Abstract
Purpose
(1) To describe the minimally invasive adjustable continence therapy (ACT)® balloon placement surgical technique. (2) To analyse the results of ACT® balloon in the treatment for female stress urinary incontinence (SUI).
Method
A review of the literature was performed by searching the PubMed database using the following search terms: ACT balloons, female urinary incontinence, and female continence.
Results
Eight studies were published between 2007 and 2013. The mean follow-up of these studies was 1–6 years. The mean age of the patients ranged between 62 and 73 years; 40–100 % of patients had already been treated surgically for their SUI. A significant reduction in the number of pads used per day was observed after ACT® balloon placement, with improvement of short pad tests from 49.6 to 77.3 g preoperatively to 11.2–25.7 g after ACT® balloon placement. Fifteen to 44 % of patients considered that their SUI had been cured and 66–78.4 % were satisfied with the result. The explantation rate ranged between 18.7 and 30.8 %. Quality of life was significantly improved, and no major complication was reported.
Conclusion
ACT® balloons constitute a reasonable, minimally invasive alternative for the treatment for female SUI due to intrinsic sphincter disorder, especially in patients who have already experienced failure of standard surgical treatment and in clinical settings incompatible with invasive surgical placement of an artificial urinary sphincter (especially women over the age of 80 years). Long-term results are essential to evaluate the efficacy of this treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the International Continence Society (ICS), stress urinary incontinence (SUI) is defined as “the complaint of involuntary leakage on effort or exertion, or on sneezing or coughing” [1].
Stress urinary incontinence is responsible for major disability in many women. The prevalence of SUI is difficult to estimate, but possibly as high as 50 % [2] depending on the definition of urinary incontinence (UI) adopted and/or the age-group studied. This prevalence is probably underestimated.
The anatomical mechanisms responsible for SUI are predominantly excessive mobility of the urethra and/or bladder neck, and intrinsic sphincter deficiency (ISD). ISD is generally due to muscle atrophy, oestrogen deficiency and/or pelvic, and perineal denervation. The main known general risk factors are age, pregnancy and childbirth, menopause, and radiotherapy [2]. ISD is assessed by a negative Marshall or submidurethral tape test and urodynamics. Concerning urodynamics, there is no current consensus on the exact urodynamic definition of ISD. In fact, the concept of ISD is a clinico-urodynamic concept [3].
Various options are currently available for the treatment for SUI, ranging from simple perineal retraining to artificial urinary sphincter (AUS), which is the last resort treatment for female SUI due to ISD after failure of several less invasive therapies [3, 4]. The surgical treatment most commonly used for urethral hypermobility is suburethral tape [TOT or tension-free vaginal tape (TVT)] [5]. Suburethral fascial slings have a real place in the treatment for SUI due to ISD [6], but may require subsequent voiding by self-catheterization.
Adjustable continence therapy (ACT)® balloons have recently extended the range of treatment options in men [7, 8] and in women [9–15]. They are indicated in women with stage III SUI (pure ISD), or stage II SUI when ISD is predominant after failure of well-conducted retraining, but the place of this modality has not been clearly defined in international guidelines [16]. The objective of this study was to describe the ACT® balloon placement surgical technique, analyse the results of ACT® balloon in the treatment for female SUI due to ISD, and therefore review the place of this option in the treatment algorithm.
Method
A review of the literature was performed by searching the PubMed database using the following search terms: ACT balloons, female urinary incontinence, and female continence. In view of the small number of published studies in peer-reviewed journals in English and French, all studies, regardless of their methodology, were included in this analysis. The main functional outcomes and complications were reported.
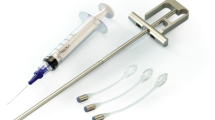
Description of the ACT® balloon [9, 17]
The ACT® balloon is an implantable medical device developed by Uromedica (Irvine, CA, USA), consisting of a silicone balloon connected to a conduit with a titanium port at its extremity. The conduit comprises two lumens, one containing a stylet to guide insertion of the conduit and the other allowing inflation of the balloon from the titanium port. The balloon has a volume of 8 ml. It is adjustable and can be inflated or deflated by simply inserting a needle into the titanium port, which is placed subcutaneously. The degree of balloon inflation is used to establish an equilibrium between continence and even dysuria, when the balloon is overinflated and persistent IU, when the balloon is underinflated.
