the development of novel targets for immunotherapy of prostate cancer. In the present study, the staining pattern and clinical significance of NGEP were evaluated in a series of prostate tissues composed of 123 prostate cancer, 19 high-grade prostatic intraepithelial neoplasia and 44 samples of benign prostate tissue included in tissue microarrays using immunohistochemistry. Our study demonstrated that NGEP localized mainly in the apical and lateral membranes and was also partially distributed in the cytoplasm of epithelial cells of normal prostate tissue. All of the examined prostate tissues expressed NGEP with a variety of intensities; the level of expression was significantly more in the benign prostate tissues compared to malignant prostate samples (P value <0.001). Among prostate adenocarcinoma samples, a significant and inverse correlation was observed between the intensity of NGEP expression and increased Gleason score (P = 0.007). Taken together, we found that NGEP protein is widely expressed in low-grade to high-grade prostate adenocarcinomas as well as benign prostate tissues, and the intensity of expression is inversely proportional to the level of malignancy. NGEP could be an attractive target for immune-based therapy of prostate cancer patients as an alternative to the conventional therapies particularly in indolent patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second most common cancer and one of the leading causes of cancer deaths among men, which affects men aged fifty or more [1, 2]. PCa most commonly metastasizes to the bones, lymph nodes, and may invade rectum, bladder and lower ureters after local extension. Standard treatment modalities for localized disease such as surgery, radiotherapy and active surveillance often prove to be ineffective, and thus, a search for therapeutic alternatives is needed for treating advanced and metastatic disease [3, 4]. Hormonal therapy and chemotherapy are often reserved for tumors that have spread beyond the prostate. However, these modalities are unable to completely eliminate androgen-independent prostate cancer cells that remain after androgen ablation of the metastatic prostate cancer [5, 6]. Therefore, novel strategies for the treatment of prostate cancer are essential. Recent studies have demonstrated that target-specific immunotherapy either alone or in combination with current treatment protocols can be an effective treatment strategy for advanced prostate cancer [7, 8]; thus, it is crucial to identify and characterize new molecular targets that are exclusively expressed in prostate cancer. Several prostate cancer-associated antigens have been identified [9–11]; some of these antigens including PSMA [12] and PSCA [13], however, are also expressed in vital normal tissues other than the prostate [14, 15].
New gene expressed in prostate (NGEP) was first identified through computer-based analysis of expressed sequence tags (ESTs) clustering. This protein is specifically expressed in prostate cancer and in normal prostate but not in other essential normal tissues [16].
NGEP, also called ANO7, is a member of an octamin/TMEM16 family that are Ca2+-activated Cl− channels and is a cell membrane protein [17]. The NGEP gene is localized on chromosome 2 band q37.3 of the human genome [16]. This family of proteins is considered to be of significant interest in cancer and developmental biology. It has been previously shown that many of the human TMEM16 genes are overexpressed in cancer and are considered as valuable tumor markers, particularly in the gene expression profiling with microarrays [18, 19]. It is also determined that TMEM16 family is expressed in taste buds localized to sweet, bitter and umami (TRPM5 positive) cells by ISH (In situ hybridization) [20].
There are two isoforms of NGEP as a result of alternative splicing of mRNA. The smaller transcript (NGEP-S) encodes a 179 amino acid located in the cell cytoplasm, and the larger transcript (NGEP-L) encodes a membrane protein comprised of 933 amino acids and contains 8 transmembrane regions with both the N- and C-termini of NGEP-L located inside the cell [16, 21].
This specific antigen, therefore, could be a promising target for a putative therapeutic antibody for prostate cancer because it is exclusively detected in prostate tissues. In addition, its cell surface expression pattern makes NGEP an ideal molecule for immune-based targeted therapy of prostate cancer [22].
