Abstract
Purpose
To compare the efficiency and safety of suctioning ureteral access sheath (UAS) and traditional UAS during flexible ureteroscopy (FURS) for treatment of renal stones.
Methods
Between January 2015 and December 2017, 165 patients who had renal stones successfully underwent FURS with suctioning UAS created by connecting a channel on the tail of the suctioning UAS to a vacuum device. The outcomes of these patients were compared with those of 165 patients undergoing FURS with traditional UAS using a 1:1 scenario matched-pair analysis. The matching parameters were age, gender and stone burden.
Results
The baseline characteristics were homogeneous between the two groups. The suctioning UAS group had significantly higher SFR one day postoperatively (82.4% vs. 71.5%; P = 0.02), but SFR 1 month postoperatively was comparable in the two groups (P = 0.13). The incidence of overall complications was significantly higher in the traditional UAS group (24.8% vs 11.5%; P < 0.001). Regarding individual complications, the traditional UAS group was associated with a significantly higher incidence of fever (13.9% vs 5.5%; P = 0.009) and urosepsis requiring only additional antibiotics (6.7% vs 1.8%; P = 0.029). No significant difference was noted in the incidence of septic shock, hematuria, steinstrasse or ureteral stricture. The suctioning UAS group had significantly shorter operative time (49.7 + 16.3 min vs. 57.0 ± 14.0 min; P < 0.001).
Conclusions
Compared to traditional UAS during FURS for treating renal stones, suctioning UAS had the advantages of higher SFR 1 day postoperatively, a lower incidence of infectious complications and a shorter operative time. Further well-designed studies are required to confirm the results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With advances in endoscopic technology coupled with the development of laser lithotripsy systems and novel endoscopic baskets, flexible ureteroscopy has become an increasingly popular option for the treatment of renal stones [1]. Despite its lower invasiveness and a satisfactory stone-free rate (SFR), it is important to note that one of the perioperative complications associated with this method is urosepsis caused by stone-colonizing bacteria and bacterial endotoxins in combination with the positive pressure of irrigation [2, 3]. Although there is evidence that the concomitant use of a ureteral access sheath (UAS) helps decrease intrarenal pressure [4, 5], the use of a small-caliber UAS is still an independent risk factor for systemic inflammatory response syndrome (SIRS) after flexible ureteroscopic lithotripsy [6]. The use of a large-caliber UAS may reduce the risk of SIRS secondary to flexible ureteroscopy (FURS) by providing improved drainage, but it significantly increases the risk of injury to the ureteral mucosa and of inducing ischemia of the ureter [7, 8]. These events can potentially lead to postoperative complications such as persistent hematuria, urinary extravasation and even ureteral stricture [9]. As a means of managing high renal pelvic pressure without increasing the incidence of complications, the application of suctioning UAS during FURS in treating upper urinary tract calculi showed the advantages of high lithotripsy efficacy and low infectious complication rate [10, 11]. Despite the acceptance of suctioning UAS in urological clinical practice, robust comparative data comparing suctioning UAS and traditional UAS during flexible ureteroscopy (FURS) are lacking. Therefore, a retrospective case–control study was performed to compare the effectiveness and safety of suctioning UAS and traditional UAS during the FURS procedure for treatment of renal stones.
Materials and methods
Patients
We retrospectively identified the data of patients with renal stones who successfully received FURS with suctioning UAS in our hospital from September 2016, when suctioning UAS was introduced, to December 2017. Patients with renal abnormalities and those who were younger than 18 years of age were excluded from the study. A total of 165 patients who successfully received FURS with suctioning UAS were included in the study; 13 of these patients had renal stones and ipsilateral ureteric calculi. We selected 165 patients who successfully received FURS with traditional UAS between January 2015 and August 2016 to serve as the control group. The control group was retrospectively matched to the intervention group at a 1:1 ratio with respect to age, gender and renal stone burden. Patients with ipsilateral ureteric calculi were matched with respect to the size and location of their ureteric calculi in addition to the above-mentioned parameters.