Two conduit lengths are available, 7 and 9 cm, chosen according to the patient’s morphology. Dedicated implant tools for balloon placement consist of a trocar, a sharp-tipped stylet, a blunt-tipped stylet, and a tissue expanding device (TED, forceps adapted to the trocar allowing creation of a compartment). Each instrument can be resterilized.
Surgical technique [9–15]
The procedure is performed under general or local anaesthesia, after ensuring a negative urine culture and under prophylactic antibiotics administered at induction. The patient is installed in the gynaecological position with the thighs flexed onto the abdomen. The image intensifier is placed over the pelvis.
The bladder neck is identified by combined fluoroscopy and urethrocystoscopy with the tip of the endoscope placed in the bladder neck. A 16 F Foley catheter is introduced, and the balloon is filled with aqueous radiopaque solution easily visible on fluoroscopy.
The incision is made in the convexity of labia majora, 2 cm anteriorly to the urethral meatus. The trocar is introduced prevaginally, laterally to the urethra, with a finger in the vagina to control progression of the trocar in order to position the trocar tip at the urethrovesical junction, over the bladder neck, ideally at 3 o’clock and 9 o’clock. Urethrocystoscopy confirms correct positioning of the trocar and the absence of urethral or bladder perforation.
When the trocar is correctly positioned, the stylet is removed and the balloon is then lubricated and introduced into the sheath introducer. The balloon port is then pierced with a syringe containing aqueous radiopaque solution (isotonic mixture of 5.7 ml of Omnipaque 300 and 6 ml of pure water). In patients allergic to iodinated contrast agents, the balloon can be filled with normal saline. The tip of the balloon comprises a radiopaque marker allowing control of its position by fluoroscopy.
Once the balloon has been correctly positioned, the trocar is slightly withdrawn (about 2 cm) while maintaining the balloon in place. The balloon is then filled with 0.2 ml of radiopaque mixture (Fig. 1).
The balloon stylet is then removed, and a subcutaneous compartment is created in the anterior part of the labium majorum to allow placement of the balloon port as accessible as possible for future adjustments.
A 16 F Foley catheter is introduced and left in place for 2–24 h depending on the type of hospitalization.
Results
Only eight studies, published between 2007 and 2013, evaluating the efficacy and complications of treatment for female urinary incontinence by ACT® balloon placement were retrieved by our literature search [9–15, 18]. Tables 1, 2, and 3 summarize these studies. All studies were prospective except for the study by Vayleux, which was retrospective [10]. All studies were open label with a 3 level of evidence, except the retrospective one which was level 4 [10]. The studies were based on comparison of endpoints before/after balloon placement. The mean follow-up ranged from 1 to 6 years. Patients included in these studies presented either isolated or mixed SUI. The mean age of these patients ranged between 62 and 73 years with a certain percentage of patients over the age of 80.
Thirty-eight to 100 % of the patients included in these studies had already undergone surgical treatment for SUI (suburethral sling, Burch colposuspension, AUS, etc.). Patients were evaluated by validated questionnaires (I-QOL, Urinary Symptom Profile®, Urinary Distress Inventory), the number of pads used per day, a urinary incontinence test (short pad test), patient impression of improvement of incontinence at the end of follow-up (improved or not improved, dry or still bothered by leaks, etc.). The specific feature of this technique is that continence is only achieved after repeated outpatient inflation of the balloons according to the symptoms reported by the patient. The results should therefore be analysed at the end of this adjustment period, ideally after 3–6 months.
Functional results
Balloon efficacy endpoints were heterogeneous and differed from one study to another. The mean number of adjustments was 1–3.8. The mean final balloon volume was not always specified in studies, but ranged between 1.97 and 3.45 ml. Preoperatively, patients presented a mean I-QOL score ranging from 30 to 40. After balloon placement, the mean I-QOL score was improved and ranged between 65.5 and 71 after 1 year, and 70.4 and 75 after 2 years of follow-up.
The number of pads used per day was significantly decreased from 4.1 to 5.4 preoperatively to 1.2–2.5 after 1 year and 1.1–1.2 after 2 years of follow-up.
The pad test was also improved from 49.6 to 77.3 g preoperatively to 11.2 to −25.7 g after ACT® balloon placement.