Increased understanding of the immune system, tumor immunology and vaccine technologies have allowed for the development of novel vaccine approaches that may be more effective. Sipuleucel-T, the first patient-specific vaccine approved in 2010 by the Food and Drug Administration (FDA) for the treatment of metastatic, castration-resistant prostate cancer (mCRPC), is formulated by incubating patient-derived peripheral blood mononuclear cells (PBMC) obtained by leukapheresis with a fusion protein consisting of GM-CSF and a tumor-derived differentiation antigen (prostatic acid phosphatase) [23, 24]. The GM-CSF serves to activate and induce maturation of the dendritic cells that initiate an immune response and potentially to direct the PAP protein into these cells [25]. Other immune therapy approaches have focused on regulating costimulatory molecules to boost the T-effector cell response to mCRPC. PROSTVAC®-VF is a poxvirus-based vaccine engineered to contain PSA and three costimulatory molecules; B7.1, ICAM-1 and LFA-3 termed TRICOM within a vaccinia or fowlpox virus vector [26]. The results from clinical trials with Sipuleucel-T and PROSTVAC®-VF have been encouraging, thus providing proof of principle for vaccines as therapeutic approach [27].
New gene expressed in prostate was also described as a potential target for T-cell-mediated immunotherapy of prostate cancer because of its potent immunogenicity and prostate-restricted expression [28]. In a previous immunohistochemical study performed by Das et al. [22], 91 % of prostate samples showed positive reactivity for NGEP-L in the cancerous region, whereas higher level of expression was detected in 100 % of normal regions of prostate specimens. However, the intensity of expression of NGEP was not related to the grade of tumors.
Considering the absence of any report in the literature concerning the association between expression of NGEP with clinicopathological characteristics of prostate cancer patients, herein we aimed for the first time to investigate the staining patterns and clinical significance of NGEP in a large series of prostate tissue specimens using tissue microarray (TMA) technique. The correlation of NGEP expression with patients’ and tumor characteristics was also examined.
Material and methods
Patients and tissue samples
This was a retrospective study on a series of 202 paraffin-embedded prostate tissue samples. Of this collection, 16 specimens were excluded from the study due to technical problems in tissue processing or absence of tumor cells within the core, leaving a total of 186 cases for the final evaluation. Our samples consisted of 123 prostate adenocarcinomas, 19 high-grade prostatic intraepithelial neoplasia (HPIN) and 44 benign prostate tissues, including 29 benign prostatic hyperplasia (BPH) and 15 normal prostate tissues adjacent to tumors which were included in the tissue microarray. All of these patients were diagnosed over the time period of 2006–2011 in Hasheminejad Kidney Center, a major university-based referral Urology Hospital in Tehran, Iran. Surgical specimens were obtained before systemic treatment, and paraffin embedding was performed within the framework of routine diagnostic procedures. Pathological reports were evaluated to obtain diagnosis and other pathologic parameters including tumor type and grade, tumor volume and site, lymph node involvement, vascular invasion, involvement of the adjacent tissue and pathologic tumor stage. Medical records of all patients were also reviewed to obtain patients’ age and serum PSA.
All hematoxylin and eosin (H and E) stained slides were reviewed to determine the best area for preparing TMA of each specimen. Tumor grade was determined according to the Gleason scoring system based on the 2005 International Society of Urological Pathology consensus on Gleason scoring of prostatic adenocarcinoma [29].
We also stratified Gleason score (GS) 7 prostate cancer into two groups (GS 3 + 4 and GS 4 + 3) in order to investigate the expression of NGEP as related to different clinical outcomes of these two groups [30, 31].
Pathologic tumor stage was defined based on the last version of AJCC/UICC TNM staging system [32]. Tumor volume was assessed based on the proportion (%) of tumor involvement in the prostate specimen.
Tumor samples were obtained from radical prostatectomy surgical specimens after obtaining informed consent from patients. The normal prostate tissues adjacent to tumors were also included in TMAs to compare the staining patterns of NGEP in a range of different tissue samples. In addition, 29 specimens from BPH diagnosed on simple prostatectomy specimens were included in our TMAs. Patients’ data were fully anonymous. This research study was approved by Iran University of Medical Sciences Research Ethics Committee. None of these patients received preoperative hormone or radiation therapy.
