Abstract
Purpose
To elucidate the impact of renal parenchymal loss and the ischemic reperfusion injury (RI) on the renal function after laparoscopic partial nephrectomy (LPN) under warm ischemia (WI).
Methods
Thirty-five patients with a single polar renal mass ≤4 cm and normal contralateral kidney underwent LPN. Transperitoneal LPN with WI using en bloc hilar occlusion was performed. The total differential renal function (T-DRF) using 99mTc-dimercaptosuccinic acid was evaluated preoperatively and postoperatively over a period of 1 year. A special region of interest (ROI) was selected on the non-tumorous pole of the involved kidney, and was compared with the same ROI in the contralateral kidney. The latter comparison was defined as partial differential renal function (P-DRF). Any postoperative decline in the P-DRF of the operated kidney was attributed to the RI. Subtraction of the P-DRF decline from the T-DRF decline was attributed to the parenchymal loss caused by the resection of the tumor and suturing of the normal parenchyma.
Results
The mean WI time was 22 min, and the mean weight of resected specimen was 18 g. The mean postoperative eGFR declined to 87 ml/min/1.73 m2 from its baseline mean value of 97 ml/min/1.73 m2 (p value = 0.075). Mean postoperative T-DRF and P-DRF of the operated kidney declined by 7 and 3 %, respectively.
Conclusions
After LPN of small renal mass, decline in renal function is primarily attributed to parenchymal loss caused by tumor resection and suturing of the normal parenchyma rather than the RI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Partial nephrectomy (PN) has become a standard of care for treatment of small renal masses. Hilar occlusion is commonly performed for a precise tumor resection and renal reconstruction. The above surgical maneuver results in warm ischemia (WI) of the remaining renal tissue and has been associated with ischemic reperfusion injury (RI) to the organ. Current evidence showed that the length of the warm ischemia time (WIT) and the subsequent RI may result in permanent renal damage [1, 2]. Moreover, the resection of the renal tumor and the suturing of the parenchyma resulted in additional reduction in the functional renal tissue [3, 4]. Thus, two mechanisms of renal function damage during PN could be proposed. Nevertheless, the importance of the mechanisms for the decline of the postoperative renal function has not been investigated. The current prospective study evaluated the split renal function and elucidated the role of renal parenchymal loss in patients with small renal mass who were treated by LPN with WI.
Patients and methods
Small renal masses have been treated by LPN at our institutions since 2005. Thirty-five patients were enrolled in a prospective pilot study between January 2012 and November 2014. Regional research ethics committee approval was received, and informed consent was obtained from all patients. The procedures were performed by two experienced laparoscopists. The exact location and dimensions of the tumor were identified by three-dimensional CT scan prior to the operation. Only patients with a single exophytic mass of ≤4 cm in diameter located in the lower or upper pole of the kidney with normal contralateral kidney were enrolled.
All operations were performed by laparoscopic transperitoneal approach with en bloc hilar occlusion using a Rumel tourniquet. Two minutes before hilar occlusion, 0.5 g/kg of 20 % mannitol was infused. The surgical technique has been previously described [5].
The renal pedicle was released only after tumor excision and completion of the renorrhaphy. The recorded parameters included the time for tumor resection, calyceal closure, hemostatic sutures, and the total WIT. After extraction of the specimen, the surrounding fatty tissue was detached and the weight of the tumor was measured. The kidney was placed in its anatomic position, and the Gerota fascia was closed.
Serum creatinine (sCr) was recorded, and estimated glomerular filtration rate (eGFR) was calculated using chronic kidney disease epidemiology collaboration (CKD-EPI) equation [6].
The above measurements were taken preoperatively, on the 1st, 3rd, and 7th postoperative days. These measurements also took place at the end of 1st, 3rd, 6th, and 12th postoperative months.
