Abstract
Gamma-aminobutyric acid (GABA) is a non-protein amino acid and has been thoroughly studied in animals, where it works as a neurotransmitter. In plants, GABA was found to be a signaling molecule after the discovery of its binding sites. GABA metabolism takes place through the GABA shunt. It occurs in mitochondria and bypasses two steps of the tricarboxylic acid (TCA) cycle. It is also produced via proline and polyamine metabolic pathways. Both abiotic and biotic stress conditions affect plant’s growth and development. These stresses impact respiration and energy production in mitochondria, resulting in the elevated production of reactive oxygen species (ROS), which ultimately leads to cell death. The synthesis of GABA aids in the restoration of respiratory processes and energy production. Its accumulation is observed during plant stress conditions. In stress conditions, GABA concentration increases which raises the tolerance level of plants. It mitigates ROS formation, improves photosynthetic machinery, regulates the opening of stomata, and activates antioxidant enzymes. The transport of GABA is crucial for its functioning throughout plants, making it important to understand its cell and organelle transport. This review describes the biosynthesis, distribution, transport, and signaling roles of GABA, and also highlights the management aspects of the GABA shunt pathway for ROS production and in the defense mechanism of plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gamma-aminobutyric acid (GABA), also called 4-aminobutyric acid, is a four-carbon non-proteinogenic amino acid having the empirical formula C4H9NO2 and a molecular mass of 103.12 g/mol, which is generally present in plants, microorganisms, as well as in animals (Jiang et al. 2020). It was first discovered in potato tubers by Steward et al. (1949). Its biosynthesis takes place in the cytoplasm and is catabolized in mitochondria (Bown et al., 2020) and its catabolism is controlled by various factors. GABA acts as an important molecule for primary and secondary metabolism as it takes part as an intermediate in amino acid biosynthesis and nitrogen metabolism (Li et al. 2021). It plays a crucial role in providing nitrogen and carbon balance (Fromm et al., 2020), stimulating plant photosynthesis (Khan et al. 2021), and mitigating reactive oxygen species (ROS) (Liu et al. 2022).
GABA and GABA shunt have been discovered to be engaged in various physiological functions, including vegetative development, control of cytosolic pH, stomatal movement, C: N balance, signal transduction, bypass of the tricarboxylic acid (TCA) cycle, and defense in biotic and abiotic stress settings (Mekonnen et al. 2016; Wang et al. 2016). It is involved in the activation of enzymes that are evolutionary-conserved, which bypass two steps of the TCA cycle in mitochondria to attenuate oxidative injury (Gilliham et al., 2016). The existence of GABA transporters was reported in Arabidopsis when it was grown efficiently with GABA as the only nitrogen source (Breitkreuz et al. 1999). Furthermore, the mitochondrial GABA transporter GABA permease (GABA-P), which helps in transferring GABA from the cytosol to mitochondria, has functionally been characterized in Arabidopsis (Michaeli et al. 2011). Moreover, plant aluminum-activated malate transporters (ALMTs), which are induced by anions and negatively controlled by GABA, are controlled by different signals (Xu et al. 2021a, b). The contribution of GABA transporters in plants is an emerging area of research, and research gaps still exist that need to be elucidated. Additionally, GABA does regulate physiological processes like root and pollen tube growth via ALMT (Ramesh et al. 2018).
It has been reported that GABA is present in different tissues, compartments, and organs in micromolar to millimolar concentrations, which suggests that it may play a role as a signaling molecule in addition to its metabolite function in plants (Ramesh et al. 2017). GABA accumulation under various biotic and abiotic conditions and the presence of its binding sites on cell membranes and gated anion channels, also gives strong proof that it behaves as a signaling molecule in plants (Ramesh et al. 2015). Additionally, during stress conditions, GABA binds to ALMT and GORK (gated outwardly rectifying K+) channels present on guard cells (Wang et al. 2017a, b). Recently, the transport of GABA across membranes in plants has received great interest, and the process of transporting it from the apoplast to cytoplasm and from the cytoplasm to different organelles is a well-received research theme (Yu et al. 2019).
In this review, we have depicted the numerous roles of GABA in plants, biosynthesis, distribution, and associated GABA transport in cells, as well as recent developments in research on the function of GABA shunt under biotic and abiotic stress responses. We will discuss GABA metabolism routes in plants, emphasizing a few major gaps in our understanding of the processes involved, and then talk about stress-related alterations in flux, ROS levels, and redox balance. Moreover, we will discuss signaling of GABA in stomata and how GABA buildup can alter K+ and malate efflux, which leads to aluminum, drought, or hypoxia tolerance. The above findings show that GABA administration can be an acceptable treatment for managing various or concurrent stressors.
Biosynthesis of GABA
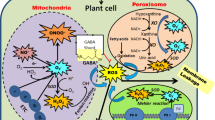
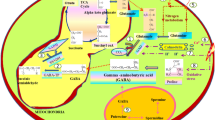
Plants synthesize GABA mainly by two methods. The first pathway takes place in the cytoplasm, in which glutamate decarboxylase (GAD) catalyzes an irreversible glutamate decarboxylation reaction and produces GABA (Yogeswara et al. 2020), and the other method is by polyamine degradation (Alcázar et al. 2010). Further, during GABA shunt, succinate is produced via succinic semialdehyde dehydrogenase (SSADH) that enters into the TCA cycle and the other product formed is succinic semialdehyde (SSA) by succinate reductase (SSR), through which gamma-hydroxybutyric acid (GHB) is produced at the end (Li et al. 2021).
Via Proline
Cytosolic GABA is indirectly synthesized from proline and produced in the plastid/cytosol by ∆1-pyrroline-5-carboxylate synthetase (NADP-dependent) and ∆1-pyrroline-5-carboxylate reductase from glutamate (Signorelli et al., 2017). The reaction of proline with the hydroxyl radical results in the abstraction of hydrogen from the amine group; afterward, proline is spontaneously decarboxylated to form pyrrolidin-1-yl. The activity of ABAL/pyrroline dehydrogenase readily converts pyrrolidin-1-yl to ∆1–pyrroline/ABAL, which is then oxidized to GABA.
Via Polyamine Degradation
The polyamine metabolic process, which involves multiple steps to convert arginine to putrescine, is an alternate method for the synthesis of GABA. Afterward, putrescine is transformed into spermidine or 4-aminobutyraldehyde by O2-dependent polyamine oxidase (PAO), which is then oxidized by NAD+-dependent 4-aminobutyraldehyde dehydrogenase to produce GABA (Shelp et al. 2012).
Putrescine, spermine, and spermidine are examples of polyamines. Putrescine is a primary polyamine that is the major component of polyamine biological metabolism, whereas spermidine and spermine are secondary and tertiary polyamines, respectively (Li et al. 2021). Putrescine is generated by arginine and ornithine, catalyzed either by arginine decarboxylase (ADC) or ornithine decarboxylase (ODC) (Kou et al. 2018). The catabolization of polyamine, namely spermidine and its precursor (putrescine), is achieved by the actions of diamine oxidase (DAO) and PAO, which result in the production of (i) hydrogen peroxide, ammonia, and 4-aminobutanal (ABAL) and (ii) 1,3-diaminopropane and Δ1-pyrroline, which cyclizes to Δ1-pyrroline (Fait et al. 2011). These enzymes have been identified as peroxisomal and cytosolic, which suggests that peroxisomes help in producing GABA via the polyamine pathway (Corpas et al. 2019). Radiolabeled GABA produced by exogenously provided radiolabeled putrescine and the addition of a CuAO inhibitor (aminoguanidine), which suppresses GABA production, provide early evidence for the presence of ABAL/pyrroline dehydrogenase in plants (Shelp et al. 2012).
GABA shunt: The Metabolic Bypass
The reaction in which succinate is formed from glutamate via GABA is known as the GABA shunt. It involves three important enzymatic reactions that are catalyzed in the cytosol by GAD and in mitochondria by SSADH and GABA transaminase (GABA-T), respectively. It has been experimentally observed that the GABA shunt plays an important role in response to different stresses (Yuan et al. 2023).
Glutamate Decarboxylase
GAD enzyme is associated with 5 phosphate pyridoxal (PLP)-dependent enzyme family, which catalyzes decarboxylation of L-glutamate to GABA. Its activity increases in the acidic medium and decreases at neutral or alkaline pH (Astegno et al. 2015). Glutamate is the precursor molecule of GABA and its production occurs in various regions in the plant cell, for example, in the cytoplasm by the action of glutamine synthetase/glutamate synthase (GS/GOGAT), and in plastids in which ammonium ions are assimilated into glutamate. So, there is an interrelationship linking glutamate content and GABA content (Ramos et al., 2019).
In plants, GAD enzyme is well-regulated by the Ca2+/calmodulin (CaM) (Ansari et al. 2021). It was first identified in petunia plants with Ca2+/CaMBD (calmodulin-binding domain) and its signaling suggests a possible role in GABA biosynthesis (Baum et al. 1996). Nuclear magnetic resonance has recently been used to establish the 3-dimensional structure of CaM that binds to the petunia GAD CaMBD, which reveals an intriguing complex of CaM with two peptides of CaMBD (Yap et al. 2003). It has been suggested that the intracellular levels of calcium rise under unfavorable environmental conditions (Behera et al. 2018), which induces the expression of CALM gene, which forms CaM protein and finally Ca2+/CaM active complex is produced (Wei et al. 2023). During osmotic stress, plasma membrane localized osmosensor (OSCA1) is identified in Arabidopsis, which is involved in increasing internal calcium (Yuan et al. 2014).