Immunohistochemistry
Immunohistochemical detection of the NGEP marker was performed on TMA slides (Superfrost plus, Thermo Scientific, Germany) using a standard chain polymer-conjugated (Envision) technique as described previously [33] on paraffin-embedded tissues of prostatectomy specimens, using an Anti-ANO7 rabbit polyclonal antibody (Sigma Aldrich, USA). After deparaffinization, tissues were rehydrated by immersion in decreasing grades of ethanol, and endogenous peroxidase activity was blocked by immersing the tissues for 15 min in methanol containing 0.3 % hydrogen peroxide. Antigens were retrieved by autoclaving for 10 min in 1 mmol/L Tris–EDTA buffer (pH 8.0). After antigen retrieval, 4 μm tissues sections were incubated overnight with primary antibody at serial dilutions of 1:40, 1:80 and 1:100 at 4 °C; finally, the optimal dilution was found to be 1:40. After washing with Tris-buffered saline (TBS), tissues were incubated in the secondary antibody which was EnVision™+/HRP, Dual Link Rabbit/Mouse (Dako, Denmark) for 30 min at room temperature with the addition of 3, 3′-diaminobenzidine (DAB, Dako) to achieve visualization of the antigen. In the final step, tissue sections were lightly counterstained with hematoxylin (Dako), dehydrated in alcohol, cleared in xylene (Dako) and mounted for visualization. The entire tissues of prostate adenocarcinoma specimen were used as positive control. Negative control, consisting of TBS instead of primary antibody, confirmed the specificity of the staining.
Tissue microarray (TMA) construction
Tissue microarray blocks were constructed as described previously [34, 35]. In each case, 5-μm H and E slides were used to identify and mark out representative areas of tumor tissue. From each corresponding paraffin-embedded block, three representative tumor regions were selected and microarray samples with a diameter of 0.6 mm were punched from selected regions of each “donor” block and precisely arrayed into a new recipient paraffin block using Tissue ArrayerMinicore (ALPHELYS, Plaisir, France). Tissue microarray (TMA) blocks were constructed in three copies for each specimen; the mean scoring of three cores was then calculated as the final score.
Evaluation of immunostaining
The immunostained tissue arrays were evaluated using a semi-quantitative scoring system (by MM) after a series was observed on a multi-headed microscope by two other observers (MA and ZM) in a coded manner without previous knowledge of clinical and pathological parameters of patients. In difficult cases, the scoring was confirmed by two observers and a consensus was achieved.
Scoring system
Intensity of staining was scored as 0 (no expression), 1 (weak), 2 (moderate) and 3 (strong). Percentage of NGEP positive cells was graded as: 0 (no staining), 1 (<50 % positive cells), 2 (50–80 % positive cells) and 3 (>80 % positive cells). The overall score was obtained by H-score (histochemical score) for each case by multiplying the intensity of staining by the percentage of positive cells, and a final score of 0–300 was given [36]. The mean of H-scores was chosen as cut-off value to classify the samples as high or low expression of NGEP, which was found to be 190.
Statistical analysis
All data were analyzed using the SPSS statistical software package version 20 (SPSS, Chicago, IL, USA). Pearson’s χ 2 and Pearson’s R tests were used to analyze the significance of correlation between NGEP expression and clinicopathological parameters. Moreover, the comparisons of NGEP expression in PCa, HPIN and benign prostatic tissues were performed using Mann–Whitney test. A P value of <0.05 was considered to be statistically significant.
Results
Study population
Overall, mean age of study population was 66 years (ranged 48–90). Mean age of prostate cancer patients was 66 years (ranged 48–90 years), and BPH cases had a mean age of 67 (ranged 52–89). Tumor volume percentage was classified as three groups: <30, 30–60 and >60 %, which ranged 5–100 % (mean 33 %) in 123 prostate cancer patients, 63 % of cases (77) had tumor volume <30, 24 % (30) had tumor volume of 30–60 %, whereas 13 % (16) had a tumor volume of >60 %. Prostate-specific antigen (PSA) levels were grouped as <4, 4–10 and >10 ng/ml (ranged 0.4–352, mean 15 ng/ml). Of 79 cases for whom the PSA data were available, 6 % (5) had a PSA of <4 ng/ml, 58 % (46) had a PSA of 4–10, whereas 36 % (28) had PSA >10 ng/ml.
Furthermore, of 123 cases of PCa, 45 % (55) showed Gleason score of 6, 49 % (60) showed Gleason score of 7 and 6 % (8) showed Gleason score of 8. Of 118 cases for which pathologic tumor stage (pTNM staging) data were available, 64 % (76) of cases were in stage pT2 and 36 % (42) of cases were classified in stage pT3.
