Abstract
Finding methods, which may improve wheat (Triticum aestivum L.) growth and tolerance under drought stress, are of significance. The hypothesis was plant growth regulators (PGRs) are able to alter wheat physiology in a way so that the plant would be able to resist the stress. Accordingly, PGRs affecting wheat response in drought stress was investigated in greater details. Different PGRs, at different drought levels (80 (S1), 100- (S2) and 120-mm (S3) evaporation from pan class A) affecting wheat biochemical and nutritional properties including nutrient uptake (N, P, K) were tested. Experimental treatments including control (water, T1), and PGRs of gibberellic acid (GA3, 110 mgL−1, T2), salicylic acid (SA, 1.5 mM, T3), and benzyl adenine (60 mgL−1, T4), GA3 + SA (T5), as well as superoxide dismutase (SOD, 5 gL−1, T6) were tested in two different locations. Wheat biochemical and nutritional parameters including grain protein, chlorophyll a and b, leaf relative water at flowering and ripening, wet and dry gluten index, soluble sugars, and grain nutrient uptake were significantly enhanced by PGRs. The grain protein was in the range of 9.90 (S3T1) and 15.01% (S1T5); however, T5 treatment significantly enhanced it at the second (14.47%) and third (13.96%) level of drought. Although the single use of PGR was effective on the alleviation of stress, the combined use of GA3 + SA followed by the single use of SOD, were the most effective ones. Treating wheat plants with the tested PGRs is recommendable to improve wheat biochemical and nutritional properties in the arid and semi-arid areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.) is one of the most important crop plants, feeding a large number of people, worldwide. Wheat is a good source of different compounds including proteins, lipids, carbohydrates and nutrients being widely used by the industry for the production of a large number of nutritional products (Tripathi et al. 2016). Wheat, compared with other crop plants including barley, is not a tolerant plant under drought stress, and its growth and yield decreases (Jamshidi and Javanmard 2018; Daei Alhag et al. 2022).
Plant response under stress is defined by the two important terms of stress alleviation and stress adaptation. Stress alleviation is a collection of the processes resulted by different parameters including the use of plant growth regulators (PGRs), which decrease the negative effects of stress on plant growth by altering plant physiological mechanisms. However, stress adaptation is a collection of processes, which alter plant physiological processes, and make the plant adapt itself to the stress conditions (Mansour 2000; Kazemi et al. 2022).
Different methods have been so far used to enhance wheat growth and yield under drought stress including the use of tolerant wheat species, genetically modified plants, plant growth promoting rhizobacteria (PGPR), and efficient irrigation methods (Farooq et al. 2014; Miransari and Smith 2019; Senapati et al. 2019). However, another method, which has been used more recently, is the exogenous use of PGRs, which are natural or synthetic products (Shourbalal et al. 2019). It was accordingly hypothesized PGRs are able to affect plant physiology in a way so that the plant can tolerate the stress, more efficiently. The responses of crop plants to the type of stress and PGRs are determined by different parameters including climate and plant species (Baena-González and Hanson 2017; Kamran et al. 2018; Li et al. 2018).
PGRs are able to enhance plant growth in drought stress conditions by maintaining plant photosynthetic potential and enhancing its efficiency, improving plant antioxidant capacity, altering plant morphological and physiological properties, regulating the expression of drought stress genes and functional proteins, improving plant osmotic potential, and promoting plant nutrient uptake and metabolism (Cao et al. 2017; Tayyab et al. 2020).
Although there has been recent research on the use of PGRs for the alleviation of drought stress on plant growth (Shourbalal et al. 2019; Askarnejad et al. 2021; Khalafi et al. 2021), more has yet to be proposed and investigated on the type and amounts of PGRs essential for the improvement of wheat physiology and quality in drought stress conditions. The objective of the present research was to investigate the effects of PGRs including gibberellic acid (GA3), salicylic acid (SA), benzyl adenine, GA3 + SA, and the antioxidant enzyme, superoxide dismutase (SOD), on wheat biochemical and nutritional properties under field drought stress.
Materials and methods
Experimental site
The experiments were conducted in two different locations (province of Yazd, Iran), namely Abarkuh (E53° 14′ and N31° 7′) with the altitude of 1530 m, and Faragheh (E53° 0′ and N31° 3′), with the altitude of 1713 m. The climate of the region according to Yazd meteorological bureau is dry and hot with the annual rainfall of 75 mm (Table 1).