On intention-to-treat analysis at the end of follow-up, considering only those patients in whom the balloons were still in place at the end of follow-up, 15–44 % of patients considered that they were cured and 66–78.4 % of patients were satisfied with the result and felt improved.
Complications
No major complications were reported. Most intraoperative complications were urethral or bladder perforations, observed in 3–17 % of cases. Note that the 17 % perforation rate was reported in the study by Chartier-Kastler published in 2007 [9], which was the first multicentre, prospective, controlled study evaluating treatment for female SUI by ACT® balloon and therefore corresponded to the early experience with this type of management in France. The most recent studies have reported intraoperative perforation rates between 3.7 and 4.5 %.
Aboseif et al. in 2009 and 2011 [11, 12] reported procedure failure rates of 2.5 and 2.2 %, respectively, without specifying the reason for these failures.
Post-operative complications, during the first year, consisted of urethral erosion (2–15 %), cutaneous erosion of the port (3–7.5 %), balloon migration (6.5–17.5 %), device infection (0.6–8.9 %), balloon dysfunction (0.6–6 %), inefficacy of treatment or even worsening of incontinence (2.5–11.7 %), dysuria or even acute urinary retention (1.5–6.8 %), and de novo urgency (10.5 %) in the study by Kocjancic et al. [14].
The explantation rate at the end of follow-up ranged between 18.7 and 30.8 %. The reimplantation rate in these explanted patients was 50 % [11, 12] in the studies by Aboseif in 2009 and 2011.
Discussion
Adjustable continence therapy® balloons are a recent treatment modality and are indicated in a specific population of patients with urinary incontinence: difficult situations of primary or secondary failure of treatment for urethral hypermobility by suburethral tape or patients unsuitable for AUS placement (for example, women over the age of 80 years, with cognitive impairments and motor disabilities). Explantation rate can reach 30 %. The risk factors for explantation of ACT® balloons have not been clearly identified in women.
In men, several studies suggested that perioperative injuries due to anatomic considerations [11, 18, 19] or difficulties of implantation due to fibrotic tissues secondary to radiotherapy could represent risk factors for explantation. One study reported a statistically significant correlation between urethral erosion and a history of pelvic radiotherapy (p = 0.005) [20].
In the light of the above results, the ideal indication for ACT® balloons in women would therefore appear to be SUI in which ISD appears to be the predominant mechanism responsible for the patient’s symptoms or detected on patient work-up. No comparative study versus another reference treatment for SUI due to ISD (AUS, suburethral fascial sling) was identified. ACT® balloons should logically be compared with AUS, although these two techniques are radically different in terms of their degree of invasiveness and their mechanism of action. Adjustable balloons should be preferred whenever the surgical setting appears to contraindicate an AUS, or when the patient refuses AUS and/or when treatment for type II SUI by suburethral sling would likely to be incomplete (see below) due to ISD. But this statement must be assessed by comparative studies. This is an important point, as the mechanism of action of these balloons must comprise bladder neck support as well as periurethral compression, as reflected by the reduction in urine flow rate observed in the majority of implanted patients [9].
No published study has compared the results of treatment for female SUI by ACT® balloon to the other conventional surgical treatments. Over the last decade, the number of colposuspensions, suburethral fascial supports or slings has decreased in favour of the use of synthetic suburethral tape. The ideal surgical treatment must be technically easy to perform, inexpensive, easy to learn, minimally invasive, and effective in the long term with no major morbidity.
Periurethral injections
There are currently no data in the literature in support of the use of periurethral injections as first-line treatment for SUI. Only limited data are available concerning comparison of the various techniques. Published studies are often poor quality with a low level of proof and limited follow-up. Improvement has been reported in 73 % of patients with cure rates ranging from 24 to 36 % [21]. Periurethral injections can sometimes have lasting effects, but repeated injections may be necessary [22, 23]. Periurethral injection is associated with few complications and adverse effects. This technique can be used because of its good benefit/risk balance in frail patients, previously operated patients, and patients refusing surgery. Consequently, after failure of surgical treatment, and/or in the presence of ISD, periurethral injections can be an alternative to another surgical procedure, although the results are considerably inferior to those of ACT® balloons or AUS.
