Abstract
Melatonin is an evolutionarily conserved multifunctional molecule that is widely found in different parts of plants. In addition to its role in regulating plant growth and development, melatonin plays an important role in improving plant biotic and abiotic stress tolerance, which is an important molecule for protecting plants from abiotic and biotic stresses. The role of melatonin in plant growth and development is manifested in the regulation of seed germination, root development, fruit maturation, and senescence. Melatonin primarily improves plant stress resistance by improving plant antioxidant efficiency, reducing the inhibition of photosynthesis, and changing the expression of stress resistance-related genes. This paper reviews the regulation of melatonin on plant growth and development and the molecular mechanisms by which melatonin improves plant tolerance to abiotic and biogenic stresses and analyzes the relationship between melatonin and other signaling molecules and signaling pathways. The future research direction of plant melatonin is prospected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melatonin (N-acetyl-5-methoxy-tryptamine) is a natural molecule widely present in organisms and is first discovered in the pineal gland of dairy cows in 1958 (Lerner et al. 1958). Melatonin in animals is secreted by the pineal gland into the cerebrospinal fluid and blood, which plays a neurohormonal role and participates in various physiological processes, such as the regulation of circadian rhythm, sleep, mood, body temperature, appetite, sexual behavior, retinal physiology, and immune system (Arnao and Hernández-Ruiz 2015). In addition, melatonin, as an antioxidant and free radical scavenger, participates in a variety of cellular activities (Mauriz et al. 2013). Melatonin is originally thought to be a signaling molecule that exists only in animals. Therefore, research on melatonin has primarily focused on animals. With the deepening of research, melatonin is found to be an ancient molecule, which also exists in photosynthetic bacteria, red and green algae, fungi, and plants (Tan 2015). Dubbels et al. (1995) discovered melatonin in higher plants. Studies have confirmed that melatonin, as a multifunctional molecule, is widely found in plants and distributed in different organs (including the roots, stems, leaves, fruits, and seeds). The concentration of melatonin in plants varies greatly in different organs (Debnath et al. 2019). Chen et al. (2003) measured melatonin in 108 Chinese medicinal herbs and found that they all contained melatonin, and the content of melatonin varied greatly. Melatonin in plants is widely involved in regulating plant growth, senescence, root organ development, and flowering. Second, melatonin is related to the stress response of plants and is an important molecule to protect plants from adversity stress (Fan et al. 2018). Given the pleiotropy of melatonin function in plants, the study of melatonin function in plants has become a rapidly developing field. This paper reviews the regulatory role of melatonin on plant growth and development and the important role and possible molecular mechanism of melatonin in relieving plant biological and abiotic stresses. In addition, this paper is focused on the signal transduction pathways and signal molecules involved in the regulation of plant stress by melatonin. This study will contribute to the comprehensive understanding of the research status of plant melatonin, and the future research direction of plant melatonin is prospected.
Melatonin Regulates Plant Growth and Development
Melatonin Regulates Plant Growth, Promotes Seed Germination, and Controls Root Development
Since the discovery of melatonin in plants, studies have found that melatonin plays an important role in regulating plant growth, seed germination, and root development (Table 1). Similar to indole-3-acetic acid (IAA), the concentration of endogenous melatonin is different in plant tissues, and the most active growth area has the highest melatonin content (Hernández-Ruiz and Arnao 2018). The application of melatonin in the germination process significantly promotes soybean growth and seed yield. The promotion of melatonin on soybean growth is related to its enhanced photosynthesis and carbohydrate metabolism (Wei et al. 2015). In winter wheat, exogenous melatonin increases wheat yield by increasing the number of ears, improving carbon assimilation capacity, and promoting root growth (Ye et al. 2020). In addition, the positive effect of exogenous melatonin on increasing seed germination and stimulating lateral root formation has been confirmed in plants such as Arabidopsis, cucumber, bermuda grass, citrus, and corn (Arnao and Hernández-Ruiz 2015; Su et al. 2018; Zhang et al. 2013, 2014a, b). Melatonin promotes plant growth and development by regulating the concentration of other hormones. For example, low concentrations of melatonin promote the germination of cotton seeds by regulating the synthesis of endogenous hormones in plants (increasing GA3 content and reducing ABA content) (Xiao et al. 2019). Melatonin can also alleviate the inhibition of various stress environments on plant growth and development, such as melatonin treatment, to reduce the toxicity of cadmium to wheat seedlings, resulting in increased root growth and biomass accumulation (Ni et al. 2018). Under salt stress, exogenous melatonin can promote root growth and hypocotyl elongation of sunflower. Meanwhile, salt stress increases endogenous melatonin in the roots and cotyledons by regulating the activity of melatonin biosynthase HIOMT (N-acetylserotonin O-methyltransferase) in sunflower seedlings (Mukherjee et al. 2014).