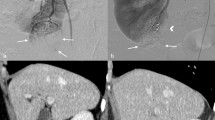
All patients underwent 99mTc-DMSA renal scintigraphy for the determination of split renal function preoperatively and at the end of 1st, 3rd, 6th, and 12th postoperative months. The 99mTc-DMSA provided the total differential renal function (T-DRF) (Fig. 1a). Any postoperative reduction in the T-DRF of the operated kidney was considered as a result of both the RI and the parenchymal loss. In an attempt to distinguish between the effect of the WI and the parenchymal loss due to the resection and suturing, the so-called partial differential renal function (P-DRF) was calculated. A special region of interest (ROI) was selected on the pole of the involved kidney without a tumor in all isotope assessments and was compared with the same ROI in the contralateral kidney (Fig. 1b).
a 99mTc-DMSA renal scintigraphy showing an imaginary tumor in the lower pole of the one kidney (red circle). The ROI is selected (purple line) to demonstrate and compare the T-DRF of both kidneys. b The ROI is selected in the non-tumorous pole of the involved kidney and compared with the same ROI in the contralateral kidney. c The graph shows the mean preoperative and postoperative eGFR values in the studied time intervals. d The graph shows the mean decline of both P-DRF and T-DRF of the operated kidney in the studied time intervals. e Comparing correlation of the T-DRF decline in the operated kidney to WI time. f Comparing correlation of the T-DRF decline in the operated kidney to the mass of the resected specimen
Any postoperative decline in the P-DRF of the operated kidney was considered as the renal functional loss related to the WI. All renal isotope tests were evaluated by a specialist doctor in nuclear medicine. For further confirmation, a linear correlation coefficient was calculated for the assessment of a possible correlation of the T-DRF decline with the WIT in the operated kidney and with the mass of the resected specimen.
Statistical evaluations
The IBM SPSS version 20 (IBM Corp., Armonk, NY, USA) was used for the calculations and statistical analysis. ANOVA and Pearson product-moment correlation were calculated as deemed necessary. A p value <0.05 was considered statistically significant.
Results
The characteristics and the surgical outcome of the LPNs are presented in Table 1. Cases related to events which could unpredictably influence the WI and the renal functional outcome were excluded from the statistical analysis. Twenty-eight patients were eventually enrolled in the statistical analysis. Mean values of the preoperative and postoperative renal function as described by sCr, eGFR, T-DRF, and P-DRF are summarized in Table 2.
Table 2 and Fig. 1c show that the mean preoperative eGFR of our patients was 97 ± 17 (range 55–122) ml/min/1.73 m2 which decreased to 81 ± 21 (range 44–114) ml/min/1.73 m2 on the 1st postoperative day (p = 0.007). Thus, there was a 16 % decline in the average eGFR which was the largest postoperative drop within the 1-year follow-up period. Conventionally, renal function status of the 1st postoperative day was described as the “transient-state” of kidney function deterioration. On the 3rd postoperative day, we observed a 7 % recovery in the average eGFR in comparison with the 1st day. Nevertheless, this trend toward recovery was statistically insignificant (p = 0.382). From the 3rd postoperative day to end of the study at 12 months, the average eGFR remained roughly the same. In these time points, the comparison of the lowest with the highest values, which were in the 7th postoperative day and 3rd month, respectively, showed insignificant alteration in the eGFR (p = 0.4483). The mean value of all postoperative eGFR values after the 1st day (transient-state) was calculated and considered as the “steady-state” of the renal function after the procedure. The average value was 87 ml/min/1.73 m2 which demonstrates a 10 % decrease in renal function compared to the baseline (p = 0.075). The mean preoperative T-DRF of the operated kidneys was 49 ± 4 % which is decreased to 42 ± 7 % on the 1st postoperative month (p < 0.001) (Table 2; Fig. 1d). This value remained almost the same in the follow-up appointments. The statistical comparison of all remaining postoperative T-DRFs to the baseline did not show any significant change (p > 0.6). Eventually, the mean value of all postoperative T-DRF which was 42 % was considered as the final postoperative result. On the other hand, the mean preoperative P-DRF of the intact pole of the operated kidney was 50 % which decreased to 47 % on the 1st postoperative month (p = 0.072). The average of all postoperative P-DRF was also 47 % without any significant alteration among the time periods (p ≥ 0.1). In addition, the linear correlation coefficient revealed a weaker correlation between the T-DRF decline and the WIT (Fig. 1e) in comparison with the resected mass (Fig. 1f) (R 2 = 0.0837 and 0.7241, respectively).