All patients’ and tumor characteristics are summarized in Table 1. Adjacent tissue involvement including extension of the tumor out of prostate to the bladder neck, seminal vesicles and vasa deferentia and lymph nodes was determined. Surgical margins, perineural and vascular invasion data are also shown in Table 2.
Expression of NGEP in PCa, HPIN and benign prostate tissues
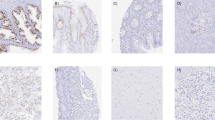
A tissue microarray-based immunohistochemical analysis was performed to investigate the expression of NGEP as a specific tissue marker of prostate. The level of expression was assessed by three scoring methods, namely the intensity of the staining, the percentage of positive cells and the overall H-score. The NGEP protein was predominantly expressed in cell membrane and cytoplasm of epithelial cells with the same pattern in the malignant and nonmalignant prostate tissues (Fig. 1).
Expression of NGEP in prostate tissues. Weak (a), moderate (b) and strong (c) expressions of NGEP were observed mainly on the cell membrane and partially on the cytoplasm of prostate adenocarcinomas, with no staining of stroma. Strong staining of NGEP in HPIN (d), moderate and strong staining in benign prostate tissues (e, f) (original magnification ×100)
The NGEP protein was expressed with a variety of intensities in epithelial cells of prostate tissues, including PCa, HPIN and benign prostate tissue, whereas no negative staining was detected in this series of tissues. Of 123 PCa cores stained for NGEP, weak, moderate and strong intensities were observed in 19 % (23), 59 % (73) and 22 % (27) of cases, respectively. Of 19 HPIN cores, weak, moderate and strong staining were detected in 0, 32 % (6) and 68 % (13), respectively. Of 44 benign prostate tissue cores, weak staining was observed in 7 % (3), whereas moderate and strong staining was detected in 68 % (30) and 25 % (11) of cases, respectively (Table 3). The majority of PCa (59 %) and benign tissues (68 %) stained moderately, whereas HPIN samples mostly (68 %) showed strong staining (Table 3). The average intensity of NGEP expression was higher among HPIN cores (mean = 3) compared to PCa (mean = 2.03) and benign (mean = 2.18) cores. The mean of H-score was 174 in PCa cases, 247 in HPIN group and 205 in benign prostate tissues.
A significant difference was observed in the level of expression of NGEP (either intensity or H-score) between PCa, HPIN and benign prostate tissues (Pearson’s χ 2, P < 0.001, Table 3).
The Mann–Whitney U test was used to compare differences between expression of NGEP in two groups, indicating a statistically significant difference between expression of NGEP (in terms of H-score and intensity) in PCa cases with HPIN group (P ≤ 0.001). A significant association was also evident between expression of NGEP, as assessed by H-score, in PCa group and benign prostatic tissues (P = 0.001) (Table 4).
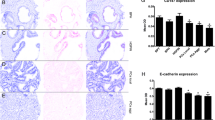
To evaluate the specific staining of NGEP antibody, we also performed immunohistochemical staining using several normal tissues such as brain, lung, kidney, liver, stomach and prostate. Only the prostate tissues specifically stained with anti-NGEP antibody (Fig. 2).
Association of NGEP expression with clinicopathological parameters
Univariate analysis showed statistically significant inverse correlation between expression of NGEP in terms of intensity of staining, percentage of positive cells and H-score (cut-off = 190) with Gleason score (P = 0.007, P < 0.001 and P = 0.005; respectively), indicating that the decreased level of NGEP expression was more often found in higher Gleason score PCa samples (Fig. 3; Table 1). Similarly, further analysis based on the subcategories of the Gleason score 7 (GS 3 + 4 and GS 4 + 3) showed a significant inverse correlation between NGEP expression in terms of intensity and H-score (cut-off = 190) with Gleason score (P = 0.008 and P = 0.01; respectively), demonstrating that the higher level of NGEP was more found in GS 3 + 4 subgroup compared to the GS 4 + 3 subgroup.
In addition, a borderline significant correlation was found between the intensity of NGEP expression and the tumor volume (P = 0.05).
However, the intensity of NGEP expression was not correlated with the level of serum PSA in prostate cancer patients (P = 0.32) (Table 1). The expression of NGEP was not also correlated with other tumor characteristics, including pTNM stage, vascular invasion, lymph node involvement or tumor invasion to adjacent tissues (Table 2).
