Abstract
Purpose
To investigate the clinical outcomes of metastatic prostate cancer patients and the relationship between nadir prostate-specific antigen (PSA) levels and different types of primary androgen deprivation therapy (PADT). This study utilized data from the Japan Study Group of Prostate Cancer registry, which is a large, multicenter, population-based database.
Methods
A total of 2982 patients treated with PADT were enrolled. Kaplan–Meier analysis was used to compare progression-free survival (PFS) and overall survival (OS) in patients treated using combined androgen blockade (CAB) and non-CAB therapies. The relationships between nadir PSA levels and PADT type according to initial serum PSA levels were also investigated.
Results
Among the 2982 enrolled patients, 2101 (70.5 %) were treated with CAB. Although CAB-treated patients had worse clinical characteristics, their probability of PFS and OS was higher compared with those treated with a non-CAB therapy. These results were due to a survival benefit with CAB in patients with an initial PSA level of 500–1000 ng/mL. Nadir PSA levels were significantly lower in CAB patients than in non-CAB patients with comparable initial serum PSA levels.
Conclusions
A small survival benefit for CAB in metastatic prostate cancer was demonstrated in a Japanese large-scale prospective cohort study. The clinical significance of nadir PSA levels following PADT was evident, but the predictive impact of PSA nadir on OS was different between CAB and non-CAB therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the efficacy of hormonal therapy for prostate cancer was first reported [1], primary androgen deprivation therapy (PADT) has become the gold-standard treatment for metastatic prostate cancer. It is also recommended as the first-choice treatment for metastatic disease in various prostate cancer clinical guidelines [2, 3]. However, there is currently no international consensus regarding the optimal type of PADT, such as combined androgen blockade (CAB) or luteinizing hormone-releasing hormone (LHRH) agonist treatment alone because various clinical randomized control trials (RCT) have reported conflicting results [4, 5].
The Japan Study Group of Prostate Cancer (J-CaP) registry is a large, multicenter, population-based database that contains information on >26,000 Japanese prostate cancer patients undergoing ADT [6, 7]. Several crucial findings, including the risk assessment tool for prostate cancer patients undergoing PADT (the Japan Cancer of the Prostate Risk Assessment; J-CAPRA), have been reported from the J-CaP database [8]. The J-CAPRA score was established based on the patient’s clinical characteristics before PADT as first-line treatment and includes pretreatment initial serum PSA level, Gleason score of biopsy specimens, and clinical TNM stage. J-CAPRA score accuracy in predicting progression-free survival (PFS), cause-specific survival (CSS), and overall survival (OS) among patients treated with PADT has been demonstrated in other cohort studies [9–11]. Recent analyses using the J-CaP registry revealed that CAB therapy might offer significant long-term survival benefits in intermediate- and high-risk prostate cancer patients, including stage IV patients and those with prostate-specific antigen (PSA) levels >500 ng/mL [12–14]. These results provide clinically important evidence regarding the indications for CAB therapy in patients with advanced prostate cancer and may explain the difference in treatment responses between CAB therapy and non-CAB therapy.
Serum PSA nadir levels, which indicate the treatment response, are strong prognostic factors for disease progression and OS after ADT [15–17]. In addition, we previously reported that patients with nadir PSA levels <0.2 ng/mL had better clinical outcomes than patients with nadir PSA levels ≥0.2 ng/mL in the J-CaP database [18]. We hypothesized that different nadir PSA levels among the various types of PADT caused the different clinical outcomes, particularly in patients with advanced disease. Therefore, we investigated the relationship between nadir PSA levels and the type of PADT used to treat metastatic prostate cancer patients in the J-CaP database. To the best of our knowledge, this is the first report assessing nadir PSA levels and clinical outcomes according to initial PSA levels in patients that received CAB and non-CAB therapy for metastatic prostate cancer.
Materials and methods
Patients and procedures
The J-CaP database includes a total of 26,272 prostate cancer patients who were diagnosed between 2001 and 2003 and underwent ADT [6, 7]. There were 384 institutions that contributed patient data to the J-CaP database, and 95 % of enrolled patients were treated with PADT. Clinical characteristics, including patient age, initial serum PSA value at diagnosis, Gleason score of biopsy specimens, clinical TNM stage [19], and treatment regiments, were reported by urologists at the participating institutions. They also reported follow-up findings every 1–3 months on an ongoing basis, including information regarding additional treatments, progression, and all-cause mortality. The present study was performed using the data set from December 2011.