All patients were imaged preoperatively using a combination of plain radiography of the kidney–ureter–bladder (KUB) and abdominal noncontrast computed tomography (CT) and/or urinary ultrasonography. The stone burden was measured based on the maximal diameter of the stone on noncontrast CT in all patients; if multiple stones were observed, the stone burden was evaluated by summing the longest axes of all the stones. Further assessment before surgery included laboratory examination and urine culture. Appropriate antibiotic prophylaxis was administered preoperatively and intra-operatively according to the patient’s positive urine culture results, and adequate control of urinary tract infections was confirmed by urine culture or urinary microscopy prior to surgical management. Prophylactic antibiotic including quinolones or cephalosporins was administered preoperatively and intra-operatively to patients with negative urine culture results.
Written informed consent to the surgical procedure was obtained preoperatively from all patients. All FURS procedures were performed by three experienced surgeons in our department. The study was approved by the local Ethics Committee (proof number: 201806889).
Surgical technique
Traditional UAS group
After successful induction of general anesthesia, the patient was placed in the lithotomy position. Under the guidance of a 0.034 inch hydrophilic safety wire (COOK, USA), a 9.8 Fr semirigid ureteroscope (Karl Storz, Germany) was used to assess the ureter. If ureteric calculi were observed, they were pushed back to the renal pelvis if feasible; otherwise, lithotripsy was performed using a Holmium laser with a 200 μm fiber at an energy range of 12–16 W and a frequency of 14–20 Hz, and when the stone was partially broken, it was pushed back to the renal pelvis. After confirming the clearance of calculi from the ureter, a 12/14 Fr traditional UAS (KYB, China; Fig. 1b) was inserted into the proximal ureter with the guide wire. Then, a 7.5 Fr flexible ureteroscope (Olympus, Japan) was passed through the UAS, and the front end of the UAS was placed in the pyeloureteral junction under direct view of the flexible ureteroscope. The perfusion flow was then set to 60–100 mL/min. Lithotripsy was performed using a holmium:yttrium aluminum garnet (Ho:YAG) laser with a 200 μm fiber at an energy range of 12–20 W and a frequency of 14–20 Hz. A nitinol basket (COOK, USA) was used to relocate stones and retrieve stone fragments if necessary. At the end of the procedure, all renal calices were inspected to confirm satisfied fragmentation before a 6F double-J stent (KYB, China) was routinely placed.
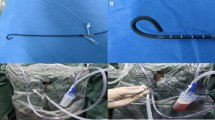
a A channel on the back end of the UAS was connected to a vacuum device to achieve a suctioning effect. b Tail of the traditional ureteral access sheath. c Tail of the suctioning ureteral access sheath: 1—channel for vacuum suction; 2—elastic rubber film with a hole; 3—flexible ureteroscope; 4—channel covered by a red cap that worked as an air door to regulate the negative pressure of the suctioning system. d Stone fragments were sucked out using suctioning UAS
Suctioning UAS group
The suction system includes a modified UAS and a general vacuum device (Fig. 1a). There is a connecting channel on the back end of the UAS; this channel was connected to the general vacuum device to achieve a suctioning effect. An elastic rubber film with a hole on the tail end of the UAS through which the flexible ureteroscope entered the UAS was designed to enhance the efficiency of suction by providing an airproof system during the FURS procedure. Additionally, on the back end of the UAS, another channel covered by a red cap worked as an air door to regulate the negative pressure of the suctioning system (Fig. 1c).