Discussion
Targeted therapy of cancer using monoclonal antibodies has become an integral part of contemporary treatment against solid tumors, including prostate and breast cancer [37, 38]. For this approach being effective, it is crucial that the antigen is expressed on the cell surface of the target cells and not expressed in other vital normal tissues. NGEP is a prostate-specific plasma membrane protein that was initially identified through computer-based analysis of EST clustering [16], thus is an attractive target for immunotherapy of prostate cancer. To our knowledge, only four studies [16, 21, 22, 28] have been previously performed regarding prostate-specific gene, NGEP, in which two studies [21, 22] examined the expression of NGEP in prostate tissues using immunohistochemistry. To apply NGEP antigen as a specific target for immunotherapy of prostate cancers, it is essential to determine the level of its expression in a larger number of prostate tissues at different stages of differentiation to determine its efficacy for specific group of patients. In the current study, we investigated the expression of NGEP protein in a well-characterized series of prostate tissues with a wide variety of differentiation consisting of PCa, HPIN and benign and normal tissues using tissue microarray technique to examine the level of expression at various stages of differentiation and to correlate NGEP expression with clinicopathological characteristics of PCa. Indeed, tumor profiling by tissue microarray technology has been developed in order to overcome some of the limitations of conventional immunohistochemical studies and allows the analysis of target protein expression for hundreds of tumors simultaneously [35].
We used the anti-ano7 antibody raised against sequence near to N-terminal of NGEP (residues 23–95), which is shared between NGEP-L and NGEP-S [16]. The C-terminus of NGEP (residues 875–933) is exclusively expressed in NGEP-L, is the most diverse among the TMEM16 members and locates in the intracellular region of prostate epithelial cells. The prior immunohistochemical studies used a polyclonal antibody raised against the C-terminus of NGEP-L. The antibody stained a protein that is highly expressed on the apical and the lateral surfaces of normal and cancer prostate epithelial cells [21, 22].
However, our immunohistochemical study illustrated that the antibody against sequence near to N-terminus of NGEP is localized to the cytoplasm (NGEP-S) and in apical and lateral membrane (NGEP-L) in epithelial cells of prostate gland, and no staining was detected in stromal cells. All of our examined prostate tissues including prostate adenocarcinoma, HPIN and benign prostate samples (BPH and normal tissues adjacent to tumors) expressed NGEP with a variety of intensities.
Our findings are consistent with earlier studies performed using In situ hybridization (ISH) and immunohistochemistry (IHC), demonstrating that NGEP is expressed in both basal and terminal epithelial cells of benign and malignant prostate tissues, whereas no signal was detectable in cells of the stromal compartment [16, 21, 22]. The above mentioned studies have also confirmed that the protein encoded by NGEP-S is localized to the cytoplasm, whereas the protein encoded by NGEP-L is present on the plasma membrane [16, 21].
Based on the RNA expression profiles and immunohistochemical analysis, NGEP is only expressed in normal prostate (non-vital for the patient) and prostate cancer tissues [21, 22]; in contrary, other putative prostate markers such as prostate-specific membrane antigen (PSMA) [39] or prostate stem cell antigen (PSCA) [40]are expressed in other normal tissues apart from the prostate. This specificity makes NGEP an exceptional immunotherapeutic target; therefore, MAb targeting of an extracellular portion of NGEP could be valuable in the immunotherapy of prostate cancer [22, 28]. Our immunohistochemical staining also illustrated specific staining of epithelial cells of prostate tissues compared to other normal tissues using a multi-tissue microarray.
In agreement with the previous studies [21, 22], we found that the level of NGEP expression was significantly higher in benign prostate tissues compared to malignant prostate samples. The expression of NGEP in normal prostate tissues as well as HPIN and PCa highlights the role of NGEP as a differentiation antigen [21], suggesting that this antigen is initially made in normal prostate and continues to be expressed in variable levels in PCa. Moreover, among the prostate carcinoma samples, the level of expression of NGEP decreased from low-grade to high-grade prostate cancers, indicating its possible value to apply as a candidate for tumor prognosis, also as an excellent target for antibody-based immunotherapy. Because of low side effect immune therapy/vaccine treatment for indolent disease, this approach would provide low-risk treatment for patients as an alternative to aggressive surgery, radiation therapy and chemotherapy with their associated morbidity [41]. The adverse effects evaluated from dendritic cell-based prostate cancer immunotherapy trials were mild and transient compared to the persistent side effects of surgery or radiation [42].