Discussion
The traditional approach for PN includes the hilar clamping in order to minimize intraoperative bleeding and to provide a bloodless surgical field. Although the hilar occlusion is not always necessary, most PNs are performed with hilar occlusion [7]. PN is related to postoperative decline in renal function due to the removal, devascularization, or incomplete recovery of the nephrons from ischemia [8]. Renal ischemia and the RI have been considered for a long time as the main factor related to postoperative renal function deterioration in patients undergoing PN under WI [1, 9–13]. Several technically challenging techniques have been introduced for the reduction in WI [14, 15]. Nevertheless, the impact of renal parenchymal mass reduction was not distinguished from the effects of WI and RI in the above literature. Parenchymal loss after PN occurs as a result of intentional tumor excision, some normal parenchyma resection and suturing. Thus, the mass or volume of the parenchymal loss should be considered and differentiated from RI when evaluating the renal functional outcome after PN. Some authors have studied the impact of the parenchymal volume reduction on the renal function. Shikanov et al. [16] assessed the influence of RI on the long-term total renal function after LPN for small renal masses. Changes in the eGFR were −16 and −11 % at the early postoperative and 1-year follow-up, respectively. The current results are similar to the above investigation and also to the literature which reported that the preservation of the total renal function after PN ranged between 88 and 91 % (approximately 10 % loss of the renal function) [7]. Mir et al. [7] also showed a higher reduction in the eGFR in patients with larger tumors which could be attributed to the excision of a larger lesions and the consequent greater loss of renal parenchyma. Sharma et al. [17] reported an average of 15 % parenchymal volume loss and 19.7 % deterioration in renal function during a midterm postoperative period in patients with solitary kidney who underwent partial nephrectomy for small renal masses. They concluded that the percent of renal parenchymal volume loss was correlated with the percent of loss in eGFR. Simmons et al. [3] showed that the volume loss had a more direct, predictable effect on ultimate eGFR than ischemia time. A similar conclusion was noted by Song et al. [18] who stated that renal volume reduction was the most significant, independent prognosticator for eGFR reduction after PN. Similarly, Mir et al. [19] revealed that the ultimate renal function after PN was primarily driven by parenchymal preservation with ischemia playing a secondary role for the cases of limited WIT.
Current literature has not concluded to the most important factor for the renal function decline after PN, and the contribution of WI to the postoperative renal function has not been well documented [7]. Some investigators advocated that the parenchymal mass preservation was strongly correlated with the functional recovery in comparison with the WI [4, 18]. The current prospective study aimed at distinguishing the impact of parenchymal loss from the WI effect on the operated kidney. The 99mTc-DMSA isotope was used for the purpose due to the fact that it allows accurate calculation of DRF [20]. The latter parameter was measured preoperatively and in different postoperative intervals in 28 patients with solitary small polar tumors. Since 99mTc-DMSA scan provided relative functional percentage of the two kidneys, the contralateral kidney served as the control for the comparison after LPN. Consequently, only patients with normal contralateral kidney were selected and a young patient population with mean age of 50.5 ± 11.9 years was eventually included in the study. Any postoperative decline in the T-DRF of the operated kidney was considered as a result of WI and WI combined with parenchymal loss. In nearly all postoperative studies, a mean decline of 7 % in the T-DRF was noted. In an attempt to distinguish the effect of WI from the parenchymal loss, the P-DRF was introduced. A ROI was selected on the non-tumorous pole of the involved kidney and was compared with the same ROI on the contralateral kidney. Any interference of the excision area to the ROI was prevented by including only patients with tumor mass of ≤4 cm in diameter located on either upper or lower pole of the kidney. Any postoperative functional decline in this intact pole of the operated kidney was considered to be as a result of WI only. The mean postoperative decline in the P-DRF of the operated kidney was only 3 % which was found to be statistically insignificant (p value = 0.072). In agreement with the previous studies [21], it could be suggested that WI may result in negligible or reversible renal damage within certain time limits of WIT such as the mean WIT of the current study. In addition, the parenchymal loss seemed to play a more important role in kidney function deterioration than WI. Considering the above, it could be advocated that the LPN surgical technique could probably focus on the precise tumor excision and suturing rather than the minimization of WIT. Nevertheless, additional studies are necessary for the confirmation of the above hypothesis.