Experimental design
The experiments were split plot on the basis of a complete randomized block design with three replicates using Sistan wheat genotype. The main plots (drought levels at 80 (S1), 100 (S2), and 120 mm (S3) evaporation from pan class A) (Fischer and Maurer 1978), and subplots (spraying) including the experimental treatments of water (control, T1), plant growth regulators (PGRs) of gibberellic acid (GA3 at 110 mgL−1, T2), salicylic acid (SA at 1.5 mM, T3), benzyl adenine (BA6 at 60 mgL−1, T4), GA3 + SA (T5), as well as super oxide dismutase (SOD at 5 gL−1, T6) were tested (Shourbalal et al. 2019; Askarnejad et al. 2021). Soil physicochemical properties were determined using the standard methods (Table 2) (Miransari et al. 2008).
The plots (including a non-treated plot as control) measuring 3 × 4 m with the plant density of 400 were established in the fields (cultivated and disked), with a 2.5 m distance from the irrigation canals. The plots were irrigated until the tillering stage and were then treated according to the experimental treatments including spraying with the PGRs at two different stages of tillering and heading. Weeds were controlled using 2, 4-D.
Sampling and measurements
The plants were considered at the physiological maturity when 50% of the plots were matured. Plant were sampled by collecting 10 plants from each plot. Different plant physiological parameters including grain protein (Gpro, %), chlorophyll a (Chla) and b (Chlb) (mgg−1 fresh weight) (the 10 recently matured leaves at flowering), leaf relative water content (%) at flowering (RWC1) and ripening (RWC2), grain wet (GI1, %) and dry gluten (GI2, %), soluble sugars (SS, %) as well as grain nutrient uptake of nitrogen (N, %), phosphorous (P, %) and potassium (K, %) were determined.
Grain protein
Grain protein (%) was measured according to the protocol presented by international association of cereal chemistry (ICC # 159) (Williams et al. 2008).
Chlorophyll contents
Chlorophyll a and chlorophyll b were determined according to the following details; 0.5 g plant fresh leaf was smashed using liquid N and porcelain mortar, and was treated with 20 ml acetone 80%. The solution was then centrifuged at 6000 g for 10 min and the supernatant was transferred to a volumetric flask. The samples were measured using a spectrophotometer at the wavelengths of 663 nm for Chla and of 645 nm for Chlb. Finally, using the following formula Chla and b were calculated (Ghassemi-Golezani et al. 2020): \({\text{chlorophyll a}} = \left( {19.3*A663 - 0.86*A645} \right)V/100W\) , \({\text{chlorophyll b}} = \left( {{19}.{3}*A{645} - {3}.{6}*A{663}} \right)V/{1}00W\), in which V is the filtrated solution (the centrifuge supernatant), A is absorbance at the wavelengths of 663 and 645 nm, and W is sample fresh weight.
Leaf relative water content
The leaf samples at flowering and ripening were randomly collected from the flag leaves, and were placed in a plastic bag containing ice. The 2-cm pieces samples from the middle of the leaf samples were weighed using a digital weight. The pieces were then soaked in distilled water in Petri dishes. The surface of the samples was wiped out using a tissue and the turgor weight of each sample was determined. The samples were dried at 75 °C for 48 h using an oven and the dry weight of each sample was determined. Finally, RWC was calculated using the following formula (Regan et al. 1992).
Gluten index
Grain gluten (wet and dry) was measured according to the method by ICC # 158 (ICC 1995) using 10 g flour (14% moisture) and 5.5 ml of a buffer solution containing NaCl, KH2PO4, NaH2PO4, and distilled water. The gluten index was determined according to the ICC method using the following formula Q = (R − W)/R, in which Q is the gluten index, R is the total weight of dehydrated gluten, and W is the meshed gluten by the gluten washer machine.
Soluble sugars
Soluble sugars were determined by collecting plant extract using a 100 mM solution of bufferic K2PO4 (pH = 7.5). The upper solution was used to measure the total soluble sugars using anthrone-sulfuric acid, and plant extract in glass tubes (1:5 v/v) using a bath (100 °C) for 10 min. The absorbance of the cool down solution was measured at 650 nm. The standard solutions were prepared using the glucose concentrations of 1–18 mg (Magné et al. 2006).
N, P, and K measurements
The dried grain samples were analysed in the lab for the uptake of N, P and K (Askarnejad et al. 2021). N was measured according to the Kjeldahl method including the stages of digestion, distillation and titration (Concon and Soltess 1973), K was determined by atomic absorption spectrophotometer, and P was measured using spectrophotometer (Kalra 1997).