Previous immunohistochemical study on 126 radical prostatectomized specimens containing benign glands along with cancerous regions also showed stronger intensity of NGEP expression in normal prostate compared to cancers. However, they could not show any correlation between the level of expression of NGEP and the Gleason scores of PCa cases [22]. In our study, we used Gleason scores of 6, 7 and 8 which is obtained from the summation of two initial Gleason scores [29], whereas the prior study used tumors with Gleason grade of 3, 4 or 5 [22]. In addition, the relation of the primary Gleason grade (in the subcategories of Gleason score 7 prostate cancers) with NGEP expression was investigated. Previous studies have shown that Gleason score 7 prostate cancers with a primary Gleason grade of 4 are more aggressive than the Gleason score 7 cancers with a primary Gleason grade of 3 [31]. Similar to the impact of NGEP expression on Gleason score, the correlation analysis of primary Gleason grade of 3 (GS 3 + 4) and primary Gleason grade of 4 (GS 4 + 3) with NGEP expression showed that the decreased level of NGEP expression was associated with GS 4 + 3 and might have an effect on the prognosis of prostate cancer.
We also observed that the expression of NGEP was positive in prostate tumors metastasized to lymph nodes as Das et al. [22] reported, suggesting that this marker can be also used as a diagnostic marker, particularly in metastatic prostate cancers.
Das et al. [21] using RT-PCR analysis illustrated that NGEP-L is detected in the androgen-dependent prostate cancer cell line, LNCaP, but it is not present in androgen-independent PC-3 and DU145 cell lines; however, our immunohistochemical study showed predominant expression of NGEP in all prostate cancer tissues.
It has been showed that in LNCaP cell line, NGEP-L plays a role in promoting cell-to-cell interactions [21]. They have also shown that, in both normal and cancerous tissues, NGEP-L is localized to the apical and the lateral surfaces of the epithelial cells of prostate. Our study confirmed the same localization for NGEP protein in epithelial cells of prostate tissues. Similar localization of NGEP-L in the LNCaP cells and in the prostate tissue suggests that NGEP-L may have an important role in the cell–cell interactions and prostate cell adhesion [21].
In addition to the feasibility of use of NGEP-L as a potential target for Ab-mediated prostate cancer immunotherapy, there is evidence that NGEP-based vaccines may be of potential use in prostate cancer in combination with T-cell-mediated immunotherapy. In this regard, Cereda et al. [28] recently identified an epitope of NGEP antigen which activates NGEP-specific T cells in the blood of prostate cancer patients. PBMC from prostate cancer patients showed higher levels of binding with NGEP-specific/HLA-A2 tetramer as compared to PBMC from normal donors. Moreover, an increase in NGEP-specific T cells was observed in the prostate cancer patients after vaccination with a PSA-based vaccine, demonstrating the immunogenicity of NGEP and feasibility of cell-mediated immunotherapy by NGEP antigen in prostate cancer patients.
Considering the NGEP as an exclusive marker of prostate tissues and its potential role for antibody-based immunotherapy, it is also essential to examine its function in prostate tumor cells. Therefore, NGEP should be investigated in cell signaling and cytotoxicity assays on prostate tumor cells in vitro and also in animal models to confirm its suitability for Ab-based therapy.
Conclusion
Our finding demonstrated that NGEP protein is widely expressed from low-grade to high-grade prostate cancers as well as benign prostate tissues with a variety of intensities. We further showed that NGEP is highly expressed in HPIN and low-grade prostate cancer, whereas the expression of NGEP was significantly decreased in high-grade prostate cancer.
These results are therefore particularly applicable to the development of immunotherapeutic strategies for treatment of prostate cancer, suggesting that early stage patients whose tumors express higher level of NGEP could be appropriate candidates for antibody-based immunotherapy. In contrast, patients with advanced tumor may achieve great benefit from NGEP-targeted therapy in combination with conventional therapy. Therefore, this approach would provide low-risk treatment for patients as an alternative to aggressive surgery or radiation therapy with their associated morbidity.