Synthetic suburethral tape
The long-term results of suburethral tape, such as TVT, show that 90 % patients remain continent [24]. Randomized studies report that retropubic suburethral tape ensures higher continence rates than Burch colposuspension. Suburethral tape is just as effective as pubovaginal slings, but slings induce a higher rate of bladder emptying disorders. Retropubic TVT tape appears to be more effective in the long term than transobturator tape [25]. ACT® balloons may be indicated after failure of suburethral tape. Table 4 compares suburethral tapes to ACT® balloons.
Colposuspension and bladder neck fascial sling
In view of the equivalent functional results obtained by suburethral tape and Burch colposuspensions and the greater morbidity of colposuspension, the indications for conventional surgery therefore correspond to contraindications to suburethral tape (marked alteration of vaginal trophicity, history of urethral repair surgery). The indications for bladder neck fascial sling now appear to be very limited.
A comprehensive review of the literature from the Cochrane database [26] indicated that the objective cure rate after colposuspension was between 59 and 100 % (median 80 %) and the subjective cure rate ranged from 71 to 100 % (median 88 %). Open colposuspension provides similar results to those of bladder neck sling and TVT, but better results than anterior colporrhaphy, Marshall-Marchetti operation, needle colposuspension or paravaginal repair [26]. With a follow-up of more than 10 years, the success rate of these techniques is between 55 and 70 % [27]. However, these techniques are invasive and can be responsible for de novo bladder capacity disorders (8–27 %), bladder emptying disorders (2–27 %), and prolapse (2.5–27 %) after colposuspension [27]. ACT® balloons could therefore constitute a valuable and less invasive alternative.
Suburethral slings
The conclusions of the Cochrane database concerning suburethral slings are not based on high levels of proof, in view of the small number of publications and their poor quality. Fascial slings are the most extensively studied technique. They represent an effective non-prosthetic technique for surgical correction of urinary incontinence, but at the price of high morbidity in terms of post-operative dysuria and wound complications [28]. The superiority of slings over colposuspensions has not been formally demonstrated. Several studies comparing TVT and fascial slings demonstrated few significant differences in terms of cure [29, 30]. Suburethral fascial sling, in view of its unfavourable efficacy/morbidity balance, is therefore now performed less and less commonly. However, in patients with persistent SUI after multiple surgical procedures, suburethral fascial sling could be considered, but may possibly require subsequent self-catheterization. AUS can also be considered in this indication.
Artificial urinary sphincter
AUS implantation in women is only performed by experienced teams [4, 31] due to the technical difficulties related to the short female urethra or a history of local surgical, responsible for the morbidity of this operation [3]. However, AUS remains the reference treatment in patients with severe SUI with ISD, often after failure of other surgical treatments. Several studies have reported the long-term results of AUS in women, with continence rates ranging between 61 and 90 % [4, 29–37]. The leading long-term complication of this treatment is explantation of the material in 3–50 % of cases due to various causes (infection, erosion) [32, 39, 40] and the need for revision of the material in 13–63 % of cases [38]. ACT® balloon placement, compared to AUS, is associated with lower morbidity, is easier to manage, and can represent a valuable alternative in elderly patients or patients with limited mental or physical capacities. The age limit of 80 years for AUS implantation [3] could constitute a very good indication for ACT® balloons. Figure 2 suggests a flow chart for the surgical management of SUI.
Conclusion
The literature concerning the long-term evaluation of treatment for female SUI by ACT® balloon is limited to only a few studies, but which nevertheless report favourable results compared to the other alternative techniques. ACT® balloons may be indicated in the management of female SUI due to predominant or isolated ISD, especially in patients with a history of failure of standard surgical treatment and in patients in whom invasive surgery would be contraindicated (age > 80 years). This technique also presents the advantage of being associated with easily managed local morbidity, which does not appear to compromise future use of the patient’s periurethral tissues. Nevertheless, the explantation rate can reach 30 %.
Long-term data are essential for more thorough evaluation of this treatment. It would also be interesting to compare the management of stage II SUI with predominant sphincter deficiency and stage III SUI by suburethral tape and first-line ACT® balloons.
