Abstract
It is important to find methods, which may improve corn physiology, including nitrogen (N) metabolism, and yield production under drought stress. The use of plant growth regulators (PGR) is among the methods, which has been found effective on the alleviation of drought stress on corn physiology and yield. However, in this research, some new PGR, which has been rarely investigated and may improve plant nitrogen (N) under drought stress including (A) control, (B) 6-benzyl adenine (10,000 mg/L), (C) proline (2.5 ml/L), (D) glutamine (1 ml/L), (E) B + C, (F) B + D, (G) B + C + D, and (H) superoxide dismutase (2.5 ml/L) were proposed and examined on corn (genotype Single Cross 640) physiology and yield components under field conditions. The experiment was a split plot on the basis of a completely randomized block design with three replicates, and in addition to PGR (subplots) the main plots (drought stress) based on 70 (D1), 90 (D2) and 110 mm (D3) of evaporation from an evaporating pan were examined. Different corn physiology- and yield-related components including relative water (RW) and proline contents (Pro), weight of 100 grains (100GW), number of grains per corn (NGC), biological yield (BY), corn fresh yield (CFY), and grain yield (GY) were determined. According to the results, corn physiology and yield components were significantly affected by drought stress as Pro increased and RW and yield-related components decreased. However, interestingly the use of PGR (treatment G) significantly improved corn physiology and yield components by increasing RW (to a maximum of 63.81%), CFY (from a minimum of 80,542 kg/ha at control to a maximum of 100,263 kg/ha), and BY (from a minimum of 49,842 kg/ha at control to a maximum of 62,277 kg/ha). Although the effect of PGR was not statistically significant on GY, treatment G resulted in a 2500-kg increase compared with control. The interaction of drought stress and PGR significantly affected different corn physiology- and yield-related components except NGC and BY. The most effective PGR treatment on the alleviation of drought stress on corn physiology and yield production was treatment G containing 6-benzyl adenine, proline, and glutamine. It is possible to improve corn physiology and yield production under drought stress using the PGR tested in this research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant growth and yield production are negatively affected under drought stress. The tolerance of different crop plants differs under drought stress as some crop plants are more tolerant and some are semi-tolerant and some are sensitive. Plants by themselves can utilize different mechanisms including morphological (Mohammadi and Asadi-Gharneh 2018) and physiological alteration, to enhance their tolerance under drought stress. The following are among the most important mechanisms utilized by plants under drought stress: (1) morphological alteration such as rolling the leaves, (2) production of different osmoprotectants such as proline, (3) activation of antioxidants including the enzymatic and non-enzymatic molecules, (4) production and alteration of different plant hormones, (5) activation of stress responsive genes, (6) activation of different signaling pathways, (7) regulation of plant stomata activity, and (8) exudation of root metabolites affecting the microbial activity as well as the symbiotic association of plant with the soil microbes (Sajedi et al. 2011; Miransari et al. 2011a, b; Khan et al. 2015; Kong et al. 2017; Nuccio et al. 2015; Miransari and Smith 2019).
The nitrogen metabolism is among the most important processes, which is negatively affected by drought stress, significantly decreasing plant growth and yield production. For example, Yang et al. (2018) indicated that under drought stress, while in corn (Zea mays L.) plants, the concentrations of simple sugars and polyunsaturated fatty acids increased, the concentration of amines, poly amines, and dipeptides decreased. Additionally, the following processes were among the most important responses of corn plants subjected to oxidative stress caused by drought: (1) activation of urea and glutathione cycles, and (2) production of carbohydrates and lipids resulting in the cellular osmo- and antioxidant protection (Yang et al. 2018). Although plants may utilize some mechanisms to alleviative the stress of drought on plant N metabolism, research has indicated that the exogenous use of plant products, which can influence plant morphology and physiology under stress, may also be a favorable method to alleviate stress on plant growth and yield production (Sami et al. 2016; Verma et al. 2016). Different plant growth regulators (PGR) have been examined on plant growth and activity under stress; however, we examined some PGR, which to our knowledge have not been previously much investigated including 6-benzyl adenine, proline, glutamine, and their combination as well as superoxide dismutase.