Studies on the molecular mechanism of melatonin in regulating growth and promoting root development have obtained potential results. Qiao et al. (2019) found that melatonin promoted the absorption and assimilation of nitrogen by increasing the activity of nitrogen absorption and metabolism-related enzymes, thereby increasing wheat yield. Zhang et al. (2017b) showed that exogenous melatonin upregulated not only stress response proteins, but also proteins in glucose metabolism (glycolysis, citric acid cycle, etc.). Therefore, under NaCl stress, melatonin promotes energy production to improve the germination of cucumber seeds. Melatonin maintains the coordination of carbon and nitrogen metabolism by enhancing the gene expression and activity of key enzymes and promotes the growth of corn (Erdal 2019). H2O2 is involved in the regulation of plant growth by melatonin. Chen et al. (2019) found that melatonin promoted lateral root development of tomatoes by stimulating polyamine oxidase (PAO) dependent H2O2 production and Rboh (respiratory burst oxidase homolog) dependent O2·− production. Similarly, H2O2 as a downstream molecule of melatonin induces the formation of lateral roots of alfalfa (Chen et al. 2018b). Melatonin also increases the activity of antioxidant enzymes and content of osmotic adjustment substances, thereby promoting the root development of rape seedlings and increasing biomass (Zeng et al. 2018). Therefore, the regulation of melatonin on plant growth is related to carbon and nitrogen metabolism, energy metabolism, and production and clearance of reactive oxygen species (ROS).
Melatonin Regulates Plant Leaf Senescence and Fruit Maturation
Leaf senescence is affected by both internal (e.g., hormone) and external (e.g., nutritional status, stress, and photoperiod) factors. The plant hormone cytokinin delays leaf senescence, whereas ABA accelerates senescence. Chlorophyll degradation and reduction are an important manifestation of leaf senescence. Research has shown that melatonin has a delaying effect on leaf senescence. Exogenous melatonin delays the senescence of barley leaves by reducing chlorophyll loss in a concentration-dependent manner (Arnao and Hernández-Ruiz 2009). The application of melatonin on maize leaves significantly increases chlorophyll content and net photosynthetic rate during leaf senescence (Ahmad et al. 2020). Melatonin does not play an independent role in regulating senescence of leaves, but a complex interaction with other hormones is observed such as ABA and cytokinin. In ryegrass, melatonin effectively alleviates heat-induced growth inhibition and leaf senescence. In addition to inhibiting the transcription of senescence-related genes, melatonin inhibits ABA synthesis and signal transduction genes and induces the synthesis of cytokinin (Zhang et al. 2017a). Similar studies have also shown that cytokinin and melatonin have synergistic or additive effects in alleviating leaf senescence in creeping bentgrass induced by drought (Ma et al. 2018). Melatonin inhibits leaf senescence by inhibiting the transcription factor ABF-mediated ABA biosynthesis, thereby revealing the molecular mechanism of ABA and melatonin-regulating leaf senescence (Tan et al. 2019). The latest research shows that the MeJA-induced upregulation of tomato chlorophyll degradation genes and leaf senescence-related genes can be suppressed by melatonin. Furthermore, melatonin affects carbon fixation during aging (Wang et al. 2019).
Melatonin is also involved in the complex regulation of fruit ripening. Exogenous melatonin increases anthocyanin accumulation-related proteins and apoptotic inhibitor (API5) proteins during fruit ripening, downregulates senescence-related proteins and antioxidant enzyme proteins, and promotes post-harvest fruit ripening of tomatoes (Sun et al. 2016). 0.1 mM of melatonin treatment can maintain the hardness, total soluble solids, and ascorbic acid content of peach fruit; reduce the rot rate of peach fruit; delay fruit senescence; and maintain its quality (Gao et al. 2016). Similarly, melatonin treatment increases antioxidant activity and ATP content, promotes accumulation of total phenols and anthocyanins, and reduces post-harvest decay of strawberry fruit (Aghdam and Fard 2017). Exogenous melatonin promotes the ripening of grape fruits by increasing ABA, H2O2, and ethylene contents, indicating that the regulation of fruit ripening requires the participation of ethylene and other signaling molecules (Xu et al. 2018).