Statistical analysis
The homogeneity of error variance between the two regions was determined using the Bartlett’s test. The results indicated the Bartlett’s test was not significant for all traits, and therefore, the null hypothesis indicating the homogeneity of error variance between the two regions is verified. Accordingly, the combined analysis of the data for the two regions was conducted assuming the random effects for the two regions. Data were subjected to analysis of variance using SAS 9.3. The significance of the main and interaction effects of the experimental treatments including the experimental field, drought stress, PGR and SOD on the measured parameters was determined. Means were compared at P ≤ 0.05 by lsmeans and were presented with their related standard deviations. The boxplots, illustrating the effects of the experimental treatments on the measured parameters, were plotted using SAS Proc Plot.
Results
Analysis of variance
According to the analysis of variance (for the three-way factorial experiment), the effects of experimental treatments and some of their interactions were significant on the measured parameters including Gpro, Chla and b, RWC1 and RWC2, G1 and G2, SS, N, P and K (Table 3). Accordingly, the effects of location or the experimental field were significant on all the measured parameters except Chlb, G1 and K. However, stress and the experimental treatments significantly affected all the measured parameters. The interaction of location and stress were significant on Gpro, RWC1 and RWC2, and G1 and G2. The interaction of location and experimental treatments was just significant on K. The interaction of stress and treatment significantly affected all the measured parameters except Gpro, G1, SS and P. However, the triple interaction of location, stress and the experimental treatments was just significant on RWC2, G2 and N (Table 3).
Grain protein
The Gpro was in the range of 9.90 (S3T1) and 15.01% (S1T5); however, the T5 treatment also significantly enhanced Gpro at the second (14.47%) and third (13.96%) level of drought. The single use of SA (T3) was more effective on the alleviation of the stress than the single use of GA3 and BA6 (Table 4, Fig. 1).
a Grain protein (Gpro), b chlorophyll a (Chla), and c chlorophyll b (Chlb) affected by the interaction of stress levels including S1 (control, 80 mm), S2 (100 mm) and S3 (120 mm), and PGR treatments including T1 (control), T2 (gibberellic acid, GA3), T3 (salicylic acid, SA), T4 (benzyl adenine), and T5 (GA3 + SA), as well as T6 (superoxide dismutase). The boxes present the range of measured data including their median and their standard errors
Chlorophyll contents
Chla was the least by S3T1 (0.87 mgg−1 fresh weight) and the highest by S1T5 (1.71 mgg−1 fresh weight) and S2T5 (1.58 mgg−1 fresh weight), followed by the T6 and T3 treatments at different levels of drought. The Chlb parameter also followed the same trend. The single use of SA treatment was also effective on the alleviation of drought stress on Chla compared with the single use of the other treatments (Table 4, Fig. 1).
Leaf relative water content
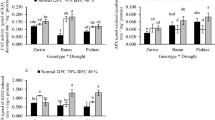
With increasing the level of stress, leaf relative water content decreased; however, the PGR and SOD treatments were able to alleviate the stress by increasing RWC1 and RWC2, as the least ones were related to treatments S1T1, S2T1 and S3T1 and the highest ones were related to treatments S1T5, S1T6, S2T5, S2T6, S3T5 and S3T6 (Table 4, Fig. 2).
Leaf relative water content at flowering (RWC1) and ripening (RWC2) affected by the interaction of stress levels including S1 (control, 80 mm), S2 (100 mm) and S3 (120 mm), and PGR treatments including T1 (control), T2 (gibberellic acid, GA3), T3 (salicylic acid, SA), T4 (benzyl adenine), and T5 (GA3 + SA), as well as T6 (superoxide dismutase). The boxes present the range of measured data including their median and their standard errors
Gluten index
Although the results indicated the significant effects of T5 and T6 on wet and dry gluten indexes, the single effects of SA and BA6 also significantly increased wet and dry gluten index compared with the control treatment. The least G1 value resulted by the S3T1 treatments was equal to 22.53%, and the highest ones were related to S3T5 (29.99%) and S3T6 (27.66%) treatments; the corresponding values for the T3 and T4 treatments were equal to 25.60% and 26.73%, respectively. Similarly, for G2 the highest values were related to the T5 and T6 treatments at different levels of drought levels, though the T3 and T4 treatments were also able to significantly enhance the G2 values (Table 4, Fig. 3).