We recently proved it is possible to alter plant physiology (using PGR) in a way so that the environmental stresses including high temperature, drought, and cool conditions can be alleviated resulting in the enhanced plant growth and yield production (Tahaei et al. 2016; Shourbalal et al. 2019; Zamani et al. 2020). Accordingly, Shourbalal et al. (2019) indicated that the most suitable method to shorten vernalization in winter wheat and avoid the stresses of drought and cool conditions is the use of PGR including gibberellic acid (GA, 100 mg/l), kinetin (100 and 200 mg/l), and 6-benzyl adenine (BA6, 50 mg/l). Tahaei et al. (2016) found that using the same PGR it is possible to enhance the seed germination and seedling growth of Fennel (Foeniculum vulgare Mill).
With respect to the above-mentioned details and because there are not much data on the use of such PGR on corn growth under field drought stress, this research was proposed and conducted. The objective was to investigate how such PGR may affect corn physiology and yield components under drought stress.
Materials and Methods
Experimental Site
The experiment was conducted in the Research Field of Islamic Azad University (Isfahan Branch), Isfahan, Iran, with the northern latitude and eastern longitude of 32° 40′ and 51° 48′, respectively, 1570 m above the sea level, and with the yearly rainfall of 150 mm. Soil physical and chemical properties were analyzed to the depths of 0–30 and 30–60 cm by the Research Complex of Kavosh, Isfahan, Iran using the standard methods (Table 1) (Miransari et al. 2008). Accordingly, the field soil was not saline and was suitable for planting silage corn. Due to the relatively high amounts of available phosphorous (P) and potassium (K) in the soil, chemical fertilization was not applied. The analysis of the irrigation water using a well is also presented in Table 1.
Experimental Treatments
The corn seeds were planted in plots measuring 10 × 10 m using a seeder on the 21st of June 2016, with the seed and row distances of 10 and 75 cm, respectively. The experiment, conducted as a split plot on the basis of a completely randomized block design, investigated the effects of drought levels (main plots), established on the basis of evaporation from an evaporating pan, including control (D1, 70 mm), medium (D2, 90 mm), and severe (D3, 110 mm), and the foliar application (sprayed at flowering and at the time of photosynthates transfer to the grains) plant growth regulators (PGR) (subplots) including (A) control, (B) 6-benzyl adenine (10,000 mg/L), (C) proline (2.5 ml/L), (D) glutamine (1 ml/L), (E) B + C, (F) B + D, 9G) B + C + D, and 9H) superoxide dismutase (2.5 ml/L) on corn (genotype Single Cross 640) growth under field conditions. A total of 96 plots, including 4 replicates, with the side distance of 5.1 m between the plots to avoid the interaction of water treatment were used for the experiment. The drought treatments were initiated at the V2–V4 growth stage and the PGR treatments were imposed at two different growth stages including (1) transition from the vegetative to the productive stage, and (2) the transition stage (Fig. 1).
Sampling
The planting rows of 1, 2, 7, and 8 were randomly selected for sampling, ignoring the 1-m distance of the two sides of each row. The samples were collected at the V6–V8, V8–V12, the milky and the maturity stages, and different parameters including plant proline content (Pro), relative water (RW), number of grains per corn (NGC), weight of 100 grains (100GW), biological yield (BY), corn fresh yield (CFY), and grain yield (GY) were determined.
