Abstract
Introduction
Treatment of recurrent stress incontinence after a failed surgical procedure is more complicated, and repeat surgeries have higher rates of complications and limited efficacy. We determined the technical feasibility, efficacy, adjustability, and safety of adjustable continence therapy device for treatment of moderate to severe recurrent urinary incontinence after failed surgical procedure.
Materials and methods
Female patients with moderate to severe recurrent stress urinary incontinence who had at least one prior surgical procedure for incontinence were enrolled. All patients underwent percutaneous placement of adjustable continence therapy (ACT) device (Uromedica, Plymouth, Minnesota). Baseline and regular follow-up tests to determine subjective and objective improvement were performed.
Results
A total of 89 patients have undergone implantation with 1–3 years of follow-up. Data are available on 77 patients at 1 year. Of the patients, 47% were dry at 1 year and 92% improved after 1-year follow-up. Stamey score improved from 2.25 to 0.94 at 1 year (P < 0.001). IQOL questionnaire scores improved from 33.9 to 71.6 at 1 year (P < 0.001). UDI scores reduced from 60.7 to 33.3 (P < 0.001) at 1 year. IIQ scores reduced from 57.0 to 21.6 (P < 0.001) at 1 year. Diary incontinence episodes per day improved from 8.1 to 3.9 (P < 0.001) at 1 year. Diary pads used per day improved from 4.3 to 1.9 (P < 0.001). Explantation was required in 21.7% of patients.
Conclusion
The ACT device is an effective, simple, safe, and minimally invasive treatment for moderate to severe recurrent female stress urinary incontinence after failed surgical treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recurrent stress urinary incontinence (SUI) after failed surgical procedure continues to be a challenging problem. It can result from either persistent hypermobility of the vesicourethral segment or intrinsic sphincter deficiency (ISD) due to lack of coaptation of the urethral wall in response to slight increase in intra-abdominal pressure.
Mid-urethral slings are considered the treatment of choice for genuine SUI; however, in recurrent cases, it can be associated with higher morbidity rates. This has led to the development of several minimally invasive options that provide the hope of reasonable efficacy associated with minimal morbidity. This includes injections of bulking agents, injection of stem cells into the rhabdosphincter, and adjustable continence therapy devices.
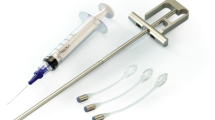
The Adjustable Continence Therapy (ACT) System (Uromedica, Plymouth, MN, USA) is a novel device that is awaiting FDA approval for the treatment of recurrent female stress urinary incontinence. The ACT device is an inflatable, silicone balloon with a titanium port connected by tubing. Two devices are implanted, one on each side of the urethra near the bladder neck. It is a minimally invasive implantable device that provides support at the bladder neck and enhances urethral coaptation. It has the unique advantage of being easily adjusted in the office with a needle injection to optimize continence. The purpose of this study was to investigate the technical feasibility, efficacy, adjustability, and safety of the ACT device for treatment of moderate to severe recurrent urinary incontinence after failed surgical procedure.
Materials and methods
Female patients with moderate to severe SUI who failed at least one surgical treatment (sling, Burch, suspension, AUS) were considered for enrollment in this prospective study at 8 centers in the United States and two in Canada from November 2001 through July 2007. The protocol was approved by the institutional review board at the respective centers and the patients signed an informed consent agreement. Moderate and severe SUI were defined as 11–50 and >50 g of urine loss during a provocative pad test, respectively. Mean pre-operative valsalva leak point pressure (VLPP) in these patients was 44.60 cm H2O (Std = 32.86). At baseline, 12.4% of patients were taking medication to control urge incontinence. Patients with insulin dependent diabetes mellitus, autoimmune disease, pregnancy, urinary tract infection, prior pelvic radiotherapy, detrusor dysfunction, untreated bladder pathology, and untreated grade 3 and 4 pelvic prolapses were excluded from participation. All patients had baseline tests including: urodynamics, cystourethroscopy, provocative pad weight [1], 3-day voiding diary, Stamey score, and validated questionnaires (IIQ-7, UDI-6, IQOL). All patients were followed every 3 months for 1 year then annually with Stamey score (primary end point), provocative pad weight, 3-day voiding diary (through 1 year), and validated questionnaires (IIQ, UDI, IQOL).