a Grain soluble sugars (SS), b wet (G1) and c dry (G2) gluten index affected by the interaction of stress levels including S1 (control, 80 mm), S2 (100 mm) and S3 (120 mm), and PGR treatments including T1 (control), T2 (gibberellic acid, GA3), T3 (salicylic acid, SA), T4 (benzyl adenine), and T5 (GA3 + SA), as well as T6 (superoxide dismutase). The boxes present the range of measured data including their median and their standard errors
Soluble sugars
The SS values were the least at the highest level of drought, and they were not different at the first and the second level of drought. The control treatments resulted in the least, and the T5 and T6 treatments, followed by the T3 and T4 treatments resulted in the highest SS values (Table 4, Fig. 3).
Grain N, P and K uptake
The stress significantly decreased plant N uptake (S3T1 = 1.19%); however, the PGR and SOD treatments including S3T3 (1.51%), S3T4 (1.45%), S3T5 (1.90%) and S3T6 (1.66%) similar to the other levels of drought, significantly increased plant N uptake, compared with the control treatment. Similarly, the stress significantly decreased plant P uptake; however the PGR treatment including T5 as well as T6, followed by the T3 and the T4 treatments significantly increased plant P uptake. For example, the least and the highest P uptake values were related to treatments S3T1 (0.31%) and S1T5 (0.56%), and the corresponding values for S3T5 and S3T6 were equal to 0.50 and 0.46%, respectively. With increasing the stress level, grain K uptake decreased; however, the use of PGR and SOD significantly alleviated the stress by enhancing grain K uptake. The least and the highest K uptake values were related to the S3T1 (0.35%) and S1T5 (0.91%) treatments, respectively. The PGR treatments and SOD at the highest level of drought resulted in the K uptake of 0.69 (S3T5), 0.61 (S3T6), 0.55 (S3T3) and 0.53% (S3T4) (Table 4, Fig. 4).
Grain N, P and K uptake affected by the interaction of stress levels including S1 (control, 80 mm), S2 (100 mm) and S3 (120 mm), and PGR treatments including T1 (control), T2 (gibberellic acid, GA3), T3 (salicylic acid, SA), T4 (benzyl adenine), and T5 (GA3 + SA), as well as T6 (superoxide dismutase). The boxes present the range of measured data including their median and their standard errors
Discussion
The effects of different PGRs and SOD on the physiology and nutrient uptake of wheat plants under drought stress indicated that it is possible to alleviate the stress using such compounds. Although according to our results, T5 (GA3 + SA) was the most effective treatment on the alleviation of the stress, followed by T6 (SOD), the single effects of SA (T3) and BA6 (T4) also indicated positive effects significantly enhancing the biochemistry and nutrient uptake of wheat plants under the stress, which are in consistent with the results by Yadav et al. (2020) and Luo et al. (2020). The tested PGR contain nitrogen, which can significantly affect plant physiology including photosynthesis, enzymatic activities, and N content.
Moreover, the effects of the tested PGR on grain protein using SA and with a less extent GA3, are by affecting plant biochemical properties through cross-talking and inducing different plant physiological processes. Research has indicated the interesting effects of SA on plant growth and biochemistry under different conditions including stress (Maghsoudi et al. 2018; Chavoushi et al. 2019). Although the role of SA in the regulation of different plant physiological processes, including plant transpiration, photosynthesis, chlorophyll synthesis, and nutrient uptake, has been indicated by research, more has to be found on the effects of SA on the mechanisms, which regulate such processes. In our just published research, it has been indicated that plant genetics and micronutrient fertilization can significantly affect corn yield, physiology, and nutrient uptake in the arid and semi-arid areas of the world (Chinipardaz et al. 2022).
The following processes by SA are among the most important ones affecting plant growth and physiology under stress: (1) activation of antioxidant enzymes, (2) reducing the damage of lipid peroxidation, (3) increasing water and nutrient uptake, (4) inducing plant systemic resistance, (5) decreasing electrolyte leakage, (6) accumulation of amino acids including serine, proline, alanine, and threonine, (7) increased production of carbohydrates including fructose, glucose, cellobiose, and mannose, and (8) metabolic regulation (Miransari and Smith 2014; Dianat et al. 2016; Saha et al. 2016; Hernández-Ruiz and Arnao 2018; Patni and Ansari 2019). Similar to our research, Kang et al. (2012) found the positive effects of 0.5 mM SA on the growth and biochemical properties of wheat seedlings under drought stress (PEG-6000, 15%).