Tobacco plants expressed with a truncated version of petunia GAD enzyme show some growth abnormalities like dwarf stems, unusual GAD complexes, low levels of glutamate, and high levels of GABA due to the absence of CaMBD (Baum et al. 1996). GAD isoform (OsGAD2), isolated for the first time in rice roots, was unable to bind to CaM, even in the presence of Ca2+ due to the lack of CaMBD at the C-terminus whereas another isoform (OsGAD1) functions normally by binding to CaM (Virdi et al. 2015).
After GABA is synthesized in the cytosol, it is transported to mitochondria by GABA carriers. At this point, active GABA metabolism takes place within the mitochondria.
GABA Transaminase: Bridging GABA and the TCA Cycle
GABA is translocated through the plasma membrane by GABA-P, which is a mitochondrial enzyme and is associated with the APC transporter family (Michaeli et al. 2011). GABA-T converts GABA into SSA, which can subsequently oxidized by NAD+-dependent succinic SSADH and ultimately transformed into succinate to enter the TCA cycle (Michaeli et al., 2015), as described in Fig. 1. GABA-Ts are of two types that use either pyruvate (GABA-TP) or α-ketoglutarate (GABA-TK) as amino acceptors, and produce alanine or glutamate (Yuan et al. 2023). GABA-TP also has GABA-TG activity, in which glyoxylic acid acts as an amino receptor that gives glycine (Ahmad & Qazi 2024). Some of the glutamate formed is used to maintain the balance of mitochondrial GABA/Glu.
GABA biosynthesis in plants mediated by GABA shunt and showing stress response. Abbreviations: GABP, GABA permease; GAD, glutamate decarboxylase; SSADH, succinic semialdehyde dehydrogenase; ETC, electron transport chain; SSR, succinic semialdehyde reductase; SSA, succinic semialdehyde; GHB, gamma-hydroxybutyric acid; GABA, gamma-aminobutyric acid; CaM, calmodulin; ROS, reactive oxygen species; GAT, GABA transporter; GDH, glutamate dehydrogenase
GABA-TP is the major enzyme that plays a role in plants, and its associated gene has been found in tobacco (Zhang et al. 2016). pH also affects the catabolism of GABA, and the optimum pH at which GABA-TP works is nine (Zheng et al. 2024). Partial purification of GABA-TP from tobacco and the homologous Arabidopsis gene was cloned, and it was seen that the recombinant GABA-TP uses pyruvate, not α-ketoglutarate (Van et al., 2002). Arabidopsis knockouts disrupted in the corresponding gene have an increased GABA level of about 100-fold in comparison to the wild type which confirms that it is the functioning enzyme during GABA shunt (Palanivelu et al. 2003).
GABA catabolism is affected by adverse environmental conditions. During stress conditions, the ratio of NAD+ to NADH is low, due to which the enzymatic activity of SSADH gets inhibited, which leads to the aggregation of SSA (Ansari et al. 2021). It has also been reported that GABA-T activity gets reduced with the accumulation of SSA and the catabolism of GABA gets inhibited (Yuan et al. 2023).
Succinic Semialdehyde Dehydrogenase (SSADH)
Succinic semialdehyde dehydrogenase (SSADH) works in the last step of GABA shunt and converts SSA to succinate by irreversibly oxidizing SSA. Its optimum activity is found to be at pH 9. In general, its localization takes place in mitochondria in many organisms. However, in yeast, it is localized within the cytosol (Coleman et al. 2001). SSADH is a homotetramer in an active state and is specific for SSA. It uses NADP for the production of NADH, where it is negatively regulated by NADH and ATP (Khan et al. 2021).
It has been reported that disrupting the SSADH gene in Arabidopsis under environmental stress leads to the necrotic cell death of plants (Bouché et al. 2003). Additionally, ssadh mutants are sensitive to ultraviolet light and heat, which causes an increase in H2O2 content in plant cells, due to which necrosis occurs (Bouché et al. 2003; Carillo 2018). The phenotype formed by ssadh mutants may be due to the absence of some metabolites like NADH or succinate, or an excess of certain metabolites like SSA or GHB, or due to an imbalance in signal molecules like GABA (Bouche and Fromm 2004).
Conversion of SSA into GHB
Alternatively, SSA is converted into γ-hydroxybutyrate (GHB) by the enzyme SSR in plants (Zarei et al. 2017). It is a reversible reaction in which SSA can be regenerated with the help of GHB dehydrogenase (GHBDH) (Li et al. 2021). The reaction may take place in the cytoplasm, plastids, and mitochondria, depending on the type of SSR.
This reaction is a reduction process that occurs in abiotic stress conditions, which indicates the function of GHBDH in stress tolerance and is controlled by the redox state of the cell (Allan et al. 2008). Stress, generally hypoxia, could promote the conversion of SSA to GHB, which helps in resisting the stress conditions by detoxifying SSA (Yin et al. 2018).
GABA Transport Mechanism
The first reports of GABA transporters date back to 1999 when Arabidopsis was grown using GABA as the sole nitrogen source in the media. This demonstrated that plants could absorb GABA externally (Breitkreuz et al. 1999). GABA can be transported between the membranes or from the cell membrane to different organelles. Some of the transporters, such as GABA transporters (GAT1), aluminum-activated malate transporters (ALMTs), cationic amino acid transporters (CATs), and bidirectional amino acid transporters (BATs), are present on organelle membrane or cell membrane, which helps in transporting GABA to various organelles and to the intracellular space.
Transcell Membrane Transporters
ALMTs
ALMT family are multigenic and are involved in processes like ion homeostasis and mineral nutrition (ZmALMT1), aluminum tolerance (TaALMT1), movement of stomata (AtALMT12), vacuolar homeostasis (AtALMT9), and mixing of soil nutrients such as phosphate (ZmALMT2) (Palmer et al. 2016). They are bidirectional transmembrane transporters (Ramesh et al. 2018), and about 12 homologous genes of ALMT have been reported in potatoes (Ma et al. 2016). The binding site for GABA on ALMTs is a stretch of 12 amino acids, which occurs at the end of the sixth transmembrane domain near the carboxy-terminus of proteins (Ramesh et al. 2017). ALMT1 is present in Arabidopsis, wheat, rice, and rape plants, but the study has been carried out mainly in wheat. To regulate ALMT transport by GABA, they must strike a delicate balance between the anion efflux in unfavorable conditions, like aluminum toxicity, and the requirement to control such flux to avoid nitrogen and carbon loss. In another study, when tobacco BY2 cells (expressing wheat ALMT1) were exposed to Al3+ and treated with the ethylene donor Ethrel, malate efflux was reduced (Tian et al. 2014). It has also been shown that GABA regulates the closing and opening of stomata under drought conditions by modulating AtALMT12 in guard cells and tonoplast localized Arabidopsis AtALMt9 (Xu et al. 2021a, b).
GAT1
It is a transcell-membrane protein, and its gene (GAT) belongs to the AAAP (amino acid/auxin protein) gene family (Ma et al. 2016). Four identical (GAT1, GAT2, GAT3, and GAT4) genes have been reported in plants. GABA is transported from the apoplast to the cytoplasm by GAT1. However, it has been seen that Al3+ can inhibit the influx of GABA transport by ALMT1 and has no negative effect on GABA by GAT1 (Long et al. 2020). Rice, Arabidopsis, potatoes, and other plants have genes encoding the GAT1 protein, and research on transporting GABA by AtGAT1 has been done in Arabidopsis. Recently, the study of Arabidopsis gat1 knockout mutants has further strengthened the importance of GAT1 in the uptake of GABA into cells. In contrast to gat1 mutants, giving GABA to C-deficient plants causes GABA to enter the TCA cycle (Batushansky et al. 2015).
AAP3 and ProT2
AAP3 and ProT2 are quaternary transporters that work partly when GAT1 becomes mutant and are unable to transport GABA. These transporters have a low affinity for GABA. They were first reported in yeast plasma membranes, namely, proline transporter 2 (ProT2) and amino acid permease 3 (AAP3) (Wipf et al. 2002). These transporter-related genes have been reported in potato, rice, Arabidopsis, and other crops (Tian et al. 2020). ProT2 is a member of the amino acid transporter (ATF) superfamily, while AAP3 is a member of the AAAP family (Ahmad and Qazi 2024).
Transorganelle Membrane Transporters
BAT1
It is a mitochondrial membrane-facilitated transporter protein that is bidirectional in nature (Dündar and Bush 2009). Some amino acids are also transported by BAT1 (Yao et al. 2020). About seven BAT genes have been reported till date in various crops (Tian et al. 2020). The study on BAT1 gene has been done only in Arabidopsis. AtBAT1 encodes this protein and has shown high transport activity for glutamate, arginine, and lysine but cannot transport GABA (Bush 1993). However, it was found by Michaeli that a splicing variant of AtBAT1 (At2g01170), that is, AtGABP (At2g01170.1), which belongs to the APC gene family, transports GABA on the mitochondrial membrane. Contrary to the two low-affinity transporters ProT2 and AAP3 discussed above, GABP has extremely similar sequence structures and can transport GABA but not proline (Michaeli et al. 2011). A study on the transport of GABA by GABP also revealed a very strong correlation between the GABP gene's co-expression and the SSADH gene, which codes for succinate semialdehyde dehydrogenase (Michaeli et al. 2011). This suggests that GABP may be involved in GABA metabolic events, including the GABA cycle and the GABA shunt.