Abbreviations
- Ano 7:
-
Anoctamin 7
- BPH:
-
Benign prostatic hyperplasia
- Epe:
-
Extraprostatic extension
- ESTs:
-
Expressed sequence tags
- FDA:
-
Food and drug administration
- HPIN:
-
High-grade prostatic intraepithelial neoplasia
- IHC:
-
Immunohistochemistry
- ISH:
-
In situ hybridization
- MAb:
-
Monoclonal antibody
- NGEP:
-
New gene expressed in prostate
- PBMC:
-
Peripheral blood mononuclear cell
- PCa:
-
Prostate cancer
- PSA:
-
Prostate-specific antigen
- PSMA:
-
Prostate-specific membrane antigen
- PSCA:
-
Prostate stem cell antigen
- TMA:
-
Tissue macroarray
- TRICOM:
-
Triad of costimulatory molecules (B7.1, ICAM-1, LFA-3)
References
Vainio P, Lehtinen L, Mirtti T, Hilvo M, Seppanen-Laakso T, Virtanen J, Sankila A, Nordling S, Lundin J, Rannikko A, Oresic M, Kallioniemi O, Iljin K (2011) Phospholipase PLA2G7, associated with aggressive prostate cancer, promotes prostate cancer cell migration and invasion and is inhibited by statins. Oncotarget 2:1176–1190
Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Colombet M, Boyle P (2007) Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 18:581–592
Shanmugam A, Suriano R, Chaudhuri D, Rajoria S, George A, Mittelman A, Tiwari RK (2011) Identification of PSA peptide mimotopes using phage display peptide library. Peptides 32:1097–1102
Crawford ED, Rosenblum M, Ziada AM, Lange PH (1999) Hormone refractory prostate cancer. Urology 54:1–7
Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, Camphausen K, Coleman CN, Sullivan F, Steinberg SM, Schlom J, Dahut W (2005) Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 11:3353–3362
Karnes RJ, Whelan CM, Kwon ED (2006) Immunotherapy for prostate cancer. Curr Pharm Des 12:807–817
Lechleider RJ, Arlen PM, Tsang KY, Steinberg SM, Yokokawa J, Cereda V, Camphausen K, Schlom J, Dahut WL, Gulley JL (2008) Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res 14:5284–5291
Nesslinger NJ, Ng A, Tsang KY, Ferrara T, Schlom J, Gulley JL, Nelson BH (2010) A viral vaccine encoding prostate-specific antigen induces antigen spreading to a common set of self-proteins in prostate cancer patients. Clin Cancer Res 16:4046–4056
Harada M, Noguchi M, Itoh K (2003) Target molecules in specific immunotherapy against prostate cancer. Int J Clin Oncol 8:193–199
Slovin SF (2005) Targeting novel antigens for prostate cancer treatment: focus on prostate-specific membrane antigen. Expert Opin Ther Targets 9:561–570
Arlen PM, Gulley JL, Tsang KY, Schlom J (2003) Strategies for the development of PSA-based vaccines for the treatment of advanced prostate cancer. Expert Rev Vaccines 2:483–493
Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD (1994) Expression of the prostate-specific membrane antigen. Cancer Res 54:1807–1811
Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, Wahl M, Nisitani S, Yamashiro J, Le Beau MM, Loda M, Witte ON (1998) Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A 95:1735–1740
Carter RE, Feldman AR, Coyle JT (1996) Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci USA 93:749–753
Yang WB, Cai F, Cheng CT, Cao G, Qing ZY (2009) Role of prostate stem cell antigen in human pancreatic carcinoma: a tissue microarray-based study. Nan Fang Yi Ke Da Xue Xue Bao 29:2135–2137
Bera TK, Das S, Maeda H, Beers R, Wolfgang CD, Kumar V, Hahn Y, Lee B, Pastan I (2004) NGEP, a gene encoding a membrane protein detected only in prostate cancer and normal prostate. Proc Natl Acad Sci U S A 101:3059–3064
Duran C, Qu Z, Osunkoya AO, Cui Y, Hartzell HC (2012) ANOs 3–7 in the anoctamin/Tmem16 Cl− channel family are intracellular proteins. Am J Physiol Cell Physiol 302:C482–C493
Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z (2009) Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol 587:2127–2139
Galindo BE, Vacquier VD (2005) Phylogeny of the TMEM16 protein family: some members are overexpressed in cancer. Int J Mol Med 16:919–924