Our results also indicated the positive effects of GA on the alleviation of drought stress. The positive role of GA on the alleviation of drought stress on plant growth is by increasing the activity of glutamine synthetase and accumulating free amino acids. It also increases plant GA content. The hormone is accordingly able to enhance plant biochemical properties (N metabolism, grain protein and the activity of antioxidants) under drought stress by altering plant physiology and yield production (Yang et al. 2013; Abid et al. 2017).
The growth of wheat plants under drought stress, investigated by Deng et al. (2019), indicated the accumulation of proteins in the non-leaf green organs of wheat glume and awn can importantly regulate wheat photosynthesis and drought resistance. However, it has yet to be indicated if such proteins are induced by SA under drought stress. Accordingly, the proteins, regulating photosynthesis and carbon metabolism, were down-regulated, and the ones regulating detoxification and plant metabolisms were up-regulated. The results also indicated that such proteins can synergistically affect plant response to stress during grain production. Such results may indicate how the single use of SA or its combination with GA3 might have enhanced grain protein in our research. According to our results, PGRs and SOD increased soluble sugars under stress. Lee et al. (2019) investigated the effects of drought stress on canola (Brassica napus) physiology and found the positive effects of stress and SA on sugars accumulation.
The tested PGRs and SOD in our experiments increased chlorophyll contents in the stressed plants. Similarly, Damalas (2019) examined the effects of drought stress on the growth and physiology of basil (Ocimum basilicum L.), treated with 200 mgL−1 SA. Although water stress significantly decreased plant growth and biochemical properties, including Chla (by 23.9%) and Chlb (by 15.8%), SA alleviated such effects. The stress also significantly decreased leaf relative water content by 29.2%; however, SA significantly improved such a parameter. The increase in leaf relative water content by SA is due to the effects of the hormone on the uptake of different nutrients, especially K, which can regulate plant water potential by controlling different physiological activities including the stomata behavior (Zamani et al. 2020).
SA can improve chlorophyll levels and photosynthesis process in plant by maintaining the structural integrity of thylakoid membrane and protecting PSII from oxidative stress and high excitation, resulted by the increased ratio of FV/FM. The use of SA also decreased the production of H2O2 and malondialdehyde, and electrolyte leakage, which indicated the alleviating effects of SA in drought stress conditions. The increase of leaf relative water content during stress by SA also indicates the role of the hormone SA in enhancing the structural integrity and protection of cellular membrane (Zulfiqar et al. 2021). Another important role of PGRs and SOD, in photosynthesis process under drought stress is by affecting N metabolism (affecting chlorophyll synthesis), which is affected by different parameters including N, P, and K uptake (Qiao et al. 2019; Tahaei et al. 2022).
Although research has indicated the effects of PGR on the biochemical and nutritional properties of different crop plants, there is not much data on the use of the PGR including GA3, SA, BA6, and GA3 + SA as well as SOD tested in the present research. For example, research has indicated, the negative effects of drought stress on the rate of gluten, however, there is not any data on the use of PGR and SOD enhancing the amount and quality of gluten, which determine dough quality (Torbica et al. 2007) under drought stress. Noori (2018) found the positive effects of NPK on the amount of gluten. Accordingly, because the tested PGR and SOD can increase wheat nutrient uptake under drought stress, they can also affect gluten quality and amount. The single effects of GA3 and BA6 on the germination of the medicinal plant fennel (Foeniculum vulgare Mill), by affecting the physiology and the biochemical properties of the seeds, has been previously indicated by research (Tahaei et al. 2016).
SOD is among the antioxidant enzymes, affecting plant growth and biochemistry under stress. The enhanced production of the enzyme under stress increases plant ability to tolerate the stress. The antioxidant enzymes are able to scavenge the free radicals (reactive oxygen species), which are produced under stress, and damage the cellular membrane (Sajedi et al. 2010; 2011; Jiang et al. 2019; Zeeshan et al. 2020). According to our results, the exogenous application of SOD was the second most effective treatment, following T5 (GA3 + SA) significantly enhancing wheat biochemical and nutritional properties under the stress. Accordingly, SOD can be favorably used to alleviate drought stress on wheat physiology and nutrient uptake.