CAT9
CATs belong to the APC gene family and are located on the membrane of vacuoles (Snowden et al. 2015). As of now, nine similar CAT genes have been identified, with CAT9 being in charge of the transport of GABA between vacuole and cytoplasm. CAT9 has been found in several plants and it has been verified experimentally that a similar gene (SlCAT9) in tomato also transports GABA (Snowden et al. 2015). Transport of GABA takes place either through the concentration gradient of the substrate transported or by the action of proton pumps present on the tonoplast (Ma et al. 2013). However, it has been found in some previous research that SlCAT9 also transports Glu/Asp and might affect some metabolic pathways in plants by converting GABA (Yang et al. 2020).
GABA Distribution
The distribution of GABA takes place in various organelles like mitochondria, peroxisomes, vacuoles, and plastids based on various metabolic processes in various organelles (Shelp et al. 2021). In the cytoplasm, GAD catalyzes GABA (Yogeswara et al. 2020) and transports it to mitochondria where it is converted into succinate via GABA shunt (Liao et al. 2021) and finally goes into the TCA cycle or some other pathway to form GHB (Allan et al. 2008). However, in mitochondria, GABA may also be converted into aspartate and glutamate (McCraw et al. 2016). The polyamine degradation pathway takes place in peroxisomes, where it produces GABA; after that, spermidine to spermine and spermine to putrescine are transformed throughout this process (Podlešáková et al. 2019).
Distribution of GABA also takes place in plastids. During this, 2-ketoglutarate and glutamine are produced by the action of GS and GOGAT, respectively (Ramos et al., 2019). Glutamate uses the urea reaction to produce arginine, which, by decarboxylation, converts to putrescine. Finally, GABA is produced by putrescine through the action of aldehyde dehydrogenase and copper amine oxidase to produce GABA (Missihoun et al. 2011).
GABA as a Signaling Molecule in Plants
The characteristic of any signaling molecule is that it is present in low concentrations, has a binding capacity to its receptor, and can acquire a cellular response. However, GABA being present in excess concentrations and its presence throughout the plant argue against it being a signaling molecule (Shelp et al. 1999; Kaspal et al. 2021). In addition, molecules that change the membrane potential in response to an event are known to be the key signaling molecules, and, as GABA changes the potential of the membrane and takes part in plant growth, it is thought to have an important signaling role in plants (Ramesh et al. 2015).
It was recently discovered that the plant anion channels (ALMTs) have a binding site (12 amino acids) for GABA and show homology with the mammalian GABAA receptor, which provides evidence that GABA can act as a signaling molecule (Ramesh et al. 2015). This binding site occurs at the sixth transmembrane domain and the C-terminus of proteins (Ramesh et al. 2017). Depolarization of the membrane takes place when anions (malate) are released due to the opening of ALMT anion channels (Barbier-Brygoo et al. 2011). Due to the binding activity of GABA, it regulates this anion efflux mediated by ALMTs, which are sensitive to small concentrations of GABA (Ramesh et al. 2015). Furthermore, there is a negative relationship between endogenous GABA and anion efflux, as studied in wheat ALMT1 (TaALMT1) (Ramesh et al. 2017). Moreover, it has been seen that aromatic amino acids like tyrosine and phenylalanine in the supposed binding site of GABA are found to be crucial for GABA regulation, and their mutation to cysteine affects the affinity of GABA in wheat ALMT1 (Ramesh et al. 2017). Different concentration ranges in various organs, compartments, and tissues suggest that GABA may be a signaling molecule along with the property of being a metabolite in plants (Ramesh et al. 2017).
It has been found in recent times that, in Arabidopsis, AtALMT9 and AtALMT12 present on the tonoplast and plasma membrane of guard cells, respectively, are regulated by GABA which works in opening and closing of stomata under drought conditions (Xu et al. 2021a, b). This provides opportunities for further investigation between signaling pathways involved in the control of stomatal apertures in response to different abiotic stresses. In Arabidopsis, GORKs play an important role under stress conditions in plants (Shabala et al. 2016) and help in the closing of stomata (Eisenach et al. 2014). Later on, it was found that these GORK channels in plants have GABA binding motifs, similar to ALMT proteins (Adem et al. 2020). The findings of GABA-gated channels confirm that GABA acts as a plant signaling molecule. These accepted plant "GABA receptors" are important in converting changes in metabolic state into physiological responses under stress conditions (Gilliham et al., 2016).
Significance of GABA Shunt
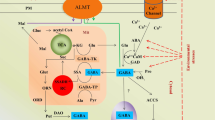
During stressful situations, GABA shunt bypasses two TCA cycle reactions, i.e., Succinyl Co-A ligase (SCOAL) and AKG dehydrogenase (AKGDH), that are blocked by ROS (Bown et al., 2016). It has long been believed that the GABA shunt plays a crucial role in controlling ROS during stressful situations, maintaining the C-N balance, and regulating pH (Xu et al. 2021a, b) (Fig. 2).
Diagram illustrating the role of GABA (Gamma-Aminobutyric Acid) in mitigating abiotic stress in plants. GABA acts as a crucial molecular player, regulating stress responses through various mechanisms, depicted here through its interactions with stress signaling pathways, ion transport regulation, and antioxidant defense systems. This diagram highlights GABA's multifaceted role in enhancing plant resilience to environmental challenges such as drought, salinity, and temperature fluctuations. ACC, Aminocyclopropane; OA, Organic anion; GORK, K+ efflux transporter; GAT1, GABA transporter; AQP, Aquaporin; GAD, Glutamate decarboxylase; ALMT, Aluminum-activated malate transporter; GABA-P, GABA permease; ROS, Reactive oxygen species—H2O2; OSCA1, Hyper osmolarity gated Ca2+ channel; ABA, Abscisic acid; NHX, Na + /H antiporter; NSCC, Non-selective cation channel; SOS, Salt overly expensive; GABA binding site. Solid lines = regulation by GABA; dotted lines = possibility of indirect or direct regulation by GABA
GABA Shunt vs Abiotic Stress
In GABA shunt, the first enzyme that works is GAD, which yields GABA. Under stressed conditions, the catalytic activity of GAD is disturbed either by rising pH due to cytosolic acidification (Allan et al. 2009; Shelp et al. 2021) or by the calcium/CaM response (Bouché et al. 2004). The majority of emphasis has been centered around GABA production under stressful conditions due to changes in the catalytic characteristics of GAD depending on Ca2+/calmodulin complex activity and cytosolic pH (Fait et al. 2005). On this premise, it has been proposed that GABA levels are primarily regulated by its production rate. However, the identification and analysis of Arabidopsis GABA-T defective mutants revealed that GABA levels could potentially be determined by the rate of breakdown (Renault et al. 2010).
GABA-P transfers the accumulated GABA from the cytosol to mitochondria and converts GABA to SSA via GABA-T and then to SSADH which later enters the TCA cycle (Michaeli et al. 2011). Since succinate traverses three TCA cycle sites of NADH generation, this final phase has a significant impact on the cell's redox status (Fait et al. 2008). It has been found that several abiotic and biotic stressors raise GABA levels in plant tissue (Scholz et al. 2017). For instance, it was found that the production of a lower level of GABA by Arabidopsis makes it more susceptible to drought stress because of stomatal closure that could be restored by elevating the internal GABA level (Mekonnen et al. 2016). Besides this, NaCl stress also increases the level of GABA content (Wang et al. 2017a, b). In contrast, Zhang et al. (2011) found a decrease in GABA levels in tobacco plants after treatment with 500 mM NaCl which may be due to the abrupt increase in externally applied NaCl. Other studies suggested that with gradually increasing NaCl concentration, GABA levels increase (Woodrow et al. 2017). However, under saline conditions and high light at high nitrate, GABA shunt might have a more significant role (Carillo 2018). It is proposed that the formation of GABA via GAD could help to dissipate excess energy and CO2 release, which allows the Calvin cycle to operate while putting less pressure on the photosynthetic electron transport chain (ETC) and decreasing photodamage and ROS (Allan et al. 2009). Furthermore, under conditions where respiration and TCA cycle are impeded and ROS levels are high, the GABA shunt delivers succinate or NADH to the mitochondrial ETC.
GABA Shunt in Low- and High-Temperature Stress
Both low and high temperatures limit plant development and growth and high levels of GABA are often reported in both stress conditions (Abdel et al., 2021; Xu et al. 2022). For example, in barley and wheat seedlings, the enhanced accumulation of GABA and induction of genes related to GABA shunt under freezing temperatures have been reported (Mazzucotelli et al. 2006). Similarly, in Brachypodium sylvaticum, increased GABA levels were observed under freezing stress (Toubiana et al. 2020). However, by applying GABA exogenously, it induces endogenous GABA also and provides tolerance against cold stress in tea plants (Zhu et al. 2019) and in Anthurium (Aghdam et al. 2016a, b). The mechanism underlying the alleviation of low-temperature stress might be due to the increased antioxidant activity of plants that mitigates ROS and malondialdehyde (MDA) (Malekzadeh et al. 2014). GABA participates in the acclimation to cold storage of zucchini fruit induced by putrescine (Palma et al. 2015) and in salicylic acid-mediated alleviation of postharvest chilling injury in anthurium cut-flowers (Aghdam et al. 2016a, b).