Regulation of Melatonin on Plant Growth and Development Has Concentration Dependence
Different concentrations of melatonin have different regulatory effects on plant root growth. 0.1 μM of exogenous melatonin can promote the growth of wild mustard roots, whereas 100 μM of melatonin inhibits root growth. Similarly, 0.1 μM of melatonin can also increase the level of endogenous IAA in roots, whereas high concentration of melatonin has no evident effect (Chen et al. 2009). Low concentration of melatonin (1 μM) promotes rooting of several sweet cherry rootstocks, whereas high concentration (10 μM) inhibits root growth (Sarropoulou et al. 2012). These studies have shown that different plants have different concentrations of suitable melatonin during the regulation of root growth. In addition, melatonin treatment at different concentrations (10–50 µM) inhibits rice radicle growth, thereby promoting lateral root formation (Liang et al. 2017). Different concentrations of melatonin have different regulation effects on plant seed germination and seedling growth. Low concentration of melatonin (20 and 5 µM) treatment can increase the germination of Stevia rebaudiana seeds, plant fresh weight, and leaf number, whereas high concentration of melatonin (500 µM) treatment promotes root development (Simlat et al. 2018). 30 µM of melatonin can reduce the inhibition of salt stress on the growth of rape seedlings, but high concentrations of melatonin (50 µM) can weaken or prevent the promotion of seedling development (Zeng et al. 2018). Similarly, moderate melatonin concentrations (10–40 µM) promote growth and development in Arabidopsis, whereas high concentrations (200 and 400 µM) inhibit growth (Bajwa et al. 2014). Therefore, when using melatonin to promote plant growth and development, selecting the appropriate concentration is important.
Overexpression or Inhibition of Melatonin Synthase Gene Affects Plant Growth and Development
Tryptophan decarboxylase (TDC) is the first enzyme that catalyzes the synthesis of tryptophan to melatonin. The overexpression of the TDC gene in rice delays the senescence of plant leaves. Conversely, the inhibition of TDC expression by RNA interference (RNAi) accelerates the senescence of leaves (Kang et al. 2009). Transgenic rice plants overexpressing rice OsSNAT1 (serotonin N-acetyltransferase) gene exhibit significant resistance to cadmium and senescence stress, which can increase yield (Lee and Back 2017b). Transgenic rice seedlings overexpressing sheep AANAT (serotonin N-acetyltransferase) enhance root elongation, increase the number of adventitious roots (Park and Back 2012), and enhance early growth of seedlings (Byeon and Back 2014). By contrast, transgenic rice that inhibits the expression of SNAT (serotonin N-acetyltransferase) or ASMT (N-acetylserotonin methyltransferase) has reduced endogenous melatonin, resulting in seedling growth retardation and decreased yield (Byeon and Back 2016). Therefore, the overexpression of melatonin synthetic gene is an effective method to improve plant growth and development, which has a good potential application in agricultural production.
Interaction of Melatonin and Auxin in the Regulation of Plant Growth
Melatonin and auxins have the same synthetic substrate (tryptophan), and they have many similarities in structure and function. Therefore, whether melatonin and auxins interact in regulating plant growth and development remains unknown. Analysis by Weeda et al. (2014) using RNA-seq showed that in melatonin-treated Arabidopsis, the expression of genes related to auxin signal transduction was mostly downregulated. Pelagio-Flores et al. (2012) found that melatonin regulated the root structure of Arabidopsis independent of IAA signaling. However, in rice, auxin-related genes are significantly activated under the treatment of melatonin, and melatonin activates the auxin signaling pathway to shape the root architecture (Liang et al. 2017). Melatonin in tomato affects auxin accumulation, transport, and signal transduction, thereby promoting adventitious root development (Wen et al. 2016). Melatonin has a concentration-dependent activation/inhibition of the IAA pathway. Melatonin regulates Arabidopsis root growth by inhibiting auxin synthesis and polar auxin transport (Wang et al. 2016). The latest research shows that melatonin and auxin have a synergistic effect, and melatonin regulates the distribution of auxin by regulating the transport of auxin and promotes the development of lateral roots (Ren et al. 2019). Although they are both indole compounds, melatonin and auxin alter Arabidopsis gene expression in different ways (Zia et al. 2019). These findings indicate that the interaction between melatonin and auxin in regulating the growth and development of plants is complicated. The current research is just the beginning, and the study of the mutual regulation of the two molecules will lead to the comprehensive understanding of plant growth and development.