Kaouthar et al. (2016) found a new SOD gene, namely TdMnSOD in durum wheat, which can regulate plant response under different types of stress including salinity, drought, and cold. The results indicated that the transgenic Arabidopsis plants expressing TdMnSOD had little levels of H2O2, and the wild type plants had higher proline under stress. According to the results the level of SOD, catalase, and peroxidases increased in the transgenic species under abiotic stress. The authors accordingly indicated durum wheat TdMnSOD increases plant tolerance under different types of abiotic stress.
The positive effects of BA6 on drought stress in the present research were also shown. The BA6 plant growth regulator can affect plant growth under drought stress by affecting the cytokinin pathway (decreasing the production of cytokinin), which eventually enhances cellular division. The conversion of benzyl adenine into 6-benzylamino-9-glucopyranosylribosyl-purine and its novel phosphorylated form, 6-benzylamino-9-glucopyranosylribosyl-purine, influences different plant physiological and morphological properties by affecting cellular division (Zhang et al. 2010; Shourbalal et al. 2019).
Conclusion
The combined use of salicylic acid (SA) + gibberellic acid (GA3) was the most effective treatment, followed by the use of superoxide dismutase (SOD); however, the single use of SA and benzyl adenine (BA6) also significantly affected wheat biochemical and nutritional properties under drought stress, indicating the importance of such plant hormones for wheat growth under stress. The possible molecular mechanisms, which may increase wheat drought tolerance by PGRs and SOD, have been presented. Accordingly, the tested PGRs and SOD are able to alter wheat physiology, in drought stress conditions, so that the plant would be able to resist the stress. The treatment of wheat plants with the PGRs and SOD tested in the presented research is recommendable to improve wheat biochemical and nutritional properties, and subsequent wheat growth and yield in the arid and semi-arid areas.
Abbreviations
- PGRs:
-
Plant growth regulators
- GA:
-
Gibberellic acid
- SA:
-
Salicylic acid
- SOD:
-
Superoxide dismutase
- BA6:
-
Benzyl adenine
- Gpro:
-
Grain protein
- Chla:
-
Chlorophyll a
- chlb:
-
Chlorophyll b
- RWC1 and RWC2:
-
Leaf relative water content at flowering and ripening
References
Abid M, Shao Y, Liu S, Wang F, Gao J, Jiang D, Tian Z, Dai T (2017) Pre-drought priming sustains grain development under post-anthesis drought stress by regulating the growth hormones in winter wheat (Triticum aestivum L.). Planta 246:509–524
Askarnejad MR, Soleymani A, Javanmard HR (2021) Barley (Hordeum vulgare L.) physiology including nutrient uptake affected by plant growth regulators under field drought conditions. J Plant Nutr 44:2201–2217
Baena-González E, Hanson J (2017) Shaping plant development through the SnRK1–TOR metabolic regulators. Curr Opin Plant Biol 35:152–157
Cao MJ, Zhang YL, Liu X, Huang H, Zhou XE, Wang WL, Zeng A, Zhao CZ, Si T, Du J, Wu WW (2017) Combining chemical and genetic approaches to increase drought resistance in plants. Nat Commun 8:1183
Chavoushi M, Najafi F, Salimi A, Angaji SA (2019) Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind Crops Prod 134:168–176
Chinipardaz F, Babaienejad T, Gholami A, Barzegari M (2022) Grain yield and micronutrient concentrations of maize parental lines of new hybrid genotypes affected by the foliar application of micronutrients. Physiol Mol Biol Plants 28:411–424
Concon JM, Soltess D (1973) Rapid micro Kjeldahl digestion of cereal grains and other biological materials. Anal Biochem 53:35–41
Daei Alhag D, Rashidi V, Aharizad S, Farahvash F, Mirshekari B (2022) The traits affecting wheat grain yield and determining tolerant genotypes using drought indices. Cereal Res Commun 50:627–636
Damalas CA (2019) Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Sci Hortic 27:360–365
Deng X, Zhen S, Liu D, Liu Y, Li M, Liu N, Yan Y (2019) Integrated proteome analyses of wheat glume and awn reveal central drought response proteins under water deficit conditions. J Plant Physiol 232:270–283