Similarly, there is a relationship between GABA and heat stress among different plant species. High level expression of GAD and reduced levels of SSADH and GABA-T were found when immature seeds of soybean were heat-dried at 40 °C. Consequently, it was observed that the GABA content increased in the treated seeds up to fivefold compared to the untreated seeds (Takahashi et al. 2013). Moreover, there was a rise in glutamate in cytoplasm (Li et al. 2016), which in combination with CaM activates GAD, and hence induces GABA accumulation under high temperatures in Arabidopsis roots (Locy et al. 2000). Upon GABA application, four-day-old seedlings of rice treated with heat stress improved their survival rate by upregulating antioxidants and osmoprotectants (Nayyar et al. 2014). Furthermore, in heat-stressed plants, there is an improvement in carbon assimilation and fixation by upregulating the osmolytes and thus reduces oxidative damage when GABA is applied (Priya et al. 2019).
GABA Shunt in Drought and Flooding
Drought and flooding severely affect plant growth and development, and has been reported in several crops that there is considerable GABA accumulation in these stressed plants (Bor et al. 2009; Ruperti et al. 2019). Arabidopsis mutants gad1/2 show low GABA content, defective stomatal closure, and a large stomatal aperture (Mekonnen et al. 2016). As a consequence, due to prolonged drought stress conditions, these mutants observed an earlier wilting than wild type. However, the complemented gad1/2 × pop2 triple mutant shows the reverse phenotype and produces increased GABA levels (Mekonnen et al. 2016). It was reported by Yong et al. (2017) that elevating endogenous GABA content by exogenous application enhances white clover drought tolerance through the upregulation of the GABA shunt, proline, and polyamine metabolism. This specifies that GABA accumulation is a stress-specific response that regulates the opening of stomata and prevents water loss (Mekonnen et al. 2016).
Waterlogging results in a reduction in crop yield. It disturbs antioxidant enzyme activity and reduces the photosynthetic rate by causing impairment of the protective enzymes (Puyang et al. 2015). However, GABA downregulates the ROS-producing enzymes, activates antioxidant enzymes, and improves the ultrastructure of chloroplast (Salah et al. 2019). Due to soil waterlogging, hypoxic condition takes place that intensify the negative effects of waterlogging on plant growth and, as a result, induce GABA accumulation (Brikis et al. 2018). Under hypoxic conditions, the GABA shunt accumulates alanine and is responsible for the induction of DAO and GAD activities, which in turn increases GABA content (Wang et al. 2018).
GABA Shunt in Salinity Stress
Soil salinity is a serious environmental stress that has a global impact on agricultural productivity. In response to salt stress, plants accumulate various protective molecules that work to mitigate it (Gong et al. 2020). It has been documented that GABA-T-deficient pop2-5 mutants accumulate more GABA in roots and become salt tolerant (Su et al. 2019), while pop2-1 mutants remain sensitive to salt (Renault et al. 2010). These contrasting outcomes of different pop2 mutants might be because of their respective GABA levels, where excessive accumulation of GABA becomes harmful for plants (Su et al. 2019). It was reported that in pop2-5 mutants and gad1,2 (having reduced ability to produce GABA), GABA reduces K+ efflux and ROS concentration induced by H2O2−, and also induces Na+ uptake and activates H+-ATPase (Su et al. 2019). GABA also modulates the activity of salt overly sensitive (SOS) genes, which mediates the efflux of Na+ and NHX antiporters for the compartmentation of Na+ into the vacuole (Li et al. 2020). As reported in one of the studies in Agrostis stolonifera (creeping bentgrass), there was a high expression of AsSOS1 that helps in sequestering excess Na+ (Li et al. 2020). When wheat leaves were salt-treated, TCA cycle enzymes were physiochemically inhibited; however, an alternative carbon source was provided by GABA shunt for the normal function of the TCA cycle in mitochondria that detour salt-sensitive enzymes to raise leaf respiration in plants (Che‐Othman et al., 2020). Additionally, as GABA mediates the water intake that is restricted by salt stress, it is thought that GABA may modulate the activity of aquaporins (AQPs) (Chang et al. 2016) (Fig. 2).
GABA Shunt in Heavy Metal Stress
Large-scale industrialization has detrimental effects on soil and crop productivity because it accumulates toxic metals, which hinder plant growth by opposing the many physiological and molecular responses of plants (Tiwari and Lata 2018). It can also cause indirect effects on plant growth. Heavy metal stress has become a major problem as it pollutes the soil as well as food due to its accumulation in crops, produces ion toxicity, and enhances ROS (Abdullah et al. 2024). Several studies indicate an augmenting role of GABA to confer tolerance in plants against toxic metals. For example, as reported in rice roots by metabolomic analysis, GABA levels increase under chromium stress (Dubey et al. 2010). However, when Nicotiana tabacum plants are treated with high (100 µM) Zinc concentrations, they show low levels of GABA accumulation, suggesting that intermediate concentrations of heavy metals induce GABA production in plants (Daş et al. 2016). In a similar study, when rice seedlings were grown under arsenic stress, GABA induces expression of genes that are GABA shunt related which activates the antioxidant enzyme system and strongly inhibits arsenic accumulation, thus providing tolerance to As (III) stress (Kumar et al. 2017). Furthermore, one of the GABA shunt-related genes, i.e., GAD genes, are upregulated in rice and maize under Cd stress, where overexpression of these genes (ZmGAD1 and ZmGAD2) in cadmium-sensitive tobacco leaves and yeast intensifies cadmium tolerance in host cells (Cheng et al. 2018). These studies indicate that the degree of GABA accumulation depends on the heavy metal, level of metal concentrations, and the type of crop (Li et al. 2021).
GABA Shunt and Carbon–Nitrogen Balance
Nitrogen and carbon are the most essential elements and their assimilation is important for plant productivity, growth, and yield (Dubey et al. 2021). Carbon enters the TCA cycle via the GABA shunt and the produced GABA acts as a nitrogen storage metabolite in plants. It has been reported in Arabidopsis that it can grow in a culture medium having GABA as a nitrogen source (Breitkreuz et al. 1999). Similarly, GABA concentration rises to triple when half of the nodules of Medicago truncatula are excised (Sulieman et al., 2010). As the level of GABA increases, there is a decline in glutamate in phloem exudates, and recovery of N2 fixation is seen after excision (Sulieman et al., 2010). GAD changes the N-to-C ratio as seen in truncated-GAD transgenic Arabidopsis; a number of amino acids and proteins accumulate, whereas the sugar as well as organic acid content decreases (Fait et al. 2011). Furthermore, any hindrance in the GABA shunt causes significant alteration in starch and sucrose levels and impacts carbon metabolism in the cell wall (Ji et al. 2020). In poplar, GABA considerably reduces the low nitrogen-induced rise of leaf antioxidant enzymes, indicating that GABA influences the C: N ratio for poplar growth by reducing energy costs in N-deficient conditions (Chen et al. 2020). Hence, GABA is adequately recognized to represent the primary role in the link between plant nitrogen and carbon metabolisms (Michaeli et al., 2015).
Role of GABA in Stomatal Functioning
During stomatal opening, two transporters, AtALMT6 (on tonoplast) and malate-activated AtALMT9, mediate the flux of malate and Cl−, respectively, into the vacuole of guard cell (Saito et al., 2019). However, during stress conditions, abscisic acid levels upregulate GORK activity with the efflux of K+ and malate, which is mediated by transporters present on the plasma membrane, namely AtGORK and AtALMT12, respectively. It then leads to stomatal closure and drought tolerance (Demidchik et al. 2018). GABA negatively regulates ALMT9 (an anion uptake channel) and suppresses its action, which limits anion absorption and stomatal opening. As a result, only a small number of stomata are open, increasing the plant’s water consumption efficiency (Xu et al. 2019).
It has been seen in Arabidopsis mutants with changing GABA levels that GABA plays an important role in drought resistance. Drought-susceptible phenotype and impaired stomatal closure are the characteristic of atgad1/2 double mutants (Mekonnen et al. 2016). Further, it was demonstrated in atalmt9 and atgad2 mutants that under drought conditions, GABA accumulation inhibits light-induced stomatal opening and has negligible effect under constant light (Xu et al. 2021a, b). Opening of stomata in almt12 showed that wild type is sensitive to GABA, while dark-induced stomatal closing is insensitive to GABA (Xu et al. 2021a, b). This showed that accumulation of GABA in guard cells inhibits stomatal reopening and water loss due to transpiration, hence improving drought tolerance (Shelp et al. 2021).
Role of GABA in Biotic Stress
Majority of the studies on GABA are carried out on abiotic stress; however, the focus on biotic stress like pathogens and pests is increasing as GABA is involved in signaling and metabolism in plant immunity (Yang et al. 2017). Recognition of microbes by plants takes place through pattern-triggered immunity (PTI) and effector-triggered immunity (ETI), which act as the first and second line of defense, respectively (Jones and Dangl 2006), and their activation takes place by an increase of Ca2+ influx, which is a primary response to recognize pathogens (Seybold et al. 2014). This increase of cytosolic Ca2+ is triggered by GADs that have CaMBD; this suggests that GABA is accumulated and regulated by Ca2+ influx as a result of the immune response of plants (Shelp et al. 2006). Furthermore, the identification of pathogens such as fungi and bacteria has been shown to stimulate GABA accumulation in various plant species, such as soybean, tomato, and beans (Copley et al. 2017; O’Leary et al., 2016; Wang et al. 2019) (Table 1). It is significant to note that the location of GABA accumulation within the cell can play a crucial role in defining how the plant–pathogen relationship plays out (Tarkowski et al. 2020). While intracellular accumulation of GABA can effectively support metabolic pathways linked to plant defense, extracellular accumulation of GABA may be advantageous for pathogens that live in apoplasts and can compete for GABA absorption, such as Pseudomonas syringae or Cladosporium fulvum (O’Leary et al., 2016).