Of the patients in the J-CaP database, 2982 metastatic cancer patients undergoing PADT with pretreatment characteristics defined by the variables in J-CAPRA and serum PSA levels reported at least every 1–3 months for the first year were enrolled in the present study. Evaluated PADT therapies included anti-androgen (AA) therapy, defined as the use of bicalutamide, flutamide, chlormadinone acetate, or diethylstilbestrol, and luteinizing hormone-releasing hormone (LHRH) agonist treatment, defined as the use of goserelin or leuprolide for 1 or 3 months [7]. In the present study, the use of AA plus LHRH agonists or surgical castration was defined as CAB, with the study cohort divided into CAB and non-CAB therapeutic groups. Of the 881 patients in the non-CAB group, 375 received LHRH monotherapy (42.6 %), 190 underwent surgical castration alone (21.6 %), 139 were administered AA alone (15.8 %), and 177 received LHRH plus short-term AA (20.1 %). Short-term AA was defined as AA given 30 days before and 60 days after the start of LHRH agonist treatment for flare prevention [7].
The nadir PSA level was defined as the lowest serum PSA value achieved by the patient during PADT [16]. Time to PSA nadir (TTN) was defined as the time taken for the PSA value to reach nadir after the commencement of PADT [17]. The disease progression reported by urologists at the participating institutions was confirmed to be consistent with the serial rise in serum PSA levels.
Statistical analysis
CAB and non-CAB group patient characteristics were compared using Mann–Whitney U tests or Fisher’s exact tests. The probability of PFS and OS was examined using the Kaplan–Meier method, with groups divided based on initial serum PSA concentrations at diagnosis and nadir PSA levels. The significance of group differences was evaluated using log-rank tests. All statistical analyses were performed using commercially available software; i.e., SPSS Statistics (IBM Corp., Armonk, NY, USA) and Prism (GraphPad Software, San Diego, CA, USA). In all analyses, p < 0.05 was accepted as indicating statistical significance.
Results
Clinical and pathological patient characteristics
The clinical and pathological characteristics of the patients eligible for inclusion in the study are shown in Table 1. Of the 2982 enrolled males, 2101 (70.5 %) were treated with CAB and 881 (29.5 %) with non-CAB. The median age of all patients was 73 (range 35–95) years, and the patients treated with CAB were significantly younger than those treated with non-CAB (73 vs 74; p = 0.002). The initial serum PSA levels at diagnosis were significantly higher in the patients treated with CAB compared with the levels in the patients treated with non-CAB (245 vs 191; p = 0.008). Regarding clinical characteristics, there were no statistically significant differences in the Gleason scores of biopsy specimens and clinical T stage between the two treatment groups, and the proportion of patients with lymph node metastasis was significantly higher in the patients treated with CAB compared with patients treated with non-CAB (43.7 vs 39.4 %; p = 0.028). The median nadir PSA level was 0.50 ng/mL (range 0.0–7700) in all patients and was significantly lower in patients treated with CAB than in patients treated with non-CAB (0.3 vs 1.0; p < 0.001). The median TTN was 7.05 months (range 0.36–90.7) in all patients, and there was no statistically significant difference in the TTN between treatment groups. The median follow-up period was 32.7 months (range 1.58–128.1), and the follow-up periods were significantly longer in the patients treated with CAB than in patients treated with non-CAB (33.5 vs 30.2; p = 0.009).
Clinical outcomes with different types of PADT according to initial PSA levels
Among all patients, 2069 (69.4 %) experienced disease progression and 1108 (37.2 %) died during the study period. Figure 1 shows the Kaplan–Meier estimates of PFS and OS among the patients according to the type of ADT used (Fig. 1a, b, respectively). The probabilities of PFS at 5 years [with 95 % confidence interval (CI)] in patients treated with CAB and non-CAB were 22.8 % (20.6–25.0 %) and 18.3 % (15.2–21.5 %), respectively. There was a significant difference in the probability of PFS among the treatment groups according to a log-rank test (hazard ratio 0.89, p = 0.0239). The probabilities of OS at 5 years with 95 % CI in patients treated with CAB and non-CAB were 54.3 % (51.5–57.0 %) and 51.5 % (47.3–55.8 %), respectively. The probability of OS was significantly different among the treatment analysis groups using a log-rank test (hazard ratio 0.88, p = 0.0424).