Relative Water Content (RW)
RW was measured according to the following. The leaf samples were randomly collected at 11–12 a.m. and were placed in paper bags containing dry ice, and were transferred to the lab. The fresh weight of the samples was weighed and then the samples were cut into 2-cm pieces and immersed in distilled water for 6–8 h at room temperature. The saturated weight of the samples was determined, and the samples were then oven dried at 70 °C for 72 h and the dry weight of the samples was determined. RW was calculated using the following formula:
Proline Content (Pro)
Proline content was measured according to the following (Bates et al. 1973). Sulfosalicylic acid was prepared by increasing the volume of 30 mL acid to the final volume of 1 L using distilled water. Ninhydrin indicator was prepared by dissolving and heating 1.25 g ninhydrin in 30 mL acetic acid and 20 mL phosphoric acid 6 M. The proline standard was prepared by dissolving 100 mg pure proline in 1 L distilled water, which was then used to prepare the standards of 1, 2, 5, 10, and 20 mg/L. Plant samples (0.5 g), which had been stored in a freezer, were smashed by a crucible, and were homogenized with 10 mL sulfosalicylic acid 3%. The solution was then centrifuged at 2000×g for 10 min, 2 mL of which was treated with 2 mL ninhydrin and 2 mL acetic acid. The tubes containing the solution were placed in a bain-marie with the temperature of 100 °C for one hour, and the reaction was terminated by putting the tubes in an ice bain-marie. The tubes were then mixed with 4 mL toluene for 30 s and the absorption of the color phase was determined by a spectrophotometer at the wavelength of 520 nm; proline concentration was calculated using the following formula:
Statistical Analyses
Data were subjected to analysis of variance and the significance of the experimental treatments and their interactions on the above-measured parameters were determined using SAS. Means were compared using Duncan’s multiple range test at P = 0.05. Using Proc Plot, the presented plots were drawn.
Results
Analysis of Variance
According to the analysis of variance, the effects of drought treatments were significant on different corn yield components excluding GY. However, PGR significantly affected RW, BY, and GFY. The interactions of irrigation and PGR were also significant on different corn components excluding NGC and BY (Table 2).
RW
Different drought treatments significantly affected plant RW, and the highest one was resulted by D1 (0.77 a), followed by D2 (1.05 b) and D3 (1.72 c). The effects of PGR were also significant on RW, and treatment G (benzyl adenine 6 + proline + Glutamine, 1.24 a) resulted in the highest RW higher than the other PGR treatments including treatment C (proline, 1.198 ab)) and treatment H (super oxide dismutase, 1.20 a) (Table 3).
Pro
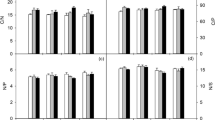
The effects of drought treatments significantly affected corn proline content (ranging from 32.16 at D1 to 63.36 mg/g fresh weight at D3) as D2 (58.24 g/g fresh weight) and D3 significantly increased this component, compared with D1. However, the effects of PGR were not significant on plant proline content ranging from 49.28 (B) to 53.30 g/g fresh weight (A). The interaction of drought treatments and PGR significantly affected plant proline content indicating that the effects of PGR on the alleviation of drought stress differ at different levels of drought stress (irrigation treatments) (Fig. 2a, Table 3).
The effects of stress and PGR on: a proline, and b the number of grains per corn; different letters indicate significant differences using Duncan’s multiple range test at P = 0.05. D1, D2, and D3 represent 70, 90, and 110 mm evaporation from the pan, respectively. a Control, b 6-benzyl adenine, c proline, d glutamine, e B + C, f B + D, g B + C + D, and h superoxide dismutase
NGC
The statistical analysis indicated the significant effects of irrigation treatments and PGR, and not their interaction, on NGC. D1 had the highest NGC (538.74a) followed by D2 (451.57b) and D3 (379.91c). The least and the highest NGC was resulted by A (414.1b) and G (517.8a), respectively (Fig. 2b, Table 3).
100GW
The effects of drought treatments including D1 (27.97 g a), D2 (23.70 g b), and D3 (20.05 g c) and its interaction with PGR were significant on the weight of 100 grains. However, the effects of PGR were not significant on the weight of 100 grains ranging from 22.03 g (A) to 25.70 g (G) (Fig. 3a, Table 3).
The effects of stress and PGR on: a the weight of 100 grains, and b grain fresh yield; different letters indicate significant differences using Duncan’s multiple range test at P = 0.05. D1, D2, and D3 represent 70, 90, and 110 mm evaporation from the pan, respectively. a Control, b 6-benzyl adenine, c proline, d glutamine, e B + C, f B + D, g B + C + D, and h superoxide dismutase
CFY
Corn fresh yield was also significantly affected by both the irrigation treatments and PGR as the highest grain fresh yield was resulted by D1 (109,048.0 kg/ha) significantly higher than D2 (92,695.0 kg/ha) and D3 (69,584.0 kg/ha). The use of PGR also significantly increased corn fresh yield from 80,542 kg/ha (A) to 100,263 kg/ha (G) (Fig. 3b, Table 3).