The anesthesia method, the position of the patient and the procedure for managing ipsilateral ureteric calculi and placing the 12/14 Fr suctioning UAS (KYB, China) were consistent with the methods used in the traditional UAS group. After confirming that the suctioning UAS was placed in the pyeloureteral junction under the direct view of a 7.5 Fr flexible ureteroscope (Olympus, Japan), a channel on the back end of the UAS was connected to the vacuum device (Fig. 2a). The perfusion flow and the negative pressure were then set to 60–140 mL/min and 3–8 kPa, respectively. To maintain a satisfactory suctioning effect during the procedure without deflating the renal pelvis, the negative pressure was dynamically regulated by manually twisting the red cap on the tail end of the suctioning UAS. The Ho:YAG laser settings and the procedure used for lithotripsy were the same as in the traditional UAS group. A nitinol basket (COOK, USA) was used to relocate stones and retrieve stone fragments if necessary. When the lithotripsy was completed, the position of the suctioning UAS in the pyeloureteral junction was reconfirmed using the flexible ureteroscope; the flexible ureteroscope was then detracted, a 5F ureteral catheter was inserted into the UAS, its tip was placed in the ureteropelvic junction, and the tail end of the ureteral catheter was injected with saline to create artificial water circulation (Fig. 2b). The flow of artificial water circulation and the negative pressure were set to approximately 180 mL/min and 5 kPa, respectively, and were maintained for 20–40 s. Next, all renal calices were inspected to confirm satisfactory fragmentation before a 6F double-J stent (KYB, China) was routinely placed.
In both groups, operation time was calculated from the time of insertion of the cystoscope to the time of successful placement of the double-J stent. The indwelling double-J stent was routinely left in place for 1 month. If the UAS failed to reach pyeloureteral junction due to ureteral stenosis, a 6F double-J (DJ) stent was inserted, and a second procedure was performed 1 month later. KUB was performed on postoperative day 1; the stone-free status was defined as radiological residue fragments < 2 mm. The complementary procedure was performed 1 month later if necessary.
Follow-up
All patients were routinely followed-up in the outpatient clinic at 1 and 6 months postoperatively. At 1 month postoperatively, KUB or noncontrast CT examination was performed to confirm the stone clearance status, in which stone-free status was defined as radiological residue fragments < 2 mm, and the DJ stent was removed in the outpatient clinic. During the remainder of the follow-up period, the patients underwent B-scan ultrasound and/or KUB and/or noncontrast CT.
Data collection
The patients’ medical records were obtained from the hospital’s electronic management system. The characteristics of the patients, including age, gender, body mass index (BMI), comorbidities, history of surgery on the ipsilateral side, urine culture results, antibiotic prophylaxis and stone parameters were identified. Postoperative data, including SFR at 1 day and at 1 month, operative time and length of postoperative hospital stay were also identified. In addition, postoperative complications were evaluated according to the Clavien system classification [12]. The stone burden and the postoperative stone-free status were assessed by one radiologist and one urologist independently, and any discrepancies were resolved by discussion or by consulting a third author. Both the radiologist and the urologist were blind to the treatment methods.
Statistical methods
Chi squared and Fisher’s exact tests, as appropriate, were applied to categorical variables that were expressed as the number of subjects (n) or percentages (%); Student’s t test was applied to continuous data that were expressed as the mean ± standard deviation. Two-sided P < 0.05 was considered statistically significant. All data analyses were performed using the Statistical Package for the Social Sciences 22.0 (SPSS for Windows, Chicago, IL, USA).
Results
The epidemiological and clinical characteristics (age, gender, BMI, comorbidities, ASA scores, history of surgery on the ipsilateral side) and renal stone parameters (stone burden, stone hardness and stone location) of the two groups were similar, showing no significant differences, and no significant difference in ipsilateral ureteric calculi size between the two groups was noted (Table 1). No significant difference (P = 0.24) was noted between the two groups in cephalosporin (79/119 vs. 75/127) or quinolone (40/119 vs. 52/127) use for patients with negative urine culture results. Additionally, there was no significant difference (P = 0.54) in the constituent ratio of patients treated by the three surgeons in the suctioning UAS group (number of cases 71, 49, and 45) and the traditional UAS group (number of cases 62, 57, and 46).