Melatonin Improves Plant Tolerance to Abiotic and Biological Stresses
To date, many studies have shown that apart from its role in regulating plant development, melatonin has played an important role in improving plant abiotic and biological stress tolerance (Table 2). The production of melatonin in plants is always parallel to the level of stress. In response to environmental stress, the expression of melatonin synthase gene, particularly the rate-limiting enzyme SNAT (serotonin N-acetyltransferase) gene, is significantly upregulated (Byeon and Back 2014). Similarly, under various stress conditions (including salinity, drought, over-watering, cold, high temperature, ultraviolet radiation, metal pollution, and pathogens), the levels of melatonin in plants are remarkably increased, indicating that melatonin is involved in plant stress tolerance (Shi et al. 2016).
Synthesis of Endogenous Melatonin is Induced by Stress and Improves the Stress Resistance of Plants
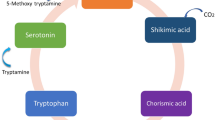
Melatonin biosynthesis starts with tryptophan and goes through four consecutive enzymatic reactions: respectively by tryptophan decarboxylase (TDC), tryptamine 5-hydroxylase (T5H), serotonin N-acetyltransferase (SNAT), and N-acetylserotonin methyltransferase (ASMT) catalysis. Under normal circumstances, the biosynthesis of melatonin is strictly regulated because of the low catalytic efficiency of SNAT and ASMT (Back 2020). Considerable evidence indicates that the synthesis of plant melatonin is induced by abiotic stress (including cold, drought, and heavy metal pollution) and biotic stresses (Tan and Reiter 2020). The overexpression of melatonin synthetic genes can increase the endogenous melatonin content of transgenic plants and enhance the tolerance to salt, cadmium, drought, pathogens, and other stresses, indicating that endogenous melatonin plays an important role in improving plant resistance to biotic and abiotic stresses (Zheng et al. 2017; Arnao and Hernández-Ruiz 2019c; Yang et al. 2019; Moustafa-Farag et al. 2020). In plants, apart from T5H, TDC, SNAT, and ASMT have several isoforms. In rice, three functional TDCs (Lee and Back 2019b), two SNAT isogenes (SNAT1 and SNAT2) (Byeon et al. 2016), and three ASMT isogenes (ASMT1-3) are identified (Kang et al. 2011). Compared with animal cells, melatonin synthesis in plant cells has various alternative pathways. Under normal conditions and abiotic stresses, the melatonin synthetic pathways are different (Ye et al. 2019). This finding may be related to the expression of different isoforms of melatonin synthase under different growth conditions. Zhang et al. (2019a) found that the synthetic isoforms of melatonin were differentially expressed in different environments, indicating that the synthesis of melatonin in plants has a great ability to adapt to environmental changes. In addition, plant melatonin biosynthesis is induced by ROS and RNS, and melatonin can act as an effective scavenger of ROS and/or RNS (Arnao and Hernández-Ruiz 2019b).
In recent years, people have studied the exact synthesis site of melatonin in cells. The current results show that intermediates for melatonin synthesis are found in the cytoplasm, endoplasmic reticulum, and chloroplast. Given the different alternative pathways for melatonin biosynthesis in plant cells, the final synthesis of melatonin occurs at different subcellular sites, which in turn affects the mode of action of melatonin in plants, enabling plants to respond more flexibly to various stress factors (Back et al. 2016). Zheng et al. (2017) have shown that purified chloroplasts can synthesize melatonin with serotonin precursors and have a protective effect on chloroplasts. The study also found that the MzASMT9 protein of apples is located in the chloroplast. The melatonin levels in the transgenic MzASMT9 Arabidopsis and its chloroplasts are significantly higher than those of wild type, and under salt stress, the levels of ROS in the chloroplasts are significantly low. The above-mentioned results indicate that the chloroplast can synthesize melatonin, and its synthesis is induced by stress. The latest research shows that, similar to the animal mitochondria, the plant mitochondria are an important place for melatonin synthesis. Melatonin synthase MzSNAT5 is located in the mitochondria of apple callus cells. The ectopic expression of MzSNAT5 can increase the synthesis of melatonin in the mitochondria and enhance the drought tolerance of transgenic plants (Wang et al. 2017). In eukaryotes, chloroplasts and mitochondria are the primary sources of ROS, and the synthesis of melatonin in these two organelles seems to protect them from oxidative stress more quickly (Zheng et al. 2017). 2-Ω (2-hydroxymelatonin), a catabolism product of melatonin, is also produced in the chloroplasts (Byeon et al. 2015). Therefore, the synthesis and catabolism of melatonin are carried out in the chloroplast, and its important biological significance needs further study.