GABA Against Bacterial Infection
GABA accumulation in plants has been associated with the activation of defense genes and pathways. It serves as a signaling molecule that triggers defense responses upon bacterial infection. Tomato plants with GAD Knock Out (KO) showed greater vulnerability to Ralstonia solanacearum, a causal organism of bacterial wilt. This suggests that GABA plays a role in plant defense against R. solanacearum (Wang et al. 2019). But after the R. solanacearum inoculation, GABA catabolism is quickly increased, whereas GABA production via GAD and the methionine cycle is inhibited (Wang et al. 2019). These findings indicate that decreased GABA buildup is a pathogen's strategy to weaken the host in the early stages of infection (Tarkowski et al. 2020). In order to increase population and pathogenicity, bacterial plant biotrophs or hemi-biotrophs majorly depend on quorum sensing (QS) (Liu et al. 2008). Interestingly, in one study on tobacco plants, it was found that derived GABA negatively regulates QS in Agrobacterium tumefaciens, and these plants were more resistant to it as compared to wild type (Chevrot et al. 2006).
It is also reported that GABA interferes with the passing of Ti plasmid (Lang et al. 2016), and a GABA binding protein (Atu4243) from A. tumefaciens reduces the intrusive property toward the host and elevates degradation of the QS signal (Planamente et al. 2012). Sometimes, GABA accumulation can also be harmful for the host, as some bacteria take advantage of it and can use it as an energy source themselves (Rico and Preston 2008). A study was carried out involving Pseudomonas syringae to determine how it utilizes GABA when other C and N sources are exhausted (McCraw et al. 2016). However, conditions promoting high concentrations of GABA can increase plant resistance by repressing the expression of the Hrp (hypersensitive response and pathogenicity) genes in the bacterium (Park et al. 2010).
GABA Against Fungi
GABA has been shown to induce resistance of treated crops toward plant pathogenic fungi. A study on tomato sitiens mutants (unable to accumulate abscisic acid) showed increased activity of GABA shunt, which plays a part in resisting necrotrophic fungus Botrytis cinerea (Seifi et al. 2013; Sun et al. 2019). It was speculated that the supply of GABA shunt by cytosolic glutamate was due to the overactivation of the GS/GOGAT cycle (Seifi et al. 2013). Further, the import of glutamate from the distal region to the infectious site was seen during infection in Helianthus annuus by B. cinerea to provide substrate for the formation of resistant compounds (Dulermo et al. 2009). This study suggests that GABA has two roles: it suppresses the spread of hypersensitive response (HR) to healthy cells by forming an HR-like ring around the spreading lesion, and it fuels the TCA cycle and supplies nitrogen to the attacked cells for the production of defense-related chemicals (Siefert et al., 2013). In another study, when rice suspensions were exposed to Pyricularia oryzae, it showed elevated GABA levels in a GAD-independent manner (Forlani et al. 2014). Similarly, high catabolism of PA was seen in grape (Vitis vinifera) varieties that possessed increased GABA levels and were resistant to B. cinerea (Hatmi et al. 2015). Likewise, it was reported in in-vitro experiments that there was a rise in catabolism of PAs when maize was infected with Aspergillus flavus in comparison to the resistant maize line (Majumdar et al. 2019). These findings suggest that GABA shunt and its related compounds help in resisting the crop from fungus infections (Fig. 3)
GABA, a crucial mediator in plant defense, surges in response to calcium influx (triggered by PTI and ETI), cytosolic pH drop, and mechanical damage. This versatile molecule disrupts bacterial quorum sensing (QS), although some pathogens adapt by exploiting GABA as a nutrient. Beyond its antimicrobial role, GABA fortifies plant resilience by powering the TCA cycle and mitigating oxidative stress from ROS bursts. This dual function underscores GABA’s pivotal role in enhancing plant endurance and systemic immune responses
GABA Limits Invertebrate Infection
Wallace's discovery that mechanically manipulating or injuring soybean leaf tissues caused a sharp rise in GABA concentration was the first indication of GABA accumulation in response to plant injury (Wallace et al. 1984). Further, GABA acts as an inhibitory neurotransmitter to feeding invertebrate pests (i.e., insects), as it prevents normal growth and development and also affects GABA-gated chloride channels (Mithöfer and Boland 2012). Many studies have suggested that a high level of GABA prevents insect larvae from feeding (Bown et al. 2002). For example, in tobacco and soybean plants, crawling of insects increases GABA synthesis within a few minutes (Scholz et al. 2017). When S. littoralis larvae were fed to Arabidopsis thaliana Col-0 plants for three hours, the local concentration of GABA doubled (Scholz et al. 2015). It is interesting to note that regular mechanical injury with the robotic caterpillar MecWorm (Mithöfer et al. 2005) raised the local endogenous GABA level by up to ten times (Scholz et al. 2015). Furthermore, it was investigated in one of the studies that GABA accumulation acts as a defense mechanism against pests (Shan et al. 2022). It mimics the effect of some insecticides and causes developmental and physiological damage to insects (Bown et al. 2006). For example, GABA inhibits the development of Bombyx mori, which could be reversed by removing GABA from an artificial diet (Pang et al. 2013).
Conclusion and Future Perspective
In conclusion, this review has illuminated the pivotal role of gamma-aminobutyric acid (GABA) in mediating plant responses to a variety of stress conditions, underscoring its importance beyond a mere metabolic byproduct to a critical signaling molecule. The intricate interplay between GABA synthesis, catabolism, and transport, as detailed herein, highlights the complexity of its involvement in maintaining cellular homeostasis and stress mitigation. Despite significant advancements in understanding GABA's multifaceted roles, several questions remain unanswered, particularly concerning the molecular mechanisms underlying GABA signaling and its interaction with other signaling pathways in plants.
Future research should focus on unraveling these mechanisms, with an emphasis on the identification and functional characterization of GABA receptors in plants. Additionally, exploring the genetic manipulation of GABA metabolism pathways could offer new insights into improving plant resilience to stress, thereby enhancing agricultural productivity. Through advanced genetic, biochemical, and physiological studies, we can further elucidate GABA's role in plant growth and development, paving the way for novel strategies to bolster plant defense mechanisms against an array of biotic and abiotic stresses. Gene-editing technologies such as CRISPR/Cas9 provide precise methods for modifying GABA-related genes under abiotic and biotic stress conditions. Investigating the effects of targeted modifications on stress tolerance could yield breakthroughs. Dynamic variations in gene expression under challenging environments can be uncovered by high-throughput RNA sequencing. Important insights will be obtained by combining transcriptome data with GABA signaling networks.