Figure 2 shows Kaplan–Meier estimates of PFS for treatment groups categorized according to initial serum PSA levels at diagnosis. In patients with initial serum PSA levels of 100–1000 ng/mL, the probability of PFS was higher in individuals treated with CAB compared with that in patients treated with non-CAB (Fig. 2c, d). There were no statistically significant differences in PFS among the groups of patients with other ranges of initial serum PSA levels (Fig. 2a, b, e, f).
Figure 3 shows Kaplan–Meier estimates of OS between the two treatment groups categorized according to initial serum PSA levels at diagnosis. In patients with initial serum PSA levels of 500–1000 ng/mL, the probability of OS was higher in those treated with CAB compared with that in patients treated with non-CAB (Fig. 3d). There were no statistically significant differences in OS among the groups of patients with other ranges of initial serum PSA levels (Fig. 3a, b, c, e, f).
Relationships between initial PSA levels and nadir PSA in PADT
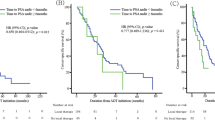
Table 2 shows the relationships between initial serum PSA levels at diagnosis and nadir PSA values according to the type of PADT administered. Nadir PSA levels were significantly lower in patients with initial serum PSA levels <5000 ng/mL who were treated with CAB compared with those in patients treated with non-CAB. Figure 4 shows that the probability of PFS and OS were significantly different among the groups of patients categorized by nadir PSA levels <0.2 ng/mL (a), 0.2–1.0 (b), 1.1–4.0 (c), 4.1–10.0 (d), and >10.0 (e). The probability of OS (with 95 % CI) at 5 years in patients treated with CAB and with nadir PSA levels <0.2 ng/mL (a), 0.2–1.0 (b), 1.1–4.0 (c), 4.1–10.0 (d), and >10.0 (e) was 82.2 % (78.9–85.4 %), 41.0 % (34.3–47.7 %), 28.8 % (22.5–35.2 %), 34.0 % (24.7–43.4 %), and 18.1 % (12.3–23.9 %), respectively (Fig. 4c). The probability of OS (with 95 % CI) at 5 years in patients treated with non-CAB and nadir PSA levels <0.2 ng/mL (a), 0.2–1.0 (b), 1.1–4.0 (c), 4.1–10.0 (d), and >10.0 (e) was 82.7 % (76.9–88.4 %), 56.0% (46.4–65.5 %), 41.6 % (31.8–51.4 %), 28.5 % (16.8–40.2 %), and 16.0 % (8.16–23.9 %), respectively (Fig. 4d). There was no statistically significant difference in the PFS and OS of patients treated using CAB and with patients with nadir PSA levels of 1.1–4.0 and 4.1–10.0 ng/mL (Fig. 4a, c).
Kaplan–Meier survival curves for progression-free survival among patients treated with CAB stratified according to nadir PSA levels (a). Overall survival among patients treated with CAB stratified by nadir PSA levels (b). Progression-free survival among patients treated with non-CAB stratified by nadir PSA levels (c). Overall survival in patients treated with CAB stratified by nadir PSA levels (d)
Discussion
In the 1990s, maximum androgen blockade (MAB), which is now known as CAB, seemed to be a useful prostate cancer therapy; however, the survival advantage of CAB compared with surgical or medical castration alone in published RCTs and meta-analyses was negligible [4, 5]. The variation in patient backgrounds may have affected the results of RCTs. Specifically, evidence from recent RCTs suggests that CAB has greater benefits than non-CAB in certain subgroups of patients, including those with stage C and D1 tumors [21, 22]. Data from the J-CaP database, which is a large-scale Japanese population-based prospective cohort, demonstrated that CAB might have significant long-term survival benefits in subgroups of intermediate- and high-risk prostate cancer patients [12], stage IV patients with a high J-CAPRA score [13], and patients with PSA levels >500 ng/mL [14]. The present study demonstrated that CAB exhibited significant PFS and OS benefits in metastatic disease, as evidenced by the better treatment response to CAB from patients with initial serum PSA levels of 500–1000 ng/mL compared with those that received non-CAB therapy.