Dianat M, Saharkhiz MJ, Tavassolian I (2016) Salicylic acid mitigates drought stress in Lippia citriodora L.: effects on biochemical traits and essential oil yield. Biocatal Agric Biotechnol 8:286–293
Farooq M, Hussain M, Siddique KH (2014) Drought stress in wheat during flowering and grain-filling periods. Crit Rev Plant Sci 33:331–349
Fischer RA, Maurer R (1978) Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust J Agric Res 29:897–912
Ghassemi-Golezani K, Hassanzadeh N, Shakiba MR, Esmaeilpour B (2020) Exogenous salicylic acid and 24-epi-brassinolide improve antioxidant capacity and secondary metabolites of Brassica nigra. Biocatal Agric Biotechnol 26:101636
Hernández-Ruiz J, Arnao MB (2018) Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agronomy 8:33
ICC (1995) Standard methods 155 and 158 of the international association of cereal chemistry and technology. ICC, Detmold
Jamshidi A, Javanmard HR (2018) Evaluation of barley (Hordeum vulgare L.) genotypes for salinity tolerance under field conditions using the stress indices. Ain Shams Eng J 9:2093–2099
Jiang W, Yang L, He Y, Zhang H, Li W, Chen H, Ma D, Yin J (2019) Genome-wide identification and transcriptional expression analysis of superoxide dismutase (SOD) family in wheat (Triticum aestivum). Peer J 7:e8062
Kalra Y (1997) Handbook of reference methods for plant analysis. CRC Press, Boca Raton
Kamran M, Cui W, Ahmad I, Meng X, Zhang X, Su W, Chen J, Ahmad S, Fahad S, Han Q, Liu T (2018) Effect of paclobutrazol, a potential growth regulator on stalk mechanical strength, lignin accumulation and its relation with lodging resistance of maize. Plant Growth Regul 84:317–332
Kang G, Li G, Xu W, Peng X, Han Q, Zhu Y, Guo T (2012) Proteomics reveals the effects of salicylic acid on growth and tolerance to subsequent drought stress in wheat. J Proteome Res 11:6066–6079
Kaouthar F, Ameny FK, Yosra K, Walid S, Ali G, Faical B (2016) Responses of transgenic Arabidopsis plants and recombinant yeast cells expressing a novel durum wheat manganese superoxide dismutase TdMnSOD to various abiotic stresses. J Plant Physiol 198:56–68
Kazemi E, Ganjali HR, Mehraban A, Ghasemi A (2022) Yield and biochemical properties of grain sorghum (Sorghum bicolor L. Moench) affected by nano-fertilizer under field drought stress. Cereal Res Commun 50:397–405
Khalafi A, Mohsenifar K, Gholami A, Barzegari M (2021) Corn (Zea mays L.) growth, yield and nutritional properties affected by fertilization methods and micronutrient use. Int J Plant Prod 15:589–597
Lee BR, Islam MT, Park SH, Lee H, Bae DW, Kim TH (2019) Antagonistic shifting from abscisic acid-to salicylic acid-mediated sucrose accumulation contributes to drought tolerance in Brassica napus. Environ Exp Bot 162:38–47
Li S, Tian Y, Wu K, Ye Y, Yu J, Zhang J, Liu Q, Hu M, Li H, Tong Y, Harberd NP (2018) Modulating plant growth–metabolism coordination for sustainable agriculture. Nature 560:595–600
Luo K, Xie C, Wang J, Du Q, Cheng P, Wang T, Wu Y, Yang W, Yong T (2020) Uniconazole, 6-benzyladenine, and diethyl aminoethyl hexanoate increase the yield of soybean by improving the photosynthetic efficiency and increasing grain filling in maize–soybean relay strip intercropping system. J Plant Growth Regul 40:1869–1880
Maghsoudi K, Emam Y, Niazi A, Pessarakli M, Arvin MJ (2018) P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J Plant Interact 13:461–471
Magné C, Saladin G, Clément C (2006) Transient effect of the herbicide flazasulfuron on carbohydrate physiology in Vitis vinifera L. Chemosphere 62:650–657
Mansour MMF (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol Plant 43:491–500
Miransari M, Smith DL (2014) Plant hormones and seed germination. Environ Exp Bot 99:110–121
Miransari M, Smith D (2019) Sustainable wheat (Triticum aestivum L.) production in saline fields: a review. Crit Rev Biotechnol 39:999–1014
Miransari M, Bahrami HA, Rejali F, Malakouti MJ (2008) Using arbuscular mycorrhiza to alleviate the stress of soil compaction on wheat (Triticum aestivum L.) growth. Soil Biol Biochem 40:1197–1206
Noori MS (2018) Nutritional and physiological studies on improvement of productivity and grain quality in wheat (Triticum aestivum L.) under drought stress condition. Ph.D. Thesis. Graduate School of Biosphere Science. Hiroshima University, Japan
