Abstract
Objectives
Our aim was to investigate the long-term preventive effect of propofol on testicular ischemia–reperfusion injury in a rat model.
Methods
Twenty-four adult male Sprague–Dawley rats were randomly divided into four groups (n = 6 for each group), control, sham-operated, torsion/detorsion (T/D) and T/D + propofol. Testicular ischemia was achieved by twisting the left testis 720° clockwise for 2 h. Half an hour before detorsion, 50 mg/kg propofol was given intraperitoneally to the T/D + propofol group. Ipsilateral orchiectomies to determine mean testicular weights and histopathological examination according to Johnsen’s mean testicular biopsy score criteria were performed 30 days after surgical procedure in all groups.
Results
Mean testicular weights were 1.57 ± 0.12 g in group I, 1.59 ± 0.36 g in group II, 0.84 ± 0.20 g in group III and 0.87 ± 0.29 g in group IV. Mean testicular weights decreased significantly in the T/D groups, but no improvement in testicular weight was observed with propofol administration (p 0.9372). Similarly, the Johnsen’s mean testicular biopsy scores of the T/D groups were lower than those of the control and sham-operated groups, but no positive effect was determined with the administration of propofol in the T/D groups (p 0.1797).
Conclusions
Our results showed that there is no apparent long-term therapeutic potential attendant on using propofol in the treatment of testicular ischemia–reperfusion injury caused by testis torsion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testicular torsion is a surgical emergency mainly affecting newborns, children and adolescent boys [1, 2]. Previous studies have demonstrated a clear correlation between duration of torsion and subsequent testicular functions [3]. Surgical detorsion should, therefore, be performed as early as possible [1, 3–6]. Although the mechanism is not completely understood, current studies have demonstrated that the primary pathophysiological event in testicular torsion is ischemia followed by reperfusion injury [1, 3–5, 7–11]. Abundant amounts of nitrogen and reactive oxygen species (ROS) are produced in the reperfusion period due to a considerable oxygen supply to the tissue [1, 11]. ROS can react with lipids in the cell and mitochondrial membranes and initiate the lipid peroxidation process. In addition, they can stimulate chain reactions by interacting with proteins and nucleic acids, causing cellular dysfunction [5, 6].
In general, formed ROS are eliminated by enzymatic and non-enzymatic antioxidants. In case of excessive ROS production or failure of the cellular antioxidant defense system, ROS may lead to cellular dysfunction and even death. In this situation, administration of antioxidants may have a potential benefit in neutralizing ROS [6]. Several anti-inflammatory drugs or antioxidants (ibuprofen, dehydroepiandrosterone, caffeic acid phenyl ester, N-acetylcysteine, morphine, erdosteine, allopurinol, resveratrol, selenium, propofol, etc.) have to date been tested to prevent ischemia–reperfusion (I/R) injury in the testis [1, 6–8, 10].
Propofol (2, 6-diisopropylphenol), a widely used intravenous anesthetic, has exhibited potent antioxidant activity against lipid peroxidation in both in vitro and in vivo studies. Its structure contains a phenolic hydroxyl group and thus resembles that of α-tocopherol (vitamin E), a natural antioxidant. As shown by previous studies, its antioxidant activity results partly from this phenolic chemical structure [12]. The antioxidant activity of propofol in testicular I/R injury has been demonstrated chemically and histologically by experiments on rats as previously shown in the intestinal mucosa, heart, cerebrum and joints [5, 6, 11, 13–19]. However, in these studies, evaluations were performed in a short period (within hours) after testicular torsion, and there has been no evaluation of the preventive effect of propofol on spermatogenesis in any long-term study.
Our aim in this experimental study was to investigate whether propofol may protect spermatogenesis from I/R injury in rats.
Materials and methods
Twenty-four mature male Sprague–Dawley rats weighing 400–425 g were selected. The animals were housed under standard conditions, in individual cages in a temperature and light-controlled room and allowed ad libitum consumption of sterile food (animal chow) with free access to food and water. For the last 12 h before the study they were given only water. All animal experiments were performed after receiving the Karadeniz Technical University Animal Care and the Ethics Committee approval, in compliance with the principles of laboratory animal care (National Institutes of Health publication no. 85–23; revised, 1985).
Study protocol and groups
Rats were randomly divided into four groups of six animals each. To maintain uniformity during the study, interventions were conducted at the same time on each rat in each group. Animals were anesthetized with an intraperitoneal injection of 50 mg/kg of ketamine hydrochloride (Ketalar, Eczacibasi, Turkey).