Limitations of this study include the reliance on DRF and the use of the non-operated kidney as a stable reference unit before and after the surgery. Any postoperative compensatory hypertrophy of the contralateral kidney may result in a false outcome of DRF. Takagi et al. [22] showed that the compensatory hypertrophy of the contralateral kidney after PN remained rather limited and less than 2.3 % in most cases. They concluded that the larger the excised volume of the kidney, the more hypertrophy of the contralateral kidney was expected. The median tumor diameter of the latter study was 3.5 cm and probably resulted in higher volume loss in comparison with our series (median of 2.6 cm). Hence, we assume that the compensatory hypertrophy may have been negligible in our study. Another limitation of our study was the lack of stratification of the results according to the length of the WIT or the tumor size. The parenchymal volume was never measured, and the current study could not provide information regarding the pre- and postoperative changes in the volume of the renal parenchyma. Nevertheless, the changes in the contour of the operated kidney may influence measurements of the renal volume and the selection of ROIs out of the excision field for measurements probably allowed for more reliable results. Moreover, the use of CT scans for the evaluation of renal volume would expose the patients in additional radiation without providing evidence that would significantly influence the results of the study.
Conclusion
In LPN, the parenchymal loss caused by the resection of the tumor and the suturing of the surrounding normal tissues resulted in kidney function deterioration which should probably be distinguished from WI effects. An average WIT of 22 min for a mean tumor diameter of 2.6 cm resulted in a 7 % kidney function decline. Four percentage could be attributed to the parenchymal loss and 3 % to WI.
References
Becker F, Van Poppel H, Hakenberg OW, Stief C, Gill I, Guazzoni G, Montorsi F, Russo P, Stöckle M (2009) Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol 56:625–634
Simmons MN, Schreiber MJ, Gill IS (2008) Surgical renal ischemia: a contemporary overview. J Urol 180:19–30
Simmons MN, Hillyer SP, Lee BH, Fergany AF, Kaouk J, Campbell SC (2012) Functional recovery after partial nephrectomy: effects of volume loss and ischemic injury. J Urol 187:1667–1673
Song C, Park S, Jeong IG, Hong JH, Park HK, Kim C-S, Ahn H (2011) Followup of unilateral renal function after laparoscopic partial nephrectomy. J Urol 186:53–58
Spaliviero M, Gill IS (2007) Laparoscopic partial nephrectomy. BJU Int 99:1313–1328
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Mir MC, Ercole C, Takagi T, Zhang Z, Velet L, Remer EM, Demirjian S, Campbell SC (2015) Decline in renal function after partial nephrectomy: etiology and prevention. J Urol 193:1889–1898
Campbell SC (2012) A nonischemic approach to partial nephrectomy is optimal. No. J Urol 187:388–390
Choi JD, Park JW, Lee SY, Jeong BC, Jeon SS, Lee HM, Choi HY, Seo SI (2012) Does prolonged warm ischemia after partial nephrectomy under pneumoperitoneum cause irreversible damage to the affected kidney? J Urol 187:802–806
Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, Devarajan P, Venkatachalam MA (2013) Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol 24:506–517