References
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U et al (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61(1):37–49
Reynolds WS, Dmochowski RR, Penson DF (2011) Epidemiology of stress urinary incontinence in women. Curr Urol Rep 12(5):370–376
Chartier-Kastler E, Van Kerrebroeck P, Olianas R, Cosson M, Mandron E, Delorme E et al (2011) Artificial urinary sphincter (AMS 800) implantation for women with intrinsic sphincter deficiency: a technique for insiders? BJU Int 107(10):1618–1626
Costa P, Poinas G, Ben Naoum K, Bouzoubaa K, Wagner L, Soustelle L et al (2013) Long-term results of artificial urinary sphincter for women with type III stress urinary incontinence. Eur Urol 63(4):753–758. doi:10.1016/j.eururo.2012.03.008
Vidart A, Cour F (2010) Guidelines for the treatment of non-neurological urinary incontinence in women using periurethral balloons. Prog Urol 20(Suppl 2):S150–S154
Novara G, Artibani W, Barber MD, Chapple CR, Costantini E, Ficarra V et al (2010) Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol 58(2):218–238
Lebret T, Cour F, Benchetrit J, Grise P, Bernstein J, Delaporte V et al (2008) Treatment of postprostatectomy stress urinary incontinence using a minimally invasive adjustable continence balloon device, ProACT: results of a preliminary, multicenter, pilot study. Urology 71(2):256–260
Mehnert U, Bastien L, Denys P, Cardot V, Even-Schneider A, Kocer S et al (2012) Treatment of neurogenic stress urinary incontinence using an adjustable continence device: 4-Year followup. J Urol 188(6):2274–2280
Chartier-Kastler E, Costa P, Ben Naoum K, Cour F, Le Normand L, Haab F (2007) French multicentre prospective study of the use of ACT balloons (Uromedica, Inc., Plymouth, Min, USA; Medtronic, Minneapolis, USA) for the treatment of female stress urinary incontinence. Prog Urol 17(7):1372–1377
Vayleux B, Luyckx F, Thélu S, Rigaud J, Bouchot O, Karam G et al (2010) Adjustable continence therapy in women, middle term follow-up and a new technique for balloon positioning. Prog Urol 20(7):520–526
Aboseif SR, Franke EI, Nash SD, Slutsky JN, Baum NH, Tu LM et al (2009) The adjustable continence therapy system for recurrent female stress urinary Incontinence: 1-year results of the North America clinical study group. J Urol 181(5):2187–2191
Aboseif S, Sassani P, Franke E, Nash S, Slutsky J, Baum N et al (2011) Treatment of moderate to severe female stress urinary incontinence with the adjustable continence therapy (ACT) device after failed surgical repair. World J Urol 29(2):249–253
Kocjancic E, Crivellaro S, Smith JJ 3rd, Ranzoni S, Bonvini D, Frea B (2008) Adjustable continence therapy for treatment of recurrent female urinary incontinence. J Endourol 22(7):1403–1407
Kocjancic E, Crivellaro S, Ranzoni S, Bonvini D, Grosseti B, Frea B (2010) Adjustable continence therapy for severe intrinsic sphincter deficiency and recurrent female stress urinary incontinence: long-term experience. J Urol 184(3):1017–1021
Wachter J, Henning A, Roehlich M, Marszalek M, Rauchenwald M, Madersbacher S (2008) Adjustable continence therapy for female urinary incontinence: a minimally invasive option for difficult cases. Urol Int 81(2):160–166
Lucas MG, Bosch RJL, Burkhard FC, Cruz F, Madden TB, Nambiar AK et al (2012) EAU guidelines on surgical treatment of urinary incontinence. Eur Urol 62(6):1118–1129
Hermieu J-F, Conquy S, Leriche B, Debodinance P, Delorme E, Boccon Gibod L et al (2010) Synthesis of the guidelines for the treatment of non-neurological urinary incontinence in women. Prog Urol 20(Suppl 2):S94–S99
Nacir M, Ballanger P, Donon L, Bernhard J-C, Douard A, Marit-Ducamp E et al (2013) ACT device: what place in the treatment of female urinary incontinence? Prog Urol 23(4):276–282
Gregori A, Romanò AL, Scieri F, Pietrantuono F, Incarbone GP, Salvaggio A et al (2010) Transrectal ultrasound–guided implantation of adjustable continence therapy (ProACT): surgical technique and clinical results after a mean follow-up of 2 years. Eur Urol 57(3):430–436
Rouprêt M, Misraï V, Gosseine P-N, Bart S, Cour F, Chartier-Kastler E (2011) Management of stress urinary incontinence following prostate surgery with minimally invasive adjustable continence balloon implants: functional results from a single center prospective study. J Urol 186(1):198–203