-
Group I: Constituted to determine baseline values of histopathological parameters.
-
Group II (sham-operated): This group was constituted to investigate the effect of surgical stress on spermatogenesis. The left testes were extracted through an ilioinguinal incision and then replaced with a fixation to the scrotum. The wound was closed using 4-0 silk suture.
-
Group III (Torsion/Detorsion): Torsion was created by rotating the left testis 720° clockwise and maintained by fixing the testis as described by Turner et al. [20]. After 2 h of torsion, the testis was counter-rotated to the natural position and replaced into the scrotum.
-
Group IV (Torsion/Detorsion plus propofol): The same surgical procedure was performed as in group III; in addition, 50 mg/kg propofol (Propofol 1% Fresenius, Fresenius Kabi AB, Uppsala, Sweden) was injected transperitoneally 30 min prior to detorsion. The propofol dose and administration method were adopted from the study by Unsal et al. [5].
Histological analysis
The study was terminated 30 days after surgical procedure [3]. All rats were killed by cervical dislocation after the left testes harvested. All testes were weighed and microscopically evaluated under a light microscope (Nikon E200) by a pathologist blinded to which groups they came from. The testicular specimens were individually immersed in Bouin’s fixative, dehydrated in alcohol and embedded in paraffin. Tissue sections (4–5 μm) were deparaffinized and stained with hematoxylin and eosin (H&E). Spermatogenesis was assessed histopathologically using Johnsen’s mean testicular biopsy score (MTBS) criteria [21]. A score of 1–10 was assigned to each tubule according to epithelial maturation under Johnsen’s classification system as follows: (10) complete spermatogenesis, (9) germinal cell disorganization, (8) few spermatozoa, (7) no spermatozoa, (6) few spermatids, (5) no spermatids, (4) few spermatocytes, (3) spermatogonia only, (2) Sertoli cells only and (1) no cells in the tubules.
The groups were compared with each other according to mean testicular weight and Johnsen’s MTBS.
Statistical analysis
Statistical analyses were performed using computer software (Prism 3.0, Graphpad Software Inc. San Diego, CA, USA.). Data were expressed as mean ± standard deviation. The Kruskal–Wallis test, Mann–Whitney U test and Spearman rank correlation coefficient test were used for statistical analyses. Statistical significance was set at p < 0.05.
Results
Twenty-four rats were evaluated in this prospective, randomized, experimental study. All rats were alive during the follow-up period of 30 days after testicular T/D.
Mean testicular weights were 1.57 ± 0.12 g (range 1.36–1.71) in group I, 1.59 ± 0.36 g (range 0.88–1.90) in group II, 0.84 ± 0.20 g (range 0.67–1.23) in group III and 0.87 ± 0.29 g (range 0.49–1.24) in group IV. There were significant differences in mean testicular weights between the groups; groups III and IV had lower mean testicular weights than groups I and II (p 0.0017). But there was no significant difference between groups III and IV (p 0.9372). Similarly, no significant difference was determined between groups I and II (p 0.3095). All comparisons of mean testicular weights are shown in Fig. 1.
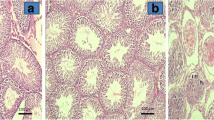
At histopathological evaluation of spermatogenesis, Johnsen’s MTBS values were 9.50 ± 0.54 (range 9–10) for group I, 9.83 ± 0.40 for group II, 5.66 ± 2.42 (range 4–10) for group III and 7.16 ± 1.83 (range 5–9) for group IV. Sample histopathological images from all groups are shown in Fig. 2. Significant differences were determined between the groups in terms of Johnsen’s MTBS values; groups III and IV had lower Johnsen’s MTBS values than groups I and II (p 0.0035). But no significant improvement with propofol administration was observed in group IV compared with group III (p 0.1797). Similarly, no significant difference was determined between groups I and II (p 0.3939). All the comparisons involving Johnsen’s MTBS values are shown in Fig. 3.
A significant correlation was determined between mean testicular weight and Johnsen’s MTBS (r 0.8870, p < 0.0001).
Discussion
Spermatic cord torsion is a urological emergency, and late presentation or failure to diagnose the condition may cause permanent testicular damage [22]. Testicular injury due to torsion and detorsion is an I/R injury attributed to neutrophil infiltration and generation of ROS [23]. Increased oxidative stress during reperfusion after ischemia, as demonstrated by greater levels of lipid peroxidation, is accompanied by a decreased level of antioxidant enzymes [10]. Lysiak et al. also reported germ cell specific apoptosis after testicular I/R and that an influx of neutrophils to the testes was essential for this pathology. This germ cell specific apoptosis leads to a decrease in testicular weight and the loss of spermatogenesis [24]. Testicular torsion of 2 h has been reported as sufficient to enhance nitric oxide (NO) levels to a point at which they are able to initiate apoptosis in germinal cells [8, 25].