BY
The effects of irrigation treatments and PGR, and not their interaction, significantly affected corn biological yield, and D1 resulted in the highest biological yield (67,868.0 kg/ha) significantly higher than the other treatments including D2 (54,257.0 kg/ha) and D3 (48,107.0 kg/ha). However, PGR was able to significantly alleviate the drought stress by increasing corn biological yield from a minimum of 49,842 kg/ha (A) to a maximum of 62,277 kg/ha (G) (Fig. 4a, Table 3).
The effects of stress and PGR on a biological yield and b grain yield; different letters indicate significant differences using Duncan’s multiple range test at P = 0.05. D1, D2, and D3 represent 70, 90, and 110 mm evaporation from the pan, respectively. a control, b 6-benzyl adenine, c proline, d glutamine, e B + C, f B + D, g B + C + D, and h) superoxide dismutase
GY
The effects of irrigation treatments, ranging from 19,385.3 kg/ha (D3) to 20,178.7 kg/ha (D1), and PGR, ranging from 18,640 (A) to 21,139.0 kg/ha (G), were not significant on grain yield; however, their interaction significantly affected grain yield (Fig. 4b, Table 3).
Discussion
The alleviation of drought stress on plant growth and yield production may contribute to the increased food production, worldwide. In this research, the effects of different PGR, which has been rarely examined, on the physiology and yield of corn under drought stress was investigated. This has been in the continuation of our previous research in which the use of PGR on shortening vernalization in wheat and increasing seed germination in fennel was determined (Tahaei et al. 2016; Shourbalal et al. 2019). This research indicated that although the effects of PGR were not significant on grain yield, it resulted in an increase of 2500 kg/ha compared with control under drought stress. However, RW, BY, and CFY were significantly increased by PGR. This indicates PGR are more effective on the increase of plant fresh tissues, which is mainly by affecting plant N metabolism. In this research, the single and combined use of Pro (amino acid), Glu (amino acid), and BA6 (plant hormone) as well as the single use of SOD (antioxidant enzyme) were tested.
Alam et al. (2016) investigated the effects of exogenous proline (sprayed at the stages of vegetative and tasseling) on corn growth under salt stress. Although salinity stress significantly decreased plant growth and negatively affected plant biochemical properties, the exogenous use of proline improved corn growth under the stress. Accordingly, the use of proline at 25 mM significantly enhanced the growth of the stressed corn by increasing the ratio of K + /Na + and plant nutrient uptake, especially phosphorus.
The effects of PGR including paclobutrazol, uniconazole, propiconazole, and gibberellic acid were tested on the yield of maize plants subjected to the single or combined effects of drought stress, nitrogen deficiency, and high plant density (Stutts et al. 2018). They indicated the response of maize plants to alleviate the stress is by altering the hormonal balance affected by genotypic differences. The PGR were able to alleviate the single and the combined effects of the tested stresses by increasing maize seed yield; however, it was a function of the stress type, environment, and plant genotypic properties.
Stutts et al. (2018) suggested finding and altering the biochemical pathways, which regulate plant hormonal balance and subsequent plant response under stress, may be a favorite method for the production of resistant maize plants in different stresses. The research is also interesting because the tested PGR were able to make a balance between N metabolism (under N deficient conditions) and plant resistance when maize was planted at high number. It is because high N levels increase maize growth resulting in plant lodging and subsequent yield reduction. However, the PGR were able to make a hormonal balance, so N deficiency was alleviated and the plants were not lodged. Such a consequence may require further research on how the tested PGR are able to make such a biochemical balance.