Application of Exogenous Melatonin Alleviates Environmental Stress
Many studies have shown that exogenous melatonin can enhance the tolerance of plants to abiotic stress; therefore, it is considered as an effective means to alleviate environmental stress. For example, exogenous melatonin effectively improves heat resistance of tall fescue (Alam et al. 2018b). Exogenous melatonin can improve plant’s ability to chelate heavy metal ions and promote the accumulation of nutrient ions and plant’s tolerance to heavy metal stress and can alleviate the effect of nutrient ion deficiency on plant growth. Melatonin alleviates Cu2+ toxicity in cucumber by improving Cu2+ chelation, carbon metabolism, and active-oxygen scavenging (Cao et al. 2019). Similarly, in tomatoes, exogenous melatonin induces the biosynthesis of phytochelates and further chelates cadmium, thereby increasing the distribution of cadmium in the cell walls and vacuoles and improving plant tolerance to cadmium (Hasan et al. 2015). Under low-potassium stress, melatonin treatment increases the transcription of K+ transport genes in bermudagrass, thereby promoting the accumulation of K+ in plant stems and root tissues, and regulates the activity of photosystem II (PSII) (Chen et al. 2017a). Exogenous melatonin improves plant tolerance to abiotic stress by slowing chlorophyll degradation, improving photosynthesis, increasing antioxidant enzyme activity, and slowing membrane damage and lipid peroxidation (Chen et al. 2018a; Huang et al. 2019; Wei et al. 2018b). Exogenous melatonin can also improve plant disease resistance. After apples (Malus prunifolia) were pretreated with melatonin, the activity of plant defense-related enzymes increased, and the resistance to Diplocarpon mali increased (Yin et al. 2013). Adding melatonin to the soil can significantly improve the growth of apple seedlings and reduce the influence of the replant disease of apples (Li et al. 2018a).
Synthetic Precursors and Metabolites of Melatonin Improve the Stress Tolerance of Plants
Among the melatonin synthase, TDC and T5H have high catalytic efficiency, whereas SNAT or ASMT is the recognized rate-limiting enzyme for melatonin biosynthesis. Therefore, under stress conditions, the content of melatonin synthetic precursors (such as serotonin) is higher than that of melatonin (Tan and Reiter 2020). Similarly, M2H and M3H, which catalyze the catabolism of melatonin, have higher catalytic efficiency than ASMT and SNAT, resulting in higher levels of melatonin metabolites (cyclic 3-hydroxymelatonin [3-Ω] and 2-hydroxymelatonin [2-Ω]) in plants than melatonin (Lee et al. 2016). Studies have shown that melatonin precursors and metabolites are also involved in plant stress resistance. Under NaCl stress, the endogenous serotonin content of sunflower roots and cotyledons increases, indicating that it is involved in salt stress (Mukherjee et al. 2014). Melatonin synthetic precursors (tryptophan and serotonin) enhance plant tolerance to heavy metals by combining with heavy metals to form stable complexes (Janice et al. 1998). In addition to alleviating heavy metal stress, serotonin can enhance a variety of abiotic stress responses by mediating the flow of ions into the chloroplast to improve the survival rate of plants under salt stress (Pickles and Sutcliffe 1955). The first metabolite of melatonin found in plants is AFMK (N1-acetyl-N2-formyl-5-methoxykynuramine). Melatonin and AFMK serve as free radical scavengers to improve the tolerance of water hyacinth to environmental pollutants (toxic chemicals and heavy metals) (Tan et al. 2007). The other two important melatonin metabolites found in plants are 3-Ω and 2-Ω. 2-Ω increases the tolerance of rice to multiple abiotic stresses by upregulating the expression of transcription factors, namely, Myb4 and AP37 (Lee and Back 2016a). Similarly, exogenous 2-Ω treatment can increase the content of non-enzymatic antioxidants and gene expression in cucumbers in response to cadmium stress (Shah et al. 2020). Therefore, under stress conditions, the direct protection against ROS-mediated oxidative stress is the comprehensive antioxidant effects of melatonin and its precursors and metabolites. Identifying the substance that plays the major role in improving plant resistance to stress is an important research field. The latest research found that, compared with melatonin, 2-hydroxymelatonin has a stronger protective effect against plant abiotic stress (Lee and Back 2019a). Therefore, in addition to melatonin, researchers must consider the role and mechanism of its synthetic precursors and metabolites in improving plant stress resistance.