Many drugs, chemical substances and physical methods have to date been used to protect testes against I/R injury in experimental animals, and some have been effective in preventing testicular damage but have been not adapted to clinical use [7]. Propofol is a widely used short-acting intravenous sedative and hypnotic agent used to provide anesthesia induction and continuous sedation. Propofol exhibits chemical structure similarities with endogenous antioxidants such as vitamin E and butylated hydroxytoluene, a phenol-based oxygen radical consumer [6, 11]. Due to the antioxidant potential of propofol, many studies have been performed on this subject. Previous studies have shown that propofol effectively blocks the formation of malonyldialdehyde (MDA), a product of lipid peroxidation [1, 5, 10, 22].
Some studies have investigated in vivo and in vitro antioxidant activity of propofol using different methods. Gulcin et al. [18] reported that propofol exhibits more effective antioxidant capacity than butylated hydroxyanisole, butylated hydroxytoluene and α-tocopherol. Ozturk et al. [15] demonstrated that prophylactic administration of propofol protects the brain from oxidative stress due to traumatic injury. Kaptanoglu et al. evaluated the effect of propofol on lipid peroxidation and ultrastructural findings in a spinal I/R injury model. They reported that propofol reduces lipid peroxidation without improving ultrastructure 1 h after spinal cord injury in rats [16]. Ergun et al. [14] observed the neuroprotective effects of propofol during global cerebral I/R injury by the use of the four-vessel occlusion method in a rat model. However, no clinical data exist to confirm whether propofol can establish neuroprotection in humans [26].
Two previous studies investigating oxidative stress in animal models of testicular torsion determined lipid peroxidation by measuring MDA, NO and histopathological injury. The results indicate that testicular MDA, NO and histopathological injury levels were significantly increased after 2 h of torsion and further increased after 2 h of detorsion. These studies suggested that propofol administration before detorsion may alleviate reperfusion injury, and although they recommended further investigation to determine the exact mechanisms responsible for the preventive effects of propofol, this protective effect was attributed to the fact that propofol appears to inhibit lipid peroxidation or prevents the formation of peroxynitrite as a result of inhibition of NO synthase [5, 11]. The long-term effect of propofol on testicular ultrastructure was not evaluated in these studies.
Our aim was to investigate the long-term preventive effect of propofol on testicular I/R injury in a rat model. The main limitation of our study is that we did not measure oxidative stress and oxidative damage biochemically in the early phase of the experiment, because of concerns over rat death due to large amounts of blood being taken. In our opinion, in terms of fertility, long-term histological changes may provide more important information than biochemical properties obtained in the early phase of I/R injury. Evaluation of the experiment was performed 30 days after detorsion, on the basis of mean ipsilateral testes weights and Johnsen’s MTBS. The testicular weight loss in the T/D groups suggests that the model of I/R injury was successfully established. In addition, there was a clear correlation between mean testes weights and Johnsen’s MTBS. Moreover, there was no impact of surgical stress observed on the mean testes weights and the mean Johnsen’s MTBS when the control and sham-operated rats were compared.
In conclusion, the results of this study show that there is no apparent long-term therapeutic potential for using propofol in the treatment of testicular I/R injury, despite the short-term beneficial effects demonstrated.