Porpiglia F, Renard J, Billia M, Musso F, Volpe A, Burruni R, Terrone C, Colla L, Piccoli G, Podio V, Scarpa RM (2007) Is renal warm ischemia over 30 minutes during laparoscopic partial nephrectomy possible? One-year results of a prospective study. Eur Urol 52:1170–1178
Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, Gill IS, Blute ML, Campbell SC (2010) Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol 58:340–345
Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, Gill IS, Campbell SC, Blute ML (2010) Comparison of warm ischemia versus no ischemia during partial nephrectomy on a solitary kidney. Eur Urol 58:331–336
Gill IS, Patil MB, Abreu ALdC, Ng C, Cai J, Berger A, Eisenberg MS, Nakamoto M, Ukimura O, Goh AC, Thangathurai D, Aron M, Desai MM (2012) Zero ischemia anatomical partial nephrectomy: a novel approach. J Urol 187:807–814
Nguyen MM, Gill IS (2008) Halving ischemia time during laparoscopic partial nephrectomy. J Urol 179:627–632
Shikanov S, Lifshitz D, Chan AA, Okhunov Z, Ordonez MA, Wheat JC, Matin SF, Landman J, Wolf JS, Eggener SE, Shalhav AL (2010) Impact of ischemia on renal function after laparoscopic partial nephrectomy: a multicenter study. J Urol 183:1714–1718
Sharma N, O’Hara J, Novick AC, Lieber M, Remer EM, Herts BR (2008) Correlation between loss of renal function and loss of renal volume after partial nephrectomy for tumor in a solitary kidney. J Urol 179:1284–1288
Song C, Bang JK, Park HK, Ahn H (2009) Factors influencing renal function reduction after partial nephrectomy. J Urol 181:48–53
Mir MC, Campbell RA, Sharma N, Remer EM, Simmons MN, Li J, Demirjian S, Kaouk J, Campbell SC (2013) Parenchymal volume preservation and ischemia during partial nephrectomy: functional and volumetric analysis. Urology 82:263–268
Kibar M, Yapar Z, Noyan A, Anarat A (2003) Technetium-99 m-N, N-ethylenedicysteine and Tc-99m DMSA scintigraphy in the evaluation of renal parenchymal abnormalities in children. Ann Nucl Med 17:219–225
Zargar H, Porpiglia F, Porter J, Quarto G, Perdona S, Bertolo R, Autorino R, Kaouk JH (2015) Achievement of trifecta in minimally invasive partial nephrectomy correlates with functional preservation of operated kidney: a multi-institutional assessment using MAG3 renal scan. World J Urol. doi:10.1007/s00345-015-1726-x
Takagi T, Mir MC, Sharma N, Remer EM, Li J, Demirjian S, Kaouk JH, Campbell SC (2014) Compensatory hypertrophy after partial and radical nephrectomy in adults. J Urol 192:1612–1618
Author’s contribution
Bagheri contributed to protocol development, data collection, data analysis, and manuscript writing and editing. Pusztai, Farkas (László), Buzogány, Szabó, Lantos, Imre, and Farkas (Nelli) helped in collecting and analyzing the data. Kallidonis helped in writing and editing the manuscript. Szántó contributed to protocol development and manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest are declared by the authors.
Ethical standard
Institutional board approval was obtained for the conduction of the study. Informed consent was obtained from all patients.
Rights and permissions
About this article
Cite this article
Bagheri, F., Pusztai, C., Farkas, L. et al. Impact of parenchymal loss on renal function after laparoscopic partial nephrectomy under warm ischemia. World J Urol 34, 1629–1634 (2016). https://doi.org/10.1007/s00345-016-1798-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1798-2