References
Abdel Razik ES, Alharbi BM, Pirzadah TB, Alnusairi GS, Soliman MH, Hakeem KR (2021) γ-Aminobutyric acid (GABA) mitigates drought and heat stress in sunflower (Helianthus annuus L.) by regulating its physiological, biochemical and molecular pathways. Phys Plantarum 172(2):505–527
Abdullah, Wani KI et al (2024) Systems biology of chromium-plant interaction: insights from omics approaches. Front Plant Sci 14:1305179
Adem GD, Chen G, Shabala L, Chen ZH, Shabala S (2020) GORK channel: a master switch of plant metabolism? Trends Plant Sci 25(5):434–445
Aghdam MS, Naderi R, Jannatizadeh A, Babalar M, Sarcheshmeh MAA, Faradonbe MZ (2016a) Impact of exogenous GABA treatments on endogenous GABA metabolism in anthurium cut flowers in response to postharvest chilling temperature. Plant Physiol Biochem 106:11–15
Aghdam MS, Naderi R, Malekzadeh P, Jannatizadeh A (2016b) Contribution of GABA shunt to chilling tolerance in anthurium cut flowers in response to postharvest salicylic acid treatment. Sci Hortic 205:90–96
Ahmad S, Qazi F (2024) Deciphering the enigmatic role of gamma-aminobutyric acid (GABA) in plants: Synthesis, transport, regulation, signaling, and biological roles in interaction with growth regulators and abiotic stresses. Plant Phys Biochem. 208:108502
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Allan WL, Simpson JP, Clark SM, Shelp BJ (2008) γ-Hydroxybutyrate accumulation in Arabidopsis and tobacco plants is a general response to abiotic stress: putative regulation by redox balance and glyoxylate reductase isoforms. J Exp Bot 59(9):2555–2564
Allan WL, Clark SM, Hoover GJ, Shelp BJ (2009) Role of plant glyoxylate reductases during stress: a hypothesis. Biochem J 423(1):15–22
Ansari MI, Jalil SU, Ansari SA, Hasanuzzaman M (2021) GABA shunt: a key-player in mitigation of ROS during stress. Plant Growth Regul 94:131–149
Astegno A, Capitani G, Dominici P (2015) Functional roles of the hexamer organization of plant glutamate decarboxylase. Biochimica et Biophys Acta (BBA)-Proteins and Proteomics 1854(9):1229–1237
Barbier-Brygoo H, De Angeli A, Filleur S, Frachisse JM, Gambale F, Thomine S, Wege S (2011) Anion channels/transporters in plants: from molecular bases to regulatory networks. Annu Rev Plant Biol 62:25–51
Batushansky A, Kirma M, Grillich N, Pham PA, Rentsch D, Galili G, Fait A (2015) The transporter GAT1 plays an important role in GABA-mediated carbon-nitrogen interactions in Arabidopsis. Front Plant Sci 6:785
Baum G, Lev-Yadun S, Fridmann Y, Arazi T, Katsnelson H, Zik M, Fromm H (1996) Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J 15(12):2988–2996
Behera S, Xu Z, Luoni L, Bonza MC, Doccula FG, De Michelis MI, Costa A (2018) Cellular Ca2+ signals generate defined pH signatures in plants. Plant Cell 30(11):2704–2719
Bor M, Seckin B, Ozgur R, Yılmaz O, Ozdemir F, Turkan I (2009) Comparative effects of drought, salt, heavy metal and heat stresses on gamma-aminobutryric acid levels of sesame (Sesamum indicum L.). Acta Physiol Plant 31:655–659
Bouché N, Fait A, Bouchez D, Møller SG, Fromm H (2003) Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc Natl Acad Sci 100(11):6843–6848
Bouché N, Fait A, Zik M, Fromm H (2004) The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol Biol 55:315–325
Bouche N, Fromm H (2004) GABA in plants: just a metabolite?. Trends Plant Sci 9(3):110–115
Bown AW, Shelp BJ (2016) Plant GABA: not just a metabolite. Trends Plant Sci 21(10):811–813
Bown AW, Shelp BJ (2020) Does the GABA shunt regulate cytosolic GABA? Trends Plant Sci 25(5):422–424
Bown AW, Hall DE, MacGregor KB (2002) Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiol 129(4):1430–1434
Bown AW, MacGregor KB, Shelp BJ (2006) Gamma-aminobutyrate: defense against invertebrate pests? Trends Plant Sci 11(9):424–427
Breitkreuz KE, Shelp BJ, Fischer WN, Schwacke R, Rentsch D (1999) Identification and characterization of GABA, proline and quaternary ammonium compound transporters from Arabidopsis thaliana. FEBS Lett 450(3):280–284
Brikis CJ, Zarei A, Chiu GZ, Deyman KL, Liu J, Trobacher CP, Shelp BJ (2018) Targeted quantitative profiling of metabolites and gene transcripts associated with 4-aminobutyrate (GABA) in apple fruit stored under multiple abiotic stresses. Hortic Res. https://doi.org/10.1038/s41438-018-0069-3
Bush DR (1993) Inhibitors of the proton-sucrose symport. Arch Biochem Biophys 307(2):355–360
Carillo P (2018) GABA shunt in durum wheat. Front Plant Sci 9:100
Chang W, Liu X, Zhu J, Fan W, Zhang Z (2016) An aquaporin gene from halophyte Sesuvium portulacastrum, SpAQP1, increases salt tolerance in transgenic tobacco. Plant Cell Rep 35:385–395
Chen W, Meng C, Ji J, Li MH, Zhang X, Wu Y, Shi S (2020) Exogenous GABA promotes adaptation and growth by altering the carbon and nitrogen metabolic flux in poplar seedlings under low nitrogen conditions. Tree Physiol 40(12):1744–1761
Cheng D, Tan M, Yu H, Li L, Zhu D, Chen Y, Jiang M (2018) Comparative analysis of Cd-responsive maize and rice transcriptomes highlights Cd co-modulated orthologs. BMC Genomics 19:1–15
Che-Othman MH, Jacoby RP, Millar AH, Taylor NL (2020) Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol 225(3):1166–1180
Chevrot R, Rosen R, Haudecoeur E, Cirou A, Shelp BJ, Ron E, Faure D (2006) GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci 103(19):7460–7464
Coleman ST, Fang TK, Rovinsky SA, Turano FJ, Moye-Rowley WS (2001) Expression of a glutamate decarboxylase homologue is required for normal oxidative stress tolerance in Saccharomyces cerevisiae. J Biol Chem 276(1):244–250
Copley TR, Aliferis KA, Kliebenstein DJ, Jabaji SH (2017) An integrated RNAseq-1 H NMR metabolomics approach to understand soybean primary metabolism regulation in response to Rhizoctonia foliar blight disease. BMC Plant Biol 17:1–18
Corpas FJ, Del Río LA, Palma JM (2019) Plant peroxisomes at the crossroad of NO and H2O2 metabolism. J Integr Plant Biol 61(7):803–816
Daş ZA, Dimlioğlu G, Bor M, Özdemir F (2016) Zinc induced activation of GABA-shunt in tobacco (Nicotiana tabaccum L.). Environ Exp Bot 122:78–84
Demidchik V, Shabala S, Isayenkov S, Cuin TA, Pottosin I (2018) Calcium transport across plant membranes: mechanisms and functions. New Phytol 220(1):49–69
Dubey S, Misra P, Dwivedi S, Chatterjee S, Bag SK, Mantri S, Tuli R (2010) Transcriptomic and metabolomic shifts in rice roots in response to Cr (VI) stress. BMC Genomics 11(1):1–19
Dubey, R. S., Srivastava, R. K., & Pessarakli, M. (2021). Physiological mechanisms of nitrogen absorption and assimilation in plants under stressful conditions. In Handbook of Plant and Crop Physiology (pp. 579–616). CRC Press.
Dulermo T, Rascle C, Chinnici G, Gout E, Bligny R, Cotton P (2009) Dynamic carbon transfer during pathogenesis of sunflower by the necrotrophic fungus Botrytis cinerea: from plant hexoses to mannitol. New Phytol 183(4):1149–1162
Dündar E, Bush DR (2009) BAT1, a bidirectional amino acid transporter in Arabidopsis. Planta 229:1047–1056
Eisenach C, Papanatsiou M, Hillert EK, Blatt MR (2014) Clustering of the K+ channel GORK of Arabidopsis parallels its gating by extracellular K+. Plant J 78(2):203–214
Fait A, Yellin A, Fromm H (2005) GABA shunt deficiencies and accumulation of reactive oxygen intermediates: insight from Arabidopsis mutants. FEBS Lett 579(2):415–420
Fait A, Fromm H, Walter D, Galili G, Fernie AR (2008) Highway or byway: the metabolic role of the GABA shunt in plants. Trends Plant Sci 13(1):14–19
Fait A, Nesi AN, Angelovici R, Lehmann M, Pham PA, Song L, Fernie AR (2011) Targeted enhancement of glutamate-to-γ-aminobutyrate conversion in Arabidopsis seeds affects carbon-nitrogen balance and storage reserves in a development-dependent manner. Plant Physiol 157(3):1026–1042
Forlani G, Bertazzini M, Giberti S (2014) Differential accumulation of γ-aminobutyric acid in elicited cells of two rice cultivars showing contrasting sensitivity to the blast pathogen. Plant Biol 16(6):1127–1132
Fromm H (2020) GABA signaling in plants: targeting the missing pieces of the puzzle. J Exp Bot 71(20):6238–6245
Gilliham M, Tyerman SD (2016) Linking metabolism to membrane signaling: the GABA–malate connection. Trends Plant Sci 21(4):295–301
Gong Z, Xiong L, Shi H, Yang S, Herrera-Estrella LR, Xu G, Zhu JK (2020) Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci 63:635–674
Hatmi S, Gruau C, Trotel-Aziz P, Villaume S, Rabenoelina F, Baillieul F, Aziz A (2015) Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to Botrytis cinerea. J Exp Bot 66(3):775–787
Ji J, Shi Z, Xie T, Zhang X, Chen W, Du C, Shi S (2020) Responses of GABA shunt coupled with carbon and nitrogen metabolism in poplar under NaCl and CdCl2 stresses. Ecotoxicol Environ Saf 193:110322
Jiang K, Yin Z, Zhou P, Guo H, Huang C, Zhang G, Ou J (2020) The scavenging capacity of γ-aminobutyric acid for acrolein and the cytotoxicity of the formed adduct. Food Funct 11(9):7736–7747
Jones JD, Dangl JL (2006) The plant immune system. Nature 444(7117):323–329
Kaspal M, Kanapaddalagamage MH, Ramesh SA (2021) Emerging roles of γ aminobutyric acid (GABA) gated channels in plant stress tolerance. Plants 10(10):2178
Khan MIR, Jalil SU, Chopra P, Chhillar H, Ferrante A, Khan NA, Ansari MI (2021) Role of GABA in plant growth, development and senescence. Plant Gene 26:100283
Kou S, Chen L, Tu W, Scossa F, Wang Y, Liu J, Xie C (2018) The arginine decarboxylase gene ADC 1, associated to the putrescine pathway, plays an important role in potato cold-acclimated freezing tolerance as revealed by transcriptome and metabolome analyses. Plant J 96(6):1283–1298
Kumar N, Dubey AK, Upadhyay AK, Gautam A, Ranjan R, Srikishna S, Mallick S (2017) GABA accretion reduces Lsi-1 and Lsi-2 gene expressions and modulates physiological responses in Oryza sativa to provide tolerance towards arsenic. Sci Rep 7(1):8786
Lang J, Gonzalez-Mula A, Taconnat L, Clement G, Faure D (2016) The plant GABA signaling downregulates horizontal transfer of the Agrobacterium tumefaciens virulence plasmid. New Phytol 210(3):974–983
Li Z, Yu J, Peng Y, Huang B (2016) Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci Rep 6(1):30338
Li Z, Cheng B, Zeng W, Zhang X, Peng Y (2020) Proteomic and metabolomic profilings reveal crucial functions of γ-aminobutyric acid in regulating ionic, water, and metabolic homeostasis in creeping bentgrass under salt stress. J Proteome Res 19(2):769–780
Li L, Dou N, Zhang H, Wu C (2021) The versatile GABA in plants. Plant Signal Behav 16(3):1862565
Liao J, Shen Q, Li R, Cao Y, Li Y, Zou Z, Zhu X (2021) GABA shunt contribution to flavonoid biosynthesis and metabolism in tea plants (Camellia sinensis). Plant Physiol Biochem 166:849–856
Liu H, Coulthurst SJ, Pritchard L, Hedley PE, Ravensdale M, Humphris S, Toth IK (2008) Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathog 4(6):e1000093
Liu P, Wu X, Gong B, Lü G, Li J, Gao H (2022) Review of the mechanisms by which transcription factors and exogenous substances regulate ROS metabolism under abiotic stress. Antioxidants 11(11):2106
Locy, R. D., Wu, S. J., Bisnette, J., Barger, T. W., McNabb, D., Zik, M., & Cherry, J. H. (2000). The regulation of GABA accumulation by heat stress in Arabidopsis. Plant Tolerance to Abiotic Stresses in Agriculture: Role of Genetic Engineering, 39–52.