Chapple CR, Wein AJ, Brubaker L, Dmochowski R, Pons ME, Haab F et al (2005) Stress incontinence injection therapy: what is best for our patients? Eur Urol 48(4):552–565
Van Kerrebroeck P (2006) The motion: injectables are justified as a first option for stress urinary incontinence. Eur Urol 50(4):857–858 discussion 860
Corcos J, Fournier C (1999) Periurethral collagen injection for the treatment of female stress urinary incontinence: 4-year follow-up results. Urology 54(5):815–818
Nilsson CG, Palva K, Rezapour M, Falconer C (2008) Eleven years prospective follow-up of the tension-free vaginal tape procedure for treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 19(8):1043–1047
Bullock TL, Ghoniem G, Klutke CG, Staskin DR (2006) Advances in female stress urinary incontinence: mid-urethral slings. BJU Int 98(Suppl 1):32–40 discussion 41–42
Lapitan MCM, Cody JD, Grant A (1996) Open retropubic colposuspension for urinary incontinence in women. In: Cochrane database of systematic reviews [Internet]. Wiley. Available on http://onlinelibrary.wiley.com.accesdistant.upmc.fr/doi/10.1002/14651858.CD002912.pub2/abstract
Norton P, Brubaker L (2006) Urinary incontinence in women. Lancet 367(9504):57–67
Bezerra CC, Bruschini H, Cody JD (1996) Traditional suburethral sling operations for urinary incontinence in women. In: Cochrane database of systematic reviews [Internet]. Wiley. Available on http://onlinelibrary.wiley.com.accesdistant.upmc.fr/doi/10.1002/14651858.CD001754.pub2/abstract
Wadie BS, Edwan A, Nabeeh AM (2005) Autologous fascial sling vs polypropylene tape at short-term follow up: a prospective randomized study. J Urol 174(3):990–993
Bai SW, Sohn WH, Chung DJ, Park JH, Kim SK (2005) Comparison of the efficacy of Burch colposuspension, pubovaginal sling, and tension-free vaginal tape for stress urinary incontinence. Int J Gynecol Obstet 91(3):246–251
Phé V, Rouprêt M, Mozer P, Chartier-Kastler E (2013) Trends in the landscape of artificial urinary sphincter implantation in men and women in France over the past decade. Eur Urol 63(2):407–408. doi:10.1016/j.eururo.2012.10.040
Vayleux B, Rigaud J, Luyckx F, Karam G, Glémain P, Bouchot O et al (2011) Female urinary incontinence and artificial urinary sphincter: study of efficacy and risk factors for failure and complications. Eur Urol 59(6):1048–1053
Revaux A, Rouprêt M, Seringe E, Misraï V, Cour F, Chartier-Kastler E (2011) Is the implantation of an artificial urinary sphincter with a large cuff in women with severe urinary incontinence associated with worse perioperative complications and functional outcomes than usual? Int Urogynecol J 22(10):1319–1324
Roupret M, Chartier-Kastler E, Richard F (2005) Artificial urinary sphincters in women: indications, techniques, results. Prog Urol 15(3):489–493
Chung E, Cartmill RA (2010) 25-year experience in the outcome of artificial urinary sphincter in the treatment of female urinary incontinence. BJU Int 106(11):1664–1667
Petero VG Jr, Diokno AC (2006) Comparison of the long-term outcomes between incontinent men and women treated with artificial urinary sphincter. J Urol 175(2):605–609
Chung E, Navaratnam A, Cartmill R (2011) Can artificial urinary sphincter be an effective salvage option in women following failed anti-incontinence surgery? Int Urogynecol J 22(3):363–366
Thomas K, Venn SN, Mundy AR (2002) Outcome of the artificial urinary sphincter in female patients. J Urol 167(4):1720–1722
Venn SN, Greenwell TJ, Mundy AR (2000) The long-term outcome of artificial urinary sphincters. J Urol 164(3, Part 1):702–707
Diokno AC, Hollander JB, Alderson TP (1987) Artificial urinary sphincter for recurrent female urinary incontinence: indications and results. J Urol 138(4):778–780
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phé, V., Nguyen, K., Rouprêt, M. et al. A systematic review of the treatment for female stress urinary incontinence by ACT® balloon placement (Uromedica, Irvine, CA, USA). World J Urol 32, 495–505 (2014). https://doi.org/10.1007/s00345-013-1117-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-013-1117-0