Interestingly, the present analysis found that a significantly higher proportion of patients with high initial serum PSA levels and N1 disease that were treated with CAB had better clinical outcomes than those that received non-CAB. In the J-CaP database, 59.0 % (11,435 of 19,409) of all patients with stage I–IV disease received CAB as PADT [7]; however, 70.5 % (2101 of 2982) of the individuals received CAB in the present cohort, which analyzed metastatic disease alone. This finding might reflect the clinical decision to select CAB rather than non-CAB for treating high-risk patients. Physicians have expected CAB to have better efficacy compared with that of non-CAB; however, evidence for and the cause of CAB superiority for advanced prostate cancer remains elusive.
Nadir serum PSA levels in prostate cancer patients undergoing ADT are a useful indicator for evaluating the response to ADT. They were also a strong prognostic factor for clinical outcome in several studies, including our previous reports using information from the J-CaP database [15–18]. However, because the type of ADT was not analyzed according to patient nadir PSA level and clinical outcome in these previous studies, the relationships between nadir PSA level and the type of ADT remains unclear. A previous RCT reported that a higher proportion of patients treated with CAB achieved nadir PSA levels of ≤1.0 ng/mL and had better OS compared with patients treated with LHRH monotherapy [22]. In the present study, we demonstrated that nadir PSA levels according to initial serum PSA levels at diagnosis showed a greater response to CAB therapy than to non-CAB therapy.
The current study reported the different survival benefits of patients treated with CAB and non-CAB according to initial PSA levels at diagnosis. A previous study reported the cause-specific benefit and overall survival benefit of CAB in patients with high initial PSA levels of 500–4999 ng/mL compared with that of non-CAB using the J-CaP database [14]. Therefore, the present results are consistent with previous findings, and the small discrepancy between these reports may be due to the differences in the eligibility criteria in the two studies. Additional findings from the present study were the varying nadir PSA levels achieved by the different types of PADT in patients with the same ranges of initial PSA levels, which may explain the greater survival benefit of CAB compared with that of non-CAB in patients with initial serum PSA levels of 500–1000 ng/mL. In this range, the median nadir PSA level in CAB patients was <1.0 ng/mL compared with >4.0 ng/mL in non-CAB patients. This difference in nadir PSA values, which may reflect the response to treatment with different types of PADT, resulted in different clinical outcomes between the two treatment groups. In patients with initial serum PSA levels <100 ng/mL, it is possible that a lower nadir PSA in the CAB patients will not lead to a survival benefit over non-CAB, since the low nadir PSA already predicted a good clinical outcome. Moreover, a low probability of PFS and OS and high nadir PSA levels were observed in patients with initial PSA levels >1000 ng/mL. The current results clearly suggest a relationship between nadir PSA levels and the type of PADT. To the best of our knowledge, this is the first report to demonstrate this relationship based on initial PSA levels.
An interesting finding from the present study was better PFS and OS clinical outcomes in non-CAB patients with nadir PSA levels of 0.2–4.0 ng/mL compared with outcomes for CAB patients within the same range of nadir PSA values (Fig. 4). This finding suggests that the clinical interpretation of PSA kinetics may depend on the type of PADT administered; therefore, it is possible that the optimal cutoff levels for nadir PSA as a prognostic factor differ between CAB and non-CAB therapies. The cutoff levels for nadir PSA after ADT differ among previous studies, with reported values including 4.0 ng/mL [15], 1.1 ng/mL [16], and 0.2 ng/mL [17, 18, 20, 23]. Although these differences were thought to depend on the development of measurement accuracy, they may also reflect variations with the use of different types of ADT in the previous studies. In the clinical setting, physicians may predict the PFS and OS of patients undergoing PADT using their initial PSA levels, as demonstrated in the present study. Moreover, they can re-evaluate the patient’s status using nadir PSA levels approximately 7 months after starting PADT, which was the median time to nadir PSA levels reported here.