References
Dokmeci D, Kanter M, Inan M, Aydogdu N, Basaran UN, Yalcin O, Turan FN (2007) Protective effects of ibuprofen on testicular torsion/detorsion-induced ischemia/reperfusion injury in rats. Arch Toxicol 81:655–663
Cuckow PM, Frank JD (2000) Torsion of the testis. BJU Int 86:349–353
Becker EJ Jr, Turner TT (1995) Endocrine and exocrine effects of testicular torsion in the prepubertal and adult rat. J Androl 16:342–351
Can C, Töre F, Tunçel N, Uysal O, Gürer F, Ak D, Tunçel M (2004) Protective effect of vasoactive intestinal peptide on testicular torsion-detorsion injury: association with heparin-containing mast cells. Urology 63:195–200
Unsal A, Devrim E, Guven C, Eroglu M, Durak I, Bozoklu A, Balbay MD (2004) Propofol attenuates reperfusion injury after testicular torsion and detorsion. World J Urol 22:461–465
Aksoy H, Yapanoglu T, Aksoy Y, Ozbey I, Turhan H, Gursan N (2007) Dehydroepiandrosterone treatment attenuates reperfusion injury after testicular torsion and detorsion in rats. J Pediatr Surg 42:1740–1744
Cay A, Alver A, Küçük M, Işik O, Eminağaoğlu MS, Karahan SC, Değer O (2006) The effects of N-acetylcysteine on antioxidant enzyme activities in experimental testicular torsion. J Surg Res 131:199–203
Koc A, Narci A, Duru M, Gergerlioglu HS, Akaydin Y, Sogut S (2005) The protective role of erdosteine on testicular tissue after testicular torsion and detorsion. Mol Cell Biochem 280:193–199
Moon C, Kim JS, Jang H, Lee HJ, Kim SH, Kang SS, Bae CS, Kim JC, Kim S, Lee Y, Shin T (2008) Activation of Akt/protein kinase B and extracellular signal-regulated kinase in rats with acute experimental testicular torsion. J Vet Med Sci 70:337–341
Salmasi AH, Beheshtian A, Payabvash S, Demehri S, Ebrahimkhani MR, Karimzadegan M, Bahadori M, Pasalar P, Dehpour AR (2005) Effect of morphine on ischemia-reperfusion injury: experimental study in testicular torsion rat model. Urology 66:1338–1342
Yagmurdur H, Ayyildiz A, Karaguzel E, Ogus E, Surer H, Caydere M, Nuhoglu B, Germiyanoglu C (2006) The preventive effects of thiopental and propofol on testicular ischemia–reperfusion injury. Acta Anaesthesiol Scand 50:1238–1243
Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, Papadimitriou L (2009) Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol 605:1–8
De La Cruz JP, Zanca A, Carmona JA, de la Cuesta FS (1999) The effect of propofol on oxidative stress in platelets from surgical patients. Anesth Analg 89:1050–1055
Ergün R, Akdemir G, Sen S, Taşçi A, Ergüngör F (2002) Neuroprotective effects of propofol following global cerebral ischemia in rats. Neurosurg Rev 25:95–98
Ozturk E, Demirbilek S, Kadir But A, Saricicek V, Gulec M, Akyol O, Ozcan Ersoy M (2005) Antioxidant properties of propofol and erythropoietin after closed head injury in rats. Prog Neuropsychopharmacol Biol Psychiatry 29:922–927
Kaptanoglu E, Sen S, Beskonakli E, Surucu HS, Tuncel M, Kilinc K, Taskin Y (2002) Antioxidant actions and early ultrastructural findings of thiopental and propofol in experimental spinal cord injury. J Neurosurg Anesthesiol 14:114–122
Adembri C, Venturi L, Pellegrini-Giampietro DE (2007) Neuroprotective effects of propofol in acute cerebral injury. CNS Drug Rev 13:333–351
Gulcin I, Alici HA, Cesur M (2005) Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull (Tokyo) 53:281–285
Azeredo MA, Azeredo LA, Eleuthério EC, Schanaider A (2008) Propofol and N-Acetylcysteine attenuate oxidative stress induced by intestinal ischemia/reperfusion in rats: protein carbonyl detection by immunoblotting. Acta Cir Bras 23:425–428
Turner TT, Tung KS, Tomomasa H, Wilson LW (1997) Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod 57:1267–1274
Johnsen SG (1970) Testicular biopsy score count–a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1:2–25
Greenstein A, Schreiber L, Matzkin H (2001) The effect of methylene blue on histological damage after spermatic cord torsion in a rat model. BJU Int 88:90–92
Atik E, Gorur S, Kiper AN (2006) The effect of caffeic acid phenethyl ester (CAPE) on histopathological changes in testicular ischemia-reperfusion injury. Pharmacol Res 54:293–297
Lysiak JJ, Nguyen QA, Turner TT (2002) Peptide and nonpeptide reactive oxygen scavengers provide partial rescue of the testis after torsion. J Androl 23:400–409
Baker LA, Turner TT (1995) Leydig cell function after experimental testicular torsion despite loss of spermatogenesis. J Androl 16:12–17
Koerner IP, Brambrink AM (2006) Brain protection by anesthetic agents. Curr Opin Anaesthesiol 19:481–486
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taşkara, E., Gör, A., Kutlu, Ö. et al. Does propofol prevent testicular ischemia–reperfusion injury due to torsion in the long term?. Pediatr Surg Int 27, 1003–1007 (2011). https://doi.org/10.1007/s00383-011-2895-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-011-2895-3