The ACT devices are placed bilaterally through two small incisions between the labia majora and minora at the level of the urethral meatus. A specially designed delivery trocar is passed under fluoroscopic and digital vaginal guidance through each incision and just distal to the bladder neck. After placement of each device, the balloons are inflated with 1.5 ml of an isotonic contrast solution and repeat fluoroscopy as well as vaginal palpation to confirm proper positioning of the balloons. Balloon adjustments followed 6 weeks postoperatively in the clinic by percutaneously accessing each subcutaneous port. Balloons were adjusted every 4 weeks (maximum of 1 ml per balloon per adjustment) until adequate continence was achieved as measured by subjective and objective criteria. Balloon adjustments typically take about 15 min and are done under local or no anesthesia. All adverse events and complications were reported and analyzed.
Statistical analyses were completed using the non-parametric Wilcoxon’s matched pairs signed ranks test to test for the significance of differences between baseline and follow-up measurements of efficacy outcomes. P values ≤ 0.05 were regarded as statistically significant. All analyses were performed using the SPSS Version 18 statistical software.
Results
A total of 89 subjects (mean age 67.9 years, range 40–86) who previously had at least one surgical procedure for treatment of stress urinary incontinence were included in our study criteria (Table 1). Of these, 47.2% (42 of 89) had two or more surgical treatments for incontinence. Subjects were followed for 1 year after implantation of the device. The mean total procedure time was 40.6 ± 16.8 min with a range of 10–124 min. Follow-up at 1 year was available for 77 patients. Four patients were explanted permanently before 1-year follow-up completed, 5 were lost to follow-up, 2 missed follow-ups, and 1 was deceased due to a non-study health issue (see Fig. 1). The mean number for adjustment visits prior to 1 year was 2.03 ± 1.6. Nineteen patients (22.1%) did not require any adjustment prior to 1 year.
We present the data both on the usual “As Followed” (AF) basis as well as the more conservative “Intent-To-Treat” (ITT) basis (where all patients that had an implant procedure and were not available at the 12-month visit are treated as failures and baseline scores are used at the 12-month end-point). The data demonstrate that 47% (AF) or 39.3% (ITT) were dry (<2 g of provocative pad weight test) and 92% (AF) or 77.5% (ITT) improved (50% or more reduction in provocative pad weight testing) after 1-year follow-up. Table 2 shows outcomes on all endpoints under both the “As Followed” analysis as well as the “Intent-to-Treat” Analysis. Improvements under both analyses are all statistically significant. In Fig. 2, we present the improvement in provocative pad weight with the corresponding confidence interval.
Thirty percent (26/86) of patients had complications reported between time of implantation and 1 year. Of those 26 patients, 19 had one complication, 5 had 2 complications, and 2 had 3 complications. Complications occurring within 1 year include port erosion (10.1%), balloon migration (7.9%), balloon erosion (4.5%), perforation at implant (4.5%), worsening incontinence or no change (3.4%), procedure failure (2.2%), pain/discomfort (1.1%), device failure (1.1%), and device infection (1.1%). Explantation, which is done under local anesthesia in the office, was required in 21.7% of patients (18 of 83). Fifty percent of those patients (9) were re-implanted before 1 year, while 28% (5) were awaiting re-implantation and 22% (4) had been explanted permanently.
Discussion
Stress urinary incontinence affects millions of women worldwide. Traditionally, surgical procedures such as slings or bladder neck suspension were the only options to treat this condition. These procedures date back to the late nineteenth century and have been refined over the years [8]. These surgical options remain the primary choice for many surgeons for treatment of SUI. Recent advances in technology led to better outcomes, shorter operative times, and decreases in complication rates. There is still no consensus on the preferred procedure; however, high success rates with mid-urethral slings such as TVT, transobturator, and percutaneous vaginal tape have made them attractive choices for primary cases [2–6]. In the literature review published by Jarvis, pubovaginal sling placement resulted in subjective and objective cure rates of 82.4 and 85.3%, respectively [9]. In 2001, Wilson et al. [5] showed an 82% cure rate using TVT for recurrent stress incontinence, which decreased to 61–77% in patients with associated intrinsic sphincter deficiency. Another study by Amaye-Obu et al. [7] reported 70–78% cure rates when performing an abdomino-vaginal polypropylene sling procedure, a modified urethral sling procedure or Burch colposuspension.