Nitrogen metabolism is an important process, affecting plant tolerance under drought stress. However, N metabolism is negatively affected under drought stress and increased N uptake can enhance plant tolerance under drought stress. Wang et al. (2017) investigated the effects of N metabolism on maize tolerance under drought stress. The authors accordingly determined the expression of the genes regulating N uptake and assimilation, plant photosynthesis, and nutrient uptake in different maize tissues subjected to drought stress. The response of different tissues was significantly different under drought stress, as the stress significantly increased activation of root genes regulating N uptake and assimilation. Accordingly, such gene activities resulted in increased N uptake and quick accumulation of amino acids indicating that such root genes may increase maize tolerance under drought stress (Zhang et al. 2016; Kant, 2018).
The higher efficiency of the combined treatments tested in this research, compared with their single use, indicates that they are synergistic and not antagonistic and each PGR can alleviate some of the negative effects of drought stress on corn growth. When plant is N deficient, for example under drought stress, different signaling pathways are activated in plant to alleviate the stress among which the production of cytokinins is one of the most important ones. Such plant hormones are derived from adenine (in different parts of the plant) determining N availability (Gu et al. 2018). This may be considered as of the main reasons indicating why the use of 6-benzyl adenine can regulate N metabolism and subsequent plant growth under drought stress.
The absorption of BA6 by plant affects the molecular pathway of cytokinins and cellular division. The absorbed BA6, which is converted to some other organic molecules such as 6-benzylamino-9-glucopyranosylribosyl-purine, can affect the activity of plant cells and the subsequent plant growth by reducing the rate of internal cytokinins (Zhang et al. 2010). Similar to the exogenous use of proline, glutamine can also alleviate drought stress on corn physiology and yield components by stimulating plant N metabolism. The exogenous use of SOD can also regulate plant growth under stress by scavenging reactive oxygen species and our results indicate that such a molecule has also been efficient on the alleviation of the stress.
The main reason for the highest impact of the combined PGR is because the three tested amino acids are required for plant growth and metabolism, and N uptake by plant results in the production of such amino acids, which can (1) regulate plant metabolic activities under different conditions including stress, and (2) can have structural roles by being incorporated in different protein structures including enzymes (Stutts et al. 2018). Accordingly, the combination of the two tested PGR can be more efficient on the alleviation of stress than the single one.
Conclusion
The drought response of corn plants (corn physiology and yield components), under field conditions, as affected by different PGR including the single and combined use of N-containing molecules including proline, glutamine, 6-benzyl adenine as well as the single use of super oxide dismutase (antioxidant enzyme) was investigated. The main reasons for the selection of such PGR were due to the effects of drought stress on N metabolism and according to our recent research (Tahaei et al. 2016; Shourbalal et al. 2019). The results indicated that the stress significantly affected corn physiology (relative water content and proline) and yield components. However, the use of PGR, especially their combined use, significantly improved plant water content as well as corn fresh and biological yields under drought stress. Although the effects of PGR (their combined use) were not statically significant on corn grain yield under stress, they increased corn grain yield by 2500 kg/ha compared with control. Such molecules are able to alleviate drought stress on corn growth by the activation of the N-stimulating genes and metabolism-related signals, which can enhance corn tolerance under drought stress. Such molecules are of economic and environmental significance.
Data Availability
The authors are not allowed to share their data.