Patni B, Ansari S (2019) Role of exogenous application of salicylic acid on medicinal plants under drought stress: a review. J Stress Physiol Biochem 15:76–85
Qiao Y, Yin L, Wang B, Ke Q, Deng X, Wang S (2019) Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiol Biochem 139:342–349
Regan KL, Siddique KHM, Turner NC, Whan BR (1992) Potential for increasing early vigour and total biomass in spring wheat. II. Characteristics associated with early vigour. Aust J Agric Res 43:541–553
Saha B, Borovskii G, Panda SK (2016) Alternative oxidase and plant stress tolerance. Plant Signal Behav 11:e1256530
Sajedi NA, Ardakani MR, Rejali F, Mohabbati F, Miransari M (2010) Yield and yield components of hybrid corn (Zea mays L.) as affected by mycorrhizal symbiosis and zinc sulfate under drought stress. Physiol Mol Biol Plants 16:343–351
Sajedi NA, Ardakani MR, Madani H, Naderi A, Miransari M (2011) The effects of selenium and other micronutrients on the antioxidant activities and yield of corn (Zea mays L.) under drought stress. Physiol Mol Biol Plants 17:215–222
Senapati N, Stratonovitch P, Paul MJ, Semenov MA (2019) Drought tolerance during reproductive development is important for increasing wheat yield potential under climate change in Europe. J Exp Bot 70:2549–2560
Shourbalal SKS, Soleymani A, Javanmard HR (2019) Shortening vernalization in winter wheat (Triticum aestivum L.) using plant growth regulators and cold stratification. J Clean Prod 219:443–450
Tahaei A, Soleymani A, Shams M (2016) Seed germination of medicinal plant, fennel (Foeniculum vulgare Mill), as affected by different priming techniques. Appl Biochem Biotechnol 180:26–40
Tahaei SA, Nasri M, Soleymani A, Ghooshchi F, Oveysi M (2022) Plant growth regulators affecting corn (Zea mays L.) physiology and rab17 expression under drought conditions. Biocatal Agric Biotechnol 41:102288
Tayyab N, Naz R, Yasmin H, Nosheen A, Keyani R, Sajjad M, Hassan MN, Roberts TH (2020) Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. PLoS ONE 15:e0232269
Torbica A, Antov M, Mastilovic J, Knezevic D (2007) The influence of changes in gluten complex structure on technological quality of wheat (Triticum aestivum L.). Food Res Int 40:1038–1045
Tripathi A, Tripathi DK, Chauhan DK, Kumar N, Singh GS (2016) Paradigms of climate change impacts on some major food sources of the world: a review on current knowledge and future prospects. Agric Ecosyst Environ 216:356–373
Williams P, Lindhauer MG, Pom RE, Wehling RL, Bergthaller W, Gaines CS (2008) The joint AACC international-ICC methods harmonization project. Cereal Food World 53:99–102
Yadav T, Kumar A, Yadav RK, Yadav G, Kumar R, Kushwaha M (2020) Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet-wheat. Saudi J Biol Sci 27:2010–2017
Yang W, Cai T, Li Y, Guo J, Peng D, Yang D, Yin Y, Wang Z (2013) Effects of exogenous abscisic acid and gibberellic acid on filling process and nitrogen metabolism characteristics in wheat grains. Aust J Crop Sci 7:58
Zamani S, Naderi MR, Soleymani A, Nasiri BM (2020) Sunflower (Helianthus annuus L.) biochemical properties and seed components affected by potassium fertilization under drought conditions. Ecotoxicol Environ Saf 190:110017
Zeeshan M, Lu M, Sehar S, Holford P, Wu F (2020) Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 10:127
Zhang H, Horgan J, Reynolds P, Jameson P (2010) 6-Benzyladenine metabolism during reinvigoration of mature Pinus radiata buds in vitro. Tree Physiol 30:514–526
Zulfiqar F, Chen J, Finnegan PM, Younis A, Nafees M, Zorrig W, Hamed KB (2021) Application of trehalose and salicylic acid mitigates drought stress in sweet basil and improves plant growth. Plants 10:1078
Funding
There was not any funding for the presented research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they do not have any conflict of interest.
Additional information
Communicated by Tibor Janda.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azizi, M.H., Soleymani, A. & Javanmard, H.R. Wheat (Triticum aestivum L.) biochemical and nutritional properties affected by plant growth regulators under field drought conditions. CEREAL RESEARCH COMMUNICATIONS 52, 591–603 (2024). https://doi.org/10.1007/s42976-023-00414-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-023-00414-1