All patients received KUB 1 day postoperatively. One month postoperatively, the number of patients lost to follow-up was 7 and 4, respectively, in the traditional and suctioning UAS groups; the percentage of patients who received noncontrast CT was 66.5% (105/158) in the traditional UAS group and 58.4% (94/161) in the suctioning UAS group (P = 0.14), and the remaining patients in both groups received KUB. Compared to the traditional UAS group, the suctioning UAS group (82.4% vs. 71.5%; P = 0.02) displayed significantly higher SFR 1 day postoperatively. However, SFR at 1 month postoperatively was comparable in the traditional UAS group (82.9%) and the suctioning UAS group (88.8%; P = 0.13).
The incidence of overall complications was significantly higher in the traditional UAS group (24.8%) than in the suctioning UAS group (11.5%; P < 0.001). Comparison of individual perioperative and postoperative complications showed that the traditional UAS group experienced a significantly higher incidence of fever (13.9% vs. 5.5%; P = 0.009) and urosepsis requiring only additional antibiotics (6.7% vs. 1.8%; P = 0.029) than the suctioning UAS group; the incidence of septic shock (P = 0.31) in the two groups was comparable. Steinstrasse was observed in one case in the traditional UAS group (1/165); it was successfully managed by axillary lithotripsy under a semirigid ureteroscope. Ureteral stricture was identified in one patient in the suctioning group (1/165) 6 months postoperatively; fortunately, it was successfully managed with ureteroureterostomy under laparoscope in our institution. Other complications such as severe bleeding, acute renal failure, and ureteral rupture or tearing were not observed in either group.
The mean operative times for the traditional UAS group and the suctioning UAS group were 57.0 ± 14.0 min and 49.7 + 16.3 min, respectively (P < 0.001). There was no significant difference between the two groups in the length of the postoperative hospital stay (P = 0.13).
Although stone analysis data were not obtained for all of the patients in this study, we found no significant difference in stone composition in the two groups based on the patients for whom this information was obtained (P = 0.56). The perioperative and postoperative outcomes are summarized in Table 2.
Discussion
Although the conventional flexible ureteroscope was first described by Marshall in 1964, it was not until 1987 that Demetrius Bagley introduced flexible ureteroscopy as we know it today [13]. In recent years, FURS has been performed at many institutions worldwide due to its acceptable SFR and limited invasiveness, and FURS is recommended by the European Association of Urology as the first choice for the removal of renal stones < 2 cm and as an alternative method for the removal of renal stones > 2 cm in patients with contraindications for percutaneous nephrolithotomy [14]. Despite the increasing popularity of FURS, due attention should be paid to the risk of perioperative sepsis caused by the entry of bacteria and endotoxins into the blood under conditions of high intrarenal pressure [15]. The management of high intrarenal pressure during FURS has been a clinical dilemma because of its difficulty. Decreasing the perfusion flow to avoid high intrarenal pressure decreases surgical visualization and results in low lithotripsy efficacy. Additionally, the use of a stone basket is time-consuming and greatly increases the medical cost. The use of a UAS has traditionally been advocated for flexible URS because it facilitates ureteroscopy and decreases intrarenal pressure, but evidence regarding the impact of UAS on perioperative infective complications and on SFR is very limited [9]. According to the theory of irrigation and suctioning, the use of suctioning UAS may help lower the intrarenal pressure and may improve surgical visualization [11]. In recent years, suctioning UAS was applied as part of the FURS procedure at our institution in the hope of decreasing the incidence of complications secondary to high renal pelvic pressure; however, because comparative data comparing suctioning UAS and traditional UAS during flexible ureteroscopy (FURS) are lacking, a pair-matched retrospective case–control study was performed.