Abbreviations
- RW:
-
Relative water content
- Pro:
-
Proline content
- BA6:
-
6-Benzyl adenine
- Glu:
-
Glutamine
- SOD:
-
Super oxide dismutase
- 100GW:
-
Weight of 100 grains
- NGC:
-
Number of grains per corn
- BY:
-
Biological yield
- CFY:
-
Corn fresh yield
- GY:
-
Grain yield
References
Alam R, Das DK, Islam MR, Murata Y, Hoque MA (2016) Exogenous proline enhances nutrient uptake and confers tolerance to salt stress in maize (Zea mays L.). Progr Agric 27:409–417
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Gu J, Li Z, Mao Y, Struik PC, Zhang H, Liu L, Wang Z, Yang J (2018) Roles of nitrogen and cytokinin signals in root and shoot communications in maximizing of plant productivity and their agronomic applications. Plant Sci 274:320–331
Kant S (2018) Understanding nitrate uptake, signaling and remobilisation for improving plant nitrogen use efficiency. Semin Cell Dev Biol 74:89–96
Khan MIR, Asgher M, Fatma M, Per TS, Khan NA (2015) Drought stress vis a vis plant functions in the era of climate change. Clim Change Environ Sustai 3:13–25
Kong F, Zhang T, Liu J, Heng S, Shi Q, Zhang H, Wang Z, Ge L, Li P, Lu X, Li G (2017) Regulation of leaf angle by auricle development in maize. Mol Plant 10:516–519
Miransari M, Bahrami HA, Rejali F, Malakouti MJ (2008) Using arbuscular mycorrhiza to alleviate the stress of soil compaction on wheat (Triticum aestivum L.) growth. Soil Biol Biochem 40:1197–1206
Miransari M (2011a) Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl Microbiol Biotechnol 89:917–930
Miransari M (2011b) Soil microbes and plant fertilization. Appl Microbiol Biotechnol 92:875–885
Miransari M, Smith D (2019) Sustainable wheat (Triticum aestivum L.) production in saline fields: a review. Crit Rev Biotechnol 39:999–1014
Mohammadi M, Asadi-Gharneh HA (2018) How the morphological properties of Mentha longifolia (L.) Huds. may be affected by geographical differences. J Photochem Photobiol B 178:237–242
Nuccio ML, Wu J, Mowers R, Zhou HP, Meghji M, Primavesi LF, Paul MJ, Chen X, Gao Y, Haque E, Basu SS, Lagrimini LM (2015) Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat Biotechnol 33:862
Sajedi NA, Ardakani MR, Madani H, Naderi A, Miransari M (2011) The effects of selenium and other micronutrients on the antioxidant activities and yield of corn (Zea mays L.) under drought stress. Physiol Mol Biol Plants 17:215–222
Sami F, Yusuf M, Faizan M, Faraz A, Hayat S (2016) Role of sugars under abiotic stress. Plant Physiol Biochem 109:54–61
Shourbalal SKS, Soleymani A, Javanmard HR (2019) Shortening vernalization in winter wheat (Triticum aestivum L.) using plant growth regulators and cold stratification. J Clean Prod 219:443–450
Stutts L, Wang Y, Stapleton AE (2018) Plant growth regulators ameliorate or exacerbate abiotic, biotic and combined stress interaction effects on Zea mays kernel weight with inbred-specific patterns. Environ Exp Bot 147:179–188
Tahaei A, Soleymani A, Shams M (2016) Seed germination of medicinal plant, fennel (Foeniculum vulgare Mill), as affected by different priming techniques. Appl Biochem Biotechnol 180:26–40
Verma V, Ravindran P, Kumar PP (2016) Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16:86
Wang H, Yang Z, Yu Y, Chen S, He Z, Wang Y, Jiang L, Wang G, Yang C, Liu B, Zhang Z (2017) Drought enhances nitrogen uptake and assimilation in maize roots. Agron J 109:39–46
Yang L, Fountain JC, Ji P, Ni X, Chen S, Lee RD, Kemerait RC, Guo B (2018) Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant Biotechnol J 16:1616–1628
Zamani S, Naderi MR, Soleymani A, Nasiri BM (2020) Sunflower (Helianthus annuus L.) biochemical properties and seed components affected by potassium fertilization under drought conditions. Ecotoxicol Environ Saf 190:110017
Zhang H, Horgan KJ, Reynolds PH, Jameson PE (2010) 6-Benzyladenine metabolism during reinvigoration of mature Pinus radiata buds in vitro. Tree Physiol 30:514–526
Zhang X, Warburton ML, Setter T, Liu H, Xue Y, Yang N, Yan J, Xiao Y (2016) Genome-wide association studies of drought-related metabolic changes in maize using an enlarged SNP panel. Theoret Appl Genet 129:1449–1463
Funding
There was no funding for this research.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest.
Additional information
Handling editor: Rhonda Peavy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noein, B., Soleymani, A. Corn (Zea mays L.) Physiology and Yield Affected by Plant Growth Regulators Under Drought Stress. J Plant Growth Regul 41, 672–681 (2022). https://doi.org/10.1007/s00344-021-10332-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10332-3