Moyer BD, Hevezi P, Gao N, Lu M, Kalabat D, Soto H, Echeverri F, Laita B, Yeh SA, Zoller M, Zlotnik A (2009) Expression of genes encoding multi-transmembrane proteins in specific primate taste cell populations. PLoS ONE 4:e7682
Das S, Hahn Y, Nagata S, Willingham MC, Bera TK, Lee B, Pastan I (2007) NGEP, a prostate-specific plasma membrane protein that promotes the association of LNCaP cells. Cancer Res 67:1594–1601
Das S, Hahn Y, Walker DA, Nagata S, Willingham MC, Peehl DM, Bera TK, Lee B, Pastan I (2008) Topology of NGEP, a prostate-specific cell:cell junction protein widely expressed in many cancers of different grade level. Cancer Res 68:6306–6312
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422
Zhou G, Levitsky H (2012) Towards curative cancer immunotherapy: overcoming posttherapy tumor escape. Clin Dev Immunol 2012:124187
Drake CG (2010) Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol 10:580–593
Arlen PM, Kaufman HL, DiPaola RS (2005) Pox viral vaccine approaches. Semin Oncol 32:549–555
Gerritsen WR (2012) The evolving role of immunotherapy in prostate cancer. Ann Oncol 23(Suppl 8):viii22–viii27
Cereda V, Poole DJ, Palena C, Das S, Bera TK, Remondo C, Gulley JL, Arlen PM, Yokokawa J, Pastan I, Schlom J, Tsang KY (2010) New gene expressed in prostate: a potential target for T cell-mediated prostate cancer immunotherapy. Cancer Immunol Immunother 59:63–71
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL (2005) The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 29:1228–1242
Pierorazio PM, Walsh PC, Partin AW, Epstein JI (2013) Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 111:753–760
Ro YK, Lee S, Jeong CW, Hong SK, Byun SS, Lee SE (2012) Biochemical recurrence in Gleason score 7 prostate cancer in Korean men: significance of the primary Gleason grade. Korean J Urol 53:826–829
Cheng L, Montironi R, Bostwick DG, Lopez-Beltran A, Berney DM (2012) Staging of prostate cancer. Histopathology 60:87–117
Madjd Z, Karimi A, Molanae S, Asadi-Lari M (2011) BRCA1 protein expression level and CD44(+) phenotype in breast cancer patients. Cell J 13:155–162
Mehrazma M, Madjd Z, Kalantari E, Panahi M, Hendi A, Shariftabrizi A (2012) Expression of stem cell markers, CD133 and CD44, in pediatric solid tumors: a study using tissue microarray. Fetal Pediatr Pathol 32:192–204
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847
McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr (1985) Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109:716–721
Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC (2005) Monoclonal antibody successes in the clinic. Nat Biotechnol 23:1073–1078
Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ (2007) Immunotoxin treatment of cancer. Annu Rev Med 58:221–237
Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, Penetrante R (2007) Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using multiple tumour tissue microarray technique. Histopathology 50:472–483
Bahrenberg G, Brauers A, Joost HG, Jakse G (2000) Reduced expression of PSCA, a member of the LY-6 family of cell surface antigens, in bladder, esophagus, and stomach tumors. Biochem Biophys Res Commun 275:783–788
Koh YT, Gray A, Higgins SA, Hubby B, Kast WM (2009) Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate 69:571–584
Ragde H, Cavanagh WA, Tjoa BA (2004) Dendritic cell based vaccines: progress in immunotherapy studies for prostate cancer. J Urol 172:2532–2538
Acknowledgments
This study was conducted as a research project and supported by a grant from Tehran University of Medical Sciences (Grant #13330).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohsenzadegan, M., Madjd, Z., Asgari, M. et al. Reduced expression of NGEP is associated with high-grade prostate cancers: a tissue microarray analysis. Cancer Immunol Immunother 62, 1609–1618 (2013). https://doi.org/10.1007/s00262-013-1463-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1463-1