Compared to the traditional UAS group, the suctioning UAS group experienced a significantly lower incidence of fever and urosepsis requiring only additional antibiotics; no significant difference in the incidence of septic shock was noted, probably due to the limited number of included cases. The results indicate that vacuum suctioning can efficiently decrease the risk of infective complications by lowering the intrarenal pressure. Similarly, the attenuation of intrarenal pressure using suctioning UAS was confirmed in a previous study conducted by Huang et al. [10]. In that study, 40 patients underwent suctioning URS with intelligent control of renal pelvic pressure provided by a UAS connected to a patented irrigation and suctioning platform; the results showed that two patients (5%) experienced postoperative complications of fever, and no sepsis was noted. With respect to complications related to infection, both our results and those of Huang et al. are superior to the results of other studies in which FURS without a suctioning system was used to deal with stones of similar size [2, 16]. Bas et al. [17] reported a single-center study that included 1571 traditional FURS procedures in which the mean stone burden was 15.15 ± 8.32 mm; the infectious complications (8.08%) included fever (5.35%), urinary tract infection (2.67%), and septic shock (0.06%), and the rates of fever and septic shock were slightly lower than those observed following the use of our suctioning FURS. A possible reason for this is that the proportion of > 20 mm renal stones in the current study (39/165; 23.6%) was considerably greater than in the study of Bas et al. (250/1571; 15.9%), although the mean stone burden was comparable. Ureteral stricture was noted in one patient (0.6%) in the suctioning UAS group; in reviewing the FURS surgical record for this patient, we found impacted stones in the middle of the ipsilateral ureter, a condition that may play a primary role in the development of ureteral stricture. Furthermore, follow-up at 6 months postoperatively showed that there was no significant difference in the incidence of urinary extravasation or ureteral stricture. Hence, there are reasons to believe that suctioning UAS can effectively lower intrarenal pressure and thus significantly decrease perioperative infectious complications without increasing other complications [3, 10].
Another advantage of the use of suctioning UAS was a significantly higher SFR 1 day postoperatively, although at 1 month postoperatively SFR was comparable in the two groups. Compared to other studies of patients with similar stone burdens, our SFR result 1 day postoperatively was superior to that reported in studies in which traditional UAS was used [16, 18]. This could be due to the fact that clinically significant fragments were more likely to remain as a cluster of residual fragments in the traditional UAS group, whereas small fragments were aspirated directly when suctioning UAS was used (Fig. 1d). Therefore, it is reasonable to investigate whether suctioning UAS permits shortening of the indwelling time of the DJ stent and to determine the optimum indwelling time for patients. Additionally, the dust produced during the procedure may hinder visualization of the clear operative field, and it may be difficult to differentiate a small fragmented stone in the midst of dust [19]; therefore, the direct aspiration of small fragments in the suctioning UAS group would provide better surgical vision and thus lead to higher lithotripsy efficiency.
To facilitate the removal of gravel particles larger than the gap between the ureteroscope and the UAS but smaller than the caliber of the UAS, a 5F ureteral catheter was inserted into the UAS, and its tip was placed in the ureteropelvic junction after withdrawing the flexible ureteroscope. The tail of the ureteral catheter was injected with saline to create artificial water circulation and facilitate direct aspiration of the fragments by suctioning UAS. This novel method can reduce the need for stone basketing; consequently, the suctioning UAS group was associated with shorter operative time. Considering its advantage of shortening the operative time, we will investigate further to determine whether the use of suctioning UAS can significantly decrease the operative time and improve one-session SFR when > 2 renal stones are managed by FURS; in the past, large renal stones have always required multiple procedures, largely due to the limitation of operative time to approximately 90 min [20].
The SFR at 1 month postoperatively in our suctioning UAS group was slightly lower than that in a similar study conducted by Huang et al. [10] in which suctioning UAS was combined with the oblique supine lithotomy position; the method used in that study was considered to facilitate the collection of gravel particles from the renal pelvic outlet by vacuum suctioning due to the effects of the high-flow perfusion and gravity. Similarly, Bryniarski et al. [21] showed that SFR in traditional FURS could also be improved by changing the position of the patient to relocate lower pole stones. Therefore, it is likely that a more satisfactory outcome of SFR in FURS can be achieved by combining suctioning UAS with the use of an optimal lithotripsy position.