There are several limitations to the present study. First, potential bias resulting from the non-randomized design must be considered. Patients were assigned to the CAB and non-CAB groups based on clinical practice. Patient background, including comorbidities, performance status, and income, may also be possible biases. Second, the non-CAB group included various treatments, e.g., LHRH monotherapy, surgical castration alone, AA alone, and LHRH plus short-term AA. Moreover, various drugs were administered as AA, including chlormadinone acetate or diethylstilbestrol, which are no longer used for prostate cancer treatment in many countries. This variation of administered drugs within the non-CAB group may be a potential bias. Third, the present study did not include data regarding second-line treatment. The patients treated with non-CAB as the PADT could receive additional treatments including CAB, which may impact OS in this group. Despite these limitations, this study demonstrated a clear survival benefit with CAB treatment of metastatic prostate cancer patients and revealed that nadir PSA levels can be used to predict PFS and OS using large-scale, multicenter registry data. The relationship between nadir PSA levels and the type of PADT is a novel, important insight into predicting the clinical outcome of patients with metastatic prostate cancer.
References
Huggins C, Hodges CV (1972) Studies on prostate cancer: i. the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin 22(4):232–240
Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D’Amico AV, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Kawachi MH, Kuettel M, Lee RJ, Macvicar GR, Malcolm AW, Miller D, Plimack ER, Pow-Sang JM, Richey S, Roach M 3rd, Rohren E, Rosenfeld S, Small EJ, Srinivas S, Stein C, Strope SA, Tward J, Walsh PC, Shead DA, Ho M (2014) National comprehensive cancer network (2013) Prostate cancer, version 1. J Natl Compr Canc Netw 11(12):1471–1479
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N, European Association of Urology (2014) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65(2):467–479
Prostate Cancer Trialists’ Collaborative Group (2000) Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 355(9241):1491–1498
Pagliarulo V, Bracarda S, Eisenberger MA, Mottet N, Schröder FH, Sternberg CN, Studer UE (2012) Contemporary role of androgen deprivation therapy for prostate cancer. Eur Urol 61(1):11–25
Akaza H, Usami M, Ogawa O, Kagawa S, Kitamura T, Tsukamoto T, Naito S, Hirao Y, Murai M, Yamanaka H (2004) Characteristics of patients with prostate cancer who have initially been treated by hormone therapy in Japan: J-CaP surveillance. Jap J Clin Oncol 34(6):329–336
Hinotsu S, Akaza H, Usami M, Ogawa O, Kagawa S, Kitamura T, Tsukamoto T, Naito S, Namiki M, Hirao Y, Murai M, Yamanaka H, Japan Study Group of Prostate Cancer (J-CaP) (2007) Current status of endocrine therapy for prostate cancer in Japan analysis of primary androgen deprivation therapy on the basis of data collected by J-CaP. Jap J Clin Oncol 37(10):775–781
Cooperberg MR, Hinotsu S, Namiki M, Ito K, Broering J, Carroll PR, Akaza H (2009) Risk assessment among prostate cancer patients receiving primary androgen deprivation therapy. J Clin Oncol 27(26):4306–4313
Kitagawa Y, Hinotsu S, Shigehara K, Nakashima K, Kawaguchi S, Yaegashi H, Mizokami A, Akaza H, Namiki M (2013) Japan Cancer of the Prostate Risk Assessment for combined androgen blockade including bicalutamide: clinical application and validation. Int J Urol 20(7):708–714
Shiota M, Yokomizo A, Takeuchi A, Imada K, Kiyoshima K, Inokuchi J, Tatsugami K, Naito S (2015) The oncological outcome and validation of Japan Cancer of the Prostate Risk Assessment score among men treated with primary androgen-deprivation therapy. J Cancer Res Clin Oncol 141(3):495–503
Yamaguchi Y, Hayashi Y, Ishizuya Y, Takeda K, Nakai Y, Arai Y, Nakayama M, Kakimoto K, Nishimura K (2015) A single-center study on predicting outcomes of primary androgen deprivation therapy for prostate cancer using the Japan Cancer of the Prostate Risk Assessment (J-CAPRA) score. Jpn J Clin Oncol 45(2):197–201