The surgical techniques above exhibit very acceptable success rates but the potential morbidity arising from these procedures has been the driving force for the introduction of less invasive surgical procedures. Postoperative complications described include bladder, bowel, and major blood vessel injuries as well as postoperative voiding difficulties [10] and de novo urgency and urge incontinence [11]. The rate of the above complications increases dramatically in secondary procedures after failed primary surgical repairs. A study by Houwert et al. [16] showed that patients who have had prior incontinence procedures have poorer outcomes. In this study, 82% of patients who have not had prior surgery were cured after mid-urethral sling, while that percentage was only 56% for patients who have had prior incontinence surgery. It appears that the proper adjustment of sling tension can be difficult in recurrent cases of stress incontinence and continues to be a challenge even in the most experienced hands. In addition, the efficacy of sling procedures is correlated to the severity of SUI. An article by Song et al. [18] on 7-year outcomes of TVT suggested that patients with high grade SUI (Stamey grade III) have a lower cure rate (50%), while patients with moderate (Stamey grade II) SUI have a cure rate of 82.8%, and patients with grade I SUI have a cure rate of 90.7%.
In recent years, multiple minimally invasive options for treatment of SUI were introduced. These include bulking agents, injection of stem cells into the rhabdosphincter, and adjustable continence therapy devices. These procedures are simple, minimally invasive and well tolerated by patients. Initially, Mittenberger et al. [17] showed promising results in using stem cells for treatment of SUI. These promising data have prompted other investigations; however, no other group was able to duplicate these results.
Many studies have described the use of adjustable continence therapy device in treatment of stress urinary incontinence. There has already been some published experience in Europe and North America in assessing the role of ACT as a minimally invasive treatment for women with urinary incontinence. Chartier-Kastler et al. [12] reported 87% clear improvement, and Kocjancic et al. [13] reported 68% dry and 16% improved,
Wachter et al. [14] reported that 59% of patients showed significant improvement, and Aboseif et al. [15] showed that 76.4% of patients with mild, moderate, and severe stress incontinence showed at least one grade improvement of the Stamey score and the mean provocative pad weight decreased by more than 50% in 80.9% of the patients.
Surgical treatment such as mid-urethral slings remains the primary treatment for SUI as discussed earlier. However, given the increased complication rate and the high risk of retention for secondary surgical treatment after failed primary therapy, we investigated the role of ACT as secondary therapy for recurrent stress urinary incontinence after failed primary therapy. Scarring and decreased flexibility of urethral wall may play a role in decreased efficacy of secondary surgical procedures after failed primary therapy. A major advantage of ACT is the ease of percutaneous adjustment to balloon volume to achieve continence with changes that may occur over time in individual patients. In our study, we reported that 47% of patients were dry and 91% improved after 1-year follow-up. The strict definition of dry in the present study of less than 2 g on provocative pad test may explain our lower dry rates compared to other studies.
With respect to safety of the ACT, there have been no major complications such as bowel perforation or vascular injury reported with the use or placement of this device. The majority of the complications were related to port erosion, balloon migration away from the bladder neck and balloon extrusion mostly into the vagina. Some of these complications may be explained by the learning curve as the majority occurred early in the study period. Of note is the minimal voiding dysfunction associated with this device. Of the patients who required explantation, 50% received a new device prior to their 12-month follow-up visit.
In addition to objective improvement after ACT implantation in our study using provocative pad weight test, Stamey scores, diary incontinence episodes and number of pads per day, three validated questionnaires were used to demonstrate subjective improvement. Our data showed significant improvement in patient’s quality of life in all three measures (Table 2).