Long Y, Tyerman SD, Gilliham M (2020) Cytosolic GABA inhibits anion transport by wheat ALMT1. New Phytol 225(2):671–678
Ma D, Lu P, Shi Y (2013) Substrate selectivity of the acid-activated glutamate/γ-aminobutyric acid (GABA) antiporter GadC from Escherichia coli. J Biol Chem 288(21):15148–15153
Ma H, Cao X, Shi S, Li S, Gao J, Ma Y, Chen Q (2016) Genome-wide survey and expression analysis of the amino acid transporter superfamily in potato (Solanum tuberosum L.). Plant Physiol Biochem 107:164–177
Majumdar R, Minocha R, Lebar MD, Rajasekaran K, Long S, Carter-Wientjes C, Cary JW (2019) Contribution of maize polyamine and amino acid metabolism toward resistance against Aspergillus flavus infection and aflatoxin production. Front Plant Sci 10:692
Malekzadeh P, Khara J, Heydari R (2014) Alleviating effects of exogenous Gamma-aminobutiric acid on tomato seedling under chilling stress. Physiol Mol Biol Plants 20:133–137
Mazzucotelli E, Tartari A, Cattivelli L, Forlani G (2006) Metabolism of γ-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J Exp Bot 57(14):3755–3766
McCraw SL, Park DH, Jones R, Bentley MA, Rico A, Ratcliffe RG, Preston GM (2016) GABA (γ-aminobutyric acid) uptake via the GABA permease GabP represses virulence gene expression in Pseudomonas syringae pv. tomato DC3000. Mol Plant-Microbe Interact 29(12):938–949
Mekonnen DW, Flügge UI, Ludewig F (2016) Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci 245:25–34
Michaeli S, Fromm H (2015) Closing the loop on the GABA shunt in plants: are GABA metabolism and signaling entwined? Front Plant Sci 6:419
Michaeli S, Fait A, Lagor K, Nunes-Nesi A, Grillich N, Yellin A, Fromm H (2011) A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J 67(3):485–498
Missihoun TD, Schmitz J, Klug R, Kirch HH, Bartels D (2011) Betaine aldehyde dehydrogenase genes from Arabidopsis with different sub-cellular localization affect stress responses. Planta 233:369–382
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63(1):431–450
Mithöfer A, Wanner G, Boland W (2005) Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Phys 137:1160–1168
Nayyar H, Kaur R, Kaur S, Singh R (2014) γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J Plant Growth Regul 33:408–419
O’Leary BM, Neale HC, Geilfus CM, Jackson RW, Arnold DL, Preston GM (2016) Early changes in apoplast composition associated with defence and disease in interactions between Phaseolus vulgaris and the halo blight pathogen Pseudomonas syringae Pv. phaseolicola. Plant, Cell Environ 39(10):2172–2184
Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114(1):47–59
Palma F, Carvajal F, Ramos JM, Jamilena M, Garrido D (2015) Effect of putrescine application on maintenance of zucchini fruit quality during cold storage: contribution of GABA shunt and other related nitrogen metabolites. Postharvest Biol Technol 99:131–140
Palmer AJ, Baker A, Muench SP (2016) The varied functions of aluminium-activated malate transporters–much more than aluminium resistance. Biochem Soc Trans 44(3):856–862
Pang S, Qi SZ, Ran ZJ, Song XY, Li XF, Wang CJ, Duan LS (2013) Synergistic effect of gamma-aminobutyric acid with avermectin on Bombyx mori. J Food Agric Environ 11(1):1022–1024
Park DH, Mirabella R, Bronstein PA, Preston GM, Haring MA, Lim CK, Schuurink RC (2010) Mutations in γ-aminobutyric acid (GABA) transaminase genes in plants or Pseudomonas syringae reduce bacterial virulence. Plant J 64(2):318–330
Planamente S, Mondy S, Hommais F, Vigouroux A, Moréra S, Faure D (2012) Structural basis for selective GABA binding in bacterial pathogens. Mol Microbiol 86(5):1085–1099
Podlešáková K, Ugena L, Spíchal L, Doležal K, De Diego N (2019) Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol 48:53–65
Priya M, Sharma L, Kaur R, Bindumadhava H, Nair RM, Siddique KHM, Nayyar H (2019) GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci Rep 9(1):7788
Puyang X, An M, Xu L, Han L, Zhang X (2015) Antioxidant responses to waterlogging stress and subsequent recovery in two Kentucky bluegrass (Poa pratensis L.) cultivars. Acta Phys Plant 37:1–12
Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Gilliham M (2015) GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun 6(1):7879
Ramesh SA, Tyerman SD, Gilliham M, Xu B (2017) γ-Aminobutyric acid (GABA) signalling in plants. Cell Mol Life Sci 74:1577–1603
Ramesh SA, Kamran M, Sullivan W, Chirkova L, Okamoto M, Degryse F, Tyerman SD (2018) Aluminum-activated malate transporters can facilitate GABA transport. Plant Cell 30(5):1147–1164
Ramos-Ruiz R, Martinez F, Knauf-Beiter G (2019) The effects of GABA in plants. Cogent Food Agric 5(1):1670553
Ramputh AI, Bown AW (1996) Rapid [gamma]-aminobutyric acid synthesis and the inhibition of the growth and development of oblique-banded leaf-roller larvae. Plant Physiol 111(4):1349–1352
Renault H, Roussel V, El Amrani A, Arzel M, Renault D, Bouchereau A, Deleu C (2010) The Arabidopsis pop2-1mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol 10(1):1–16
Rico A, Preston GM (2008) Pseudomonas syringae pv. tomato DC3000 uses constitutive and apoplast-induced nutrient assimilation pathways to catabolize nutrients that are abundant in the tomato apoplast. Mol Plant-Microbe Interact 21(2):269–282
Ruperti B, Botton A, Populin F, Eccher G, Brilli M, Quaggiotti S, Meggio F (2019) Flooding responses on grapevine: a physiological, transcriptional, and metabolic perspective. Front Plant Sci 10:339
Saito S, Uozumi N (2019) Guard cell membrane anion transport systems and their regulatory components: an elaborate mechanism controlling stress-induced stomatal closure. Plants 8(1):9
Salah A, Zhan M, Cao C, Han Y, Ling L, Liu Z, Jiang Y (2019) γ-Aminobutyric acid promotes chloroplast ultrastructure, antioxidant capacity, and growth of waterlogged maize seedlings. Sci Rep 9(1):484
Scholz SS, Reichelt M, Mekonnen DW, Ludewig F, Mithöfer A (2015) Insect herbivory-elicited GABA accumulation in plants is a wound-induced, direct, systemic, and jasmonate-independent defense response. Front Plant Sci 6:1128
Scholz SS, Malabarba J, Reichelt M, Heyer M, Ludewig F, Mithöfer A (2017) Evidence for GABA-induced systemic GABA accumulation in Arabidopsis upon wounding. Front Plant Sci 8:388
Seifi HS, Curvers K, De Vleesschauwer D, Delaere I, Aziz A, Höfte M (2013) Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the ABA-deficient sitiens mutant of tomato leads to resistance against Botrytis cinerea. New Phytol 199(2):490–504
Seybold H, Trempel F, Ranf S, Scheel D, Romeis T, Lee J (2014) Ca2+ signalling in plant immune response: from pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol 204(4):782–790
Shabala L, Zhang J, Pottosin I, Bose J, Zhu M, Fuglsang AT, Shabala S (2016) Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol 172(4):2445–2458
Shan Q, Liu M, Li R, Shi Q, Li Y, Gong B (2022) γ-Aminobutyric acid (GABA) improves pesticide detoxification in plants. Sci Total Environ 835:155404
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4(11):446–452
Shelp BJ, Bown AW, Faure D (2006) Extracellular γ-aminobutyrate mediates communication between plants and other organisms. Plant Physiol 142(4):1350–1352
Shelp BJ, Bozzo GG, Trobacher CP, Zarei A, Deyman KL, Brikis CJ (2012) Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci 193:130–135
Shelp BJ, Aghdam MS, Flaherty EJ (2021) γ-Aminobutyrate (GABA) regulated plant defense: Mechanisms and opportunities. Plants 10(9):1939
Signorelli S, Monza J (2017) Identification of Δ1-pyrroline 5-carboxylate synthase (P5CS) genes involved in the synthesis of proline in Lotus japonicus. Plant Signal Behav 12(11):e1367464