Akaza H, Hinotsu S, Usami M, Ogawa O, Kitamura T, Suzuki K, Tsukamoto T, Naito S, Namiki M, Hirao Y, Murai M (2013) Evaluation of primary androgen deprivation therapy in prostate cancer patients using J-CAPRA risk score. Prostate Int 1(2):81–88
Mastuoka T, Kawai K, Kimura T, Kojima T, Onozawa M, Miyazaki J, Nishiyama H, Hinotsu S, Akaza H (2014) Long-term outcomes of combined androgen blockade therapy in stage IV prostate cancer. J Cancer Res Clin Oncol 141(4):759–765
Sugihara T, Yu C, Kattan MW, Yasunaga H, Ihara H, Onozawa M, Hinotsu S, Akaza H (2014) Long-term survival of extremely advanced prostate cancer patients diagnosed with prostate-specific antigen over 500 ng/mL. Jpn J Clin Oncol 44(12):1227–1232
Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, Wilding G, Akdas A, Small EJ, Donnelly B, MacVicar G, Raghavan D, Southwest Oncology Group Trial 9346 (INT-0162) (2006) Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from southwest oncology group trial 9346 (INT-0162). J Clin Oncol 24(24):3984–3990
Kwak C, Jeon SJ, Park MS, Lee E, Lee SE (2002) Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol 168(3):995–1000
Morote J, Trilla E, Esquena S, Abascal JM, Reventos J (2004) Nadir prostate-specific antigen best predicts the progression to androgen-independent prostate cancer. Int J Cancer 108(6):877–881
Kitagawa Y, Ueno S, Izumi K, Mizokami A, Hinotsu S, Akaza H, Namiki M (2014) Nadir prostate-specific antigen (PSA) level and tome to PSA nadir following primary androgen deprivation therapy as independent prognostic factors in a Japanese large-scale prospective cohort study (J-CaP). J Cancer Res Clin Oncol 140(4):673–679
International Union Against Cancer (1997) Urologic Tumors: Prostate. In: Sobin LH, Wittekind CH (eds) TNM Classification of Malignant Tumours, 5th edn. Wiley, New York, pp 170–173
Choueiri TK, Xie W, D’Amico AV, Ross RW, Hu JC, Pomerantz M, Regan MM, Taplin ME, Kantoff PW, Sartor O, Oh WK (2009) Time to prostate-specific antigen nadir independently predicts overall survival in patients who have metastatic hormone-sensitive prostate cancer treated with androgen-deprivation therapy. Cancer 115(5):981–987
Usami M, Akaza H, Arai Y, Hirano Y, Kagawa S, Kanetake H, Naito S, Sumiyoshi Y, Takimoto Y, Terai A, Yoshida H, Ohashi Y (2007) Bicalutamide 80 mg combined with a luteinizing hormone-releasing hormone agonist (LHRH-A) versus LHRH-A monotherapy in advanced prostate cancer: findings from a phase III randomized, double-blind, multicenter trial in Japanese patients. Prostate Cancer Prostat Dis 10(2):194–201
Akaza H, Hinotsu S, Usami M, Arai Y, Kanetake H, Naito S, Hirao Y, Study Group for the Combined Androgen Blockade Therapy of Prostate Cancer (2009) Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 115(15):3437–3445
Hori S, Jabbar T, Kachroo N, Vasconcelos JC, Robson CN, Gnanapragasam VJ (2011) Outcomes and predictive factors for biochemical relapse following primary androgen deprivation therapy in men with bone scan negative prostate cancer. J Cancer Res Clin Oncol 137(2):235–241
Acknowledgments
We thank Dr. Michiyuki Usami, Dr. Osamu Ogawa, Dr. Tadaichi Kitamura, Dr. Kazuhiro Suzuki, Dr. Taiji Tsukamoto, Dr. Seiji Naito, Dr. Yoshihiko Hirao, Dr. Masaru Murai, and the Japan Study Group of Prostate Cancer (J-CaP) for providing clinical data from the J-CaP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethical standard
This study has been approved by Japanese multicenter institutional review board for research (UMIN 000000570). There is no patient identifying information included in this manuscript.
Rights and permissions
About this article
Cite this article
Kitagawa, Y., Ueno, S., Izumi, K. et al. Clinical outcomes and nadir prostate-specific antigen (PSA) according to initial PSA levels in primary androgen deprivation therapy for metastatic prostate cancer. World J Urol 34, 319–327 (2016). https://doi.org/10.1007/s00345-015-1621-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-015-1621-5