Conclusion
The ACT device is an effective, simple, safe, and minimally invasive treatment for moderate to severe recurrent female stress urinary incontinence after failed surgical treatment. The device can be easily adjusted percutaneously to enhance efficacy. Complications are usually easily manageable. Explantation is an easy office procedure and does not preclude future repeat implantation or other treatments. Continuing follow-up of these patients is ongoing to assess the long-term viability of this therapy. Future studies should focus on predictors of success and reduction in complications.
References
Abrams P, Blaivis JG (1988) Stanton Sl et.al. for the International Continence Committee on Standardization of Terminology: the standardization of terminology of lower tract function. Scand J Urol Nephrol 114(suppl):5–19
Agarwala N, Liu CY (2002) Minimally invasive management of urinary incontinence. Current Opin Obstet Gynecol 14:429–433
Bullock TL, Ghoniem G, Klutke CG et al (2006) Advances in female stress urinary incontinence: mid urethral sling. BJU 98:32–40
Walters MD, Daneshgari F (2004) Surgical management of stress urinary incontinence. Clin Obstet Gynecol 47:93–103
Wilson TS, Lemack GE, Zimmern PE (2003) Management of intrinsic sphincter deficiency in women. J Urol 169:1662–1669
Kuschel S, Schussler B (2008) Results of function and safety of the Safyre-t, a hybrid transobturator vaginal sling for treatment of stress urinary incontinence. Neurourol Urodyn 27:403–406
Amaye-Obu FA, Drutz HP (1999) Surgical management of recurrent stress urinary incontinence: a 12 year experience. Am J Obstet Gynecol 181:1296–1307
Harding CK, Thorpe AC (2008) Surgical treatment for stress urinary incontinence. Int J of Urol 15:27–34
Jarvis GJ (1994) Surgery for genuine stress incontinence. BJOG 101:371–374
de Tayrac R, Deffieux D, Droupy S et al (2004) A prospective randomized trial comparing tension free vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol 190:602–608
David-Montefiore E, Frobert JL, Grisard-Anaf M et al (2006) Functional results after suburethral sling procedure for urinary stress incontinence at 1 year: a French prospective multicenter study comparing the retropubic and transobturator routs (abstract). Int Urogynecol J Pelvic Floor Dysfunct 17(suppl 2):S95
Chartier-Kastler E, Costa P, Ben Naoum K et al (2007) French multicenter prospective study of the use of ACT balloons (Uromedica, inc, Plymouth, Min, USA; Medtronic, Minneapolis, USA) for the treatment of female stress urinary incontinence. Prog Urol 17:1372–1377
Kocjancic E, Crivellaro S, Smith JJ et al (2008) Adjustable continence therapy for treatment of recurrent female urinary incontinence. J Endourol 22:1403–1407
Wachter J, Henning A, Roehlich M et al (2008) Adjustable continence therapy for female urinary incontinence: a minimally invasive option for difficult cases. Urol Int 81:160–166
Aboseif SR, Franke EI, Nash SD et al (2009) The adjustable continence therapy system for recurrent female stress urinary incontinence: 1-year results of the North America clinical study group. J Urol 181:2187–2191
Houwert RM, Venema PL, Aquarius AE et al (2009) Predictive value of urodynamics on outcome after midurethral sling surgery for female stress urinary incontinence. Am J of Obstet and Gynecol, www.AJOG.org
Mittenberger M, Pinggera GM, Marksteiner R et al (2008) Adult stem cell therapy for female stress urinary incontinence. Eur Urol 53:169–175
Song PH, Kim YD, Kim HD et al (2009) The 7-year outcome of the tension free vaginal tape procedure for treating female stress urinary incontinence. BJU Int 104(8):1113–1117
Acknowledgments
John F Bressette, Lahey Clinics.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aboseif, S.R., Sassani, P., Franke, E.I. et al. Treatment of moderate to severe female stress urinary incontinence with the adjustable continence therapy (ACT) device after failed surgical repair. World J Urol 29, 249–253 (2011). https://doi.org/10.1007/s00345-010-0589-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-010-0589-4