Certain limitations of the current study must be acknowledged. First, the current study with a limited number of cases was a retrospective design conducted at a single center; the matching parameters were only age, gender and stone burden; thus, it is worth noting that potential selection bias could not be eliminated. Second, although there was no significant difference between the two groups in the constituent ratio of patients treated by the three surgeons, a possible influence of surgeon preference on the outcome could not be eliminated. Third, noncontrast CT is superior to KUB and ultrasonography for measuring the SFR. Due to the preferences of individual patients, SFR was not assessed by noncontrast CT in all patients during the follow-up. Fourth, the system used in this study lacks pressure feedback devices and does not allow real-time recording of intrarenal pressure. During the removal of fragments by suction after extracting the ureteroscope, the perfusion flow created by saline injection was approximately 180 mL/min, significantly higher than the perfusion flow in the traditional UAS group, and this may have resulted in high intrarenal pressure despite the assistance provided by the vacuum device. For this reason, a pressure monitoring feedback device will be used in subsequent studies. Considering the limitations of the retrospective observational nature of our study, studies involving a large population of patients in a prospective randomized design are needed to confirm the current results.
Conclusions
According to our findings, compared to traditional UAS during FURS for treating renal stones, suctioning UAS has the advantages of higher SFR one day postoperatively, fewer infectious complications and shorter operative time. Considering the limitations of the retrospective observational nature of our study, further well-designed studies are required to evaluate the safety and efficiency of this procedure.
References
Desai M, Sun Y, Buchholz N et al (2017) Treatment selection for urolithiasis: percutaneous nephrolithotomy, ureteroscopy, shock wave lithotripsy, and active monitoring. World J Urol 35:1395–1399. https://doi.org/10.1007/s00345-017-2030-8
Berardinelli F, De Francesco P, Marchioni M et al (2016) Infective complications after retrograde intrarenal surgery: a new standardized classification system. Int Urol Nephrol 48:1757–1762. https://doi.org/10.1007/s11255-016-1373-1
Deng X, Song L, Xie D et al (2016) A novel flexible ureteroscopy with intelligent control of renal pelvic pressure: an initial experience of 93 cases. J Endourol 30:1067–1072. https://doi.org/10.1089/end.2015.0770
Auge BK, Pietrow PK, Lallas CD et al (2004) Ureteral access sheath provides protection against elevated renal pressures during routine flexible ureteroscopic stone manipulation. J Endourol 18:33–36. https://doi.org/10.1089/089277904322836631
Rehman J, Monga M, Landman J et al (2003) Characterization of intrapelvic pressure during ureteropyeloscopy with ureteral access sheaths. Urology 61:713–718
Zhong W, Leto G, Wang L, Zeng G (2015) Systemic inflammatory response syndrome after flexible ureteroscopic lithotripsy: a study of risk factors. J Endourol 29:25–28. https://doi.org/10.1089/end.2014.0409
Traxer O, Thomas A (2013) Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J Urol 189:580–584. https://doi.org/10.1016/j.juro.2012.08.197
Lallas CD, Auge BK, Raj GV et al (2002) Laser Doppler flowmetric determination of ureteral blood flow after ureteral access sheath placement. J Endourol 16:583–590. https://doi.org/10.1089/089277902320913288
Huang J, Zhao Z, AlSmadi JK et al (2018) Use of the ureteral access sheath during ureteroscopy: a systematic review and meta-analysis. PLoS One 13:e0193600. https://doi.org/10.1371/journal.pone.0193600