Snowden CJ, Thomas B, Baxter CJ, Smith JAC, Sweetlove LJ (2015) A tonoplast Glu/Asp/GABA exchanger that affects tomato fruit amino acid composition. Plant J 81(5):651–660
Steward FC (1949) γ-Aminobutyric acid: a constituent of potato tubers? Science 110:439–440
Su N, Wu Q, Chen J, Shabala L, Mithöfer A, Wang H, Shabala S (2019) GABA operates upstream of H+-ATPase and improves salinity tolerance in Arabidopsis by enabling cytosolic K+ retention and Na+ exclusion. J Exp Bot 70(21):6349–6361
Sulieman S, Schulze J (2010) Phloem-derived γ-aminobutyric acid (GABA) is involved in upregulating nodule N2 fixation efficiency in the model legume Medicago truncatula. Plant, Cell Environ 33(12):2162–2172
Sun C, Jin L, Cai Y, Huang Y, Zheng X, Yu T (2019) L-Glutamate treatment enhances disease resistance of tomato fruit by inducing the expression of glutamate receptors and the accumulation of amino acids. Food Chem 293:263–270
Takahashi Y, Sasanuma T, Abe T (2013) Accumulation of gamma-aminobutyrate (GABA) caused by heat-drying and expression of related genes in immature vegetable soybean (edamame). Breed Sci 63(2):205–210
Tarkowski ŁP, Signorelli S, Höfte M (2020) γ-Aminobutyric acid and related amino acids in plant immune responses: emerging mechanisms of action. Plant, Cell Environ 43(5):1103–1116
Tian Q, Zhang X, Ramesh S, Gilliham M, Tyerman SD, Zhang WH (2014) Ethylene negatively regulates aluminium-induced malate efflux from wheat roots and tobacco cells transformed with TaALMT1. J Exp Bot 65(9):2415–2426
Tian R, Yang Y, Chen M (2020) Genome-wide survey of the amino acid transporter gene family in wheat (Triticum aestivum L.): Identification, expression analysis and response to abiotic stress. Int J Biol Macromol 162:1372–1387
Tiwari S, Lata C (2018) Heavy metal stress, signaling, and tolerance due to plant-associated microbes: an overview. Front Plant Sci 9:452
Toubiana D, Sade N, Liu L, Rubio Wilhelmi MDM, Brotman Y, Luzarowska U, Blumwald E (2020) Correlation-based network analysis combined with machine learning techniques highlight the role of the GABA shunt in Brachypodium sylvaticum freezing tolerance. Sci Rep 10(1):4489
Virdi AS, Singh S, Singh P (2015) Abiotic stress responses in plants: roles of calmodulin-regulated proteins. Front Plant Sci 6:809
Wallace W, Secor J, Schrader LE (1984) Rapid accumulation of γ-aminobutyric acid and alanine in soybean leaves in response to an abrupt transfer to lower temperature, darkness, or mechanical manipulation. Plant Physiol 75(1):170–175
Wang Y, Luo Z, Mao L, Ying T (2016) Contribution of polyamines metabolism and GABA shunt to chilling tolerance induced by nitric oxide in cold-stored banana fruit. Food Chem 197:333–339
Wang F, Chen ZH, Liu X, Colmer TD, Shabala L, Salih A, Shabala S (2017a) Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J Exp Bot 68(12):3191–3204
Wang Y, Gu W, Meng Y, Xie T, Li L, Li J, Wei S (2017b) γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci Rep 7(1):43609
Wang P, Liu K, Gu Z, Yang R (2018) Enhanced γ-aminobutyric acid accumulation, alleviated componential deterioration and technofunctionality loss of germinated wheat by hypoxia stress. Food Chem 269:473–479
Wang G, Kong J, Cui D, Zhao H, Niu Y, Xu M, Wang W (2019) Resistance against Ralstonia solanacearum in tomato depends on the methionine cycle and the γ-aminobutyric acid metabolic pathway. Plant J 97(6):1032–1047
Wei Q, Xie K, Wang H, Shao X, Wei Y, Chen Y, Xu F (2023) Calcium involved in the enrichment of γ-Aminobutyric acid (GABA) in broccoli sprouts under fructose treatment. Plants 12(2):224
Wipf D, Ludewig U, Tegeder M, Rentsch D, Koch W, Frommer WB (2002) Conservation of amino acid transporters in fungi, plants and animals. Trends Biochem Sci 27(3):139–147
Woodrow P, Ciarmiello LF, Annunziata MG, Pacifico S, Iannuzzi F, Mirto A, Carillo P (2017) Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol Plant 159(3):290–312
Xu B, Long Y, Feng X, Zhu X, Sai N, Chirkova L, Gilliham M (2021a) GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat Commun 12(1):1952
Xu B, Sai N, Gilliham M (2021b) The emerging role of GABA as a transport regulator and physiological signal. Plant Physiol 187(4):2005–2016
Xu M, Yang Q, Bai G, Li P, Yan J (2022) Polyamine pathways interconnect with GABA metabolic processes to mediate the low-temperature response in plants. Front Plant Sci 13:1035414
Xu, B., Long, Y., Feng, X., Zhu, X., Sai, N., Chirkova, L., & Gilliham, M. (2019). GABA signalling in guard cells acts as a ‘stress memory’to optimise plant water loss. bioRxiv, 2019–12.
Yang J, Sun C, Zhang Y, Fu D, Zheng X, Yu T (2017) Induced resistance in tomato fruit by γ-aminobutyric acid for the control of alternaria rot caused by Alternaria alternata. Food Chem 221:1014–1020
Yang G, Wei Q, Huang H, Xia J (2020) Amino acid transporters in plant cells: a brief review. Plants 9(8):967
Yao X, Nie J, Bai R, Sui X (2020) Amino acid transporters in plants: Identification and function. Plants 9(8):972
Yap KL, Yuan T, Mal TK, Vogel HJ, Ikura M (2003) Structural basis for simultaneous binding of two carboxy-terminal peptides of plant glutamate decarboxylase to calmodulin. J Mol Biol 328(1):193–204
Yin Y, Cheng C, Fang W (2018) Effects of the inhibitor of glutamate decarboxylase on the development and GABA accumulation in germinating fava beans under hypoxia-NaCl stress. Royal Soc Chem Adv 8(36):20456–20461
Yogeswara IBA, Maneerat S, Haltrich D (2020) Glutamate decarboxylase from lactic acid bacteria—A key enzyme in GABA synthesis. Microorganisms 8(12):1923
Yong B, Xie H, Li Z, Li YP, Zhang Y, Nie G, Peng Y (2017) Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front Physiol 8:1107
Yu P, Ren Q, Wang X, Huang X (2019) Enhanced biosynthesis of γ-aminobutyric acid (GABA) in Escherichia coli by pathway engineering. Biochem Eng J 141:252–258
Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Pei ZM (2014) OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514(7522):367–371
Yuan D, Wu X, Gong B, Huo R, Zhao L, Li J, Gao H (2023) GABA Metabolism, transport and their roles and mechanisms in the regulation of abiotic stress (hypoxia, salt, drought) resistance in plants. Metabolites 13(3):347
Zarei A, Brikis CJ, Bajwa VS, Chiu GZ, Simpson JP, DeEll JR, Shelp BJ (2017) Plant glyoxylate/succinic semialdehyde reductases: comparative biochemical properties, function during chilling stress, and subcellular localization. Front Plant Sci 8:1399
Zhang J, Zhang Y, Du Y, Chen S, Tang H (2011) Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J Proteome Res 10(4):1904–1914
Zhang X, Lin HM, Hu H, Hu X, Hu L (2016) Gamma-aminobutyric acid mediates nicotine biosynthesis in tobacco under flooding stress. Plant Diversity 38(1):53–58
Zheng J, Zhang Z, Zhang N, Liang Y, Gong Z, Wang J, Li X (2024) Identification and function analysis of GABA branch three gene families in the cotton related to abiotic stresses. BMC Plant Biol 24(1):57
Zhu X, Liao J, Xia X, Xiong F, Li Y, Shen J, Fang W (2019) Physiological and iTRAQ-based proteomic analyses reveal the function of exogenous γ-aminobutyric acid (GABA) in improving tea plant (Camellia sinensis L.) tolerance at cold temperature. BMC Plant Biol 19(1):1–20
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Author: Ravi Gupta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdullah, Wani, K.I., Naeem, M. et al. From Neurotransmitter to Plant Protector: The Intricate World of GABA Signaling and its Diverse Functions in Stress Mitigation. J Plant Growth Regul (2024). https://doi.org/10.1007/s00344-024-11470-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-024-11470-0