Huang J, Xie D, Xiong R et al (2018) The application of suctioning flexible ureteroscopy with intelligent pressure control in treating upper urinary tract calculi on patients with a solitary kidney. Urology 111:44–47. https://doi.org/10.1016/j.urology.2017.07.042
Zeng G, Wang D, Zhang T, Wan SP (2016) Modified access sheath for continuous flow ureteroscopic lithotripsy: a preliminary report of a novel concept and technique. J Endourol 30:992–996. https://doi.org/10.1089/end.2016.0411
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Doizi S, Traxer O (2018) Flexible ureteroscopy: technique, tips and tricks. Urolithiasis 46:47–58. https://doi.org/10.1007/s00240-017-1030-x
Turk C, Petrik A, Sarica K et al (2016) EAU guidelines on interventional treatment for urolithiasis. Eur Urol 69:475–482. https://doi.org/10.1016/j.eururo.2015.07.041
Somani BK, Giusti G, Sun Y et al (2017) Complications associated with ureterorenoscopy (URS) related to treatment of urolithiasis: the Clinical Research Office of Endourological Society URS Global study. World J Urol 35:675–681. https://doi.org/10.1007/s00345-016-1909-0
Knoll T, Jessen JP, Honeck P, Wendt-Nordahl G (2011) Flexible ureterorenoscopy versus miniaturized PNL for solitary renal calculi of 10–30 mm size. World J Urol 29:755–759. https://doi.org/10.1007/s00345-011-0784-y
Bas O, Tuygun C, Dede O et al (2017) Factors affecting complication rates of retrograde flexible ureterorenoscopy: analysis of 1571 procedures-a single-center experience. World J Urol 35:819–826. https://doi.org/10.1007/s00345-016-1930-3
Atis G, Gurbuz C, Arikan O et al (2012) Ureteroscopic management with laser lithotripsy of renal pelvic stones. J Endourol 26:983–987. https://doi.org/10.1089/end.2011.0664
Multescu R, Geavlete B, Georgescu D, Geavlete P, Chiutu L (2014) Holmium laser intrarenal lithotripsy in pyelocaliceal lithiasis treatment: to dust or to extractable fragments? Chirurgia (Bucharest 1990). Romania 109:95–98
Eswara JR, Shariftabrizi A, Sacco D (2013) Positive stone culture is associated with a higher rate of sepsis after endourological procedures. Urolithiasis 41:411–414. https://doi.org/10.1007/s00240-013-0581-8
Bryniarski P, Paradysz A, Zyczkowski M et al (2012) A randomized controlled study to analyze the safety and efficacy of percutaneous nephrolithotripsy and retrograde intrarenal surgery in the management of renal stones more than 2 cm in diameter. J Endourol 26:52–57. https://doi.org/10.1089/end.2011.0235
Acknowledgements
We would like to thank the National Natural Science Foundation of China, the Nature Science Foundation of Hunan Province, and the Key Research and Development Projects of the Hunan Science and Technology Department for providing funding for this study.
Funding
Funding was provided by the National Natural Science Foundation of China (81770705 to Hequn Chen), the Nature Science Foundation of Hunan Province, China (2017JJ2395 to Yang Li) and Key Research and Development Projects of the Hunan Science and Technology Department (2016JC2041 to Hequn Chen).
Author information
Authors and Affiliations
Contributions
ZZ: project development, data collection and manuscript writing. YC: data collection and analysis. FZ: data collection. YL: data collection. ZC: data analysis. CH: project development.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Written informed consent to surgical procedures and to the publication of clinical data on the condition of anonymity was obtained preoperatively from all included patients. The study was approved by the Ethics Committee of the Xiangya Hospital of Central South University (proof number: 201806889).
Rights and permissions
About this article
Cite this article
Zhu, Z., Cui, Y., Zeng, F. et al. Comparison of suctioning and traditional ureteral access sheath during flexible ureteroscopy in the treatment of renal stones. World J Urol 37, 921–929 (2019). https://doi.org/10.1007/s00345-018-2455-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2455-8