Overexpression of the Melatonin Synthetic Gene Improves Plant Resistance to Stress
Current research has shown that the overexpression of melatonin synthetic genes can significantly improve plant stress tolerance. Compared with wild type, the overexpression of MzASMT in Arabidopsis results in increased endogenous melatonin levels, decreased ROS content, increased biomass, and greater tolerance to drought stress (Zuo et al. 2014). The overexpression of ASMT in tomatoes can reduce the level of ubiquitinated proteins under high-temperature stress, enhance the expression of heat shock proteins (HSPs), reduce the accumulation of aggregated proteins, and promote the protection of cellular proteins (Xu et al. 2016). Similarly, the overexpression of the SlSNAT gene in tomatoes significantly increases the endogenous melatonin levels, decreases reactive oxygen accumulation and Fv/Fm levels, and increases heat resistance (Wang et al. 2020b). ASMT is considered to be the rate-limiting enzyme in melatonin synthesis, but only a few monocotyledons have ASMT homologs. In Arabidopsis, caffeic acid o-methyltransferase (COMT) can replace ASMT enzyme to convert N-acetyl serotonin to melatonin; therefore, it is considered as the last step of plant melatonin biosynthesis (Lee et al. 2014b). COMT homologs have also been isolated in other plants, and studies have shown that the overexpression of COMT can also increase the stress resistance of transgenic plants. The overexpression of wheat TaCOMT increases endogenous melatonin and enhances drought tolerance of transgenic Arabidopsis (Yang et al. 2019). In addition, the overexpression of the SlCOMT1 gene in tomatoes activates the salt overly sensitive (SOS) pathway, maintains Na+/K+ balance, upregulates stress-related gene expression, and improves salt tolerance (Sun et al. 2019). Moreover, the overexpression of animal melatonin synthesis-related genes in plants can improve the resistance of transgenic plants. Transgenic rice that overexpresses sheep AANAT gene has significantly increased endogenous melatonin, active oxygen-scavenging ability, and resistance to oxidative stress (Park et al. 2013). Similarly, the overexpression of sheep AANAT and HIOMT genes can improve drought tolerance of transgenic tomatoes (Wang et al. 2014) and salt tolerance of transgenic switchgrass (Huang et al. 2017). Given the competition between melatonin and IAA for the co-synthetic precursor tryptophan, AANAT transgenic plants have high levels of melatonin and low IAA content (Wang et al. 2014).
Mechanism of Melatonin Improving Plant Stress Tolerance
Melatonin Removes ROS and Improves Antioxidant Capacity
Research on the interaction of melatonin with stress signaling mechanisms has shown a complex relationship between melatonin and ROS. First, melatonin is a highly effective free radical scavenger and a broad spectrum of direct antioxidants. Melatonin can directly interact with ROS to stabilize biofilms and reduce cellular oxidative stress. Both endogenous and exogenous melatonin can efficiently remove active oxygen and relieve oxidative stress caused by drought, salt, cold, and high temperature (Cui et al. 2018; Gao et al. 2018; Liang et al. 2015; Zhang et al. 2015). Melatonin treatment reduces the accumulation of oxidized proteins, increases autophagy induction capacity, and reduces photooxidative damage (Wang et al. 2015). These results indicate that melatonin serves as a powerful antioxidant and detoxifying free radical, thereby improving tolerance of plants to oxidative stress. Second, apart from the role of melatonin in directly removing free radicals and ROS, it can improve its efficiency as an antioxidant by regulating the activity of antioxidant enzymes. Under various abiotic stresses, exogenous melatonin controls the accumulation of ROS and reduces oxidative stress by inducing antioxidant enzyme activities (including CAT, APX, POD, and SOD), which has been demonstrated in a variety of plants (Alam et al. 2018a; Chen et al. 2018a, 2017b; Li et al. 2017a; Nawaz et al. 2018; Qi et al. 2018; Shi et al. 2015d; Sun et al. 2020a). Therefore, in addition to directly interacting with ROS, melatonin activates the enzymatic antioxidant system and improves plant tolerance to various stresses. Third, melatonin also enhances plant resistance to stress by regenerating endogenous antioxidants such as glutathione (GSH) and ascorbic acid (AsA). The AsA-GSH cycle has a protective effect on oxidative stress in plants. Melatonin improves plant’s resistance to adversity stress by regulating the AsA–GSH cycle. Under different stresses, melatonin increases the activity of non-enzymatic antioxidants by regulating AsA–GSH cycle-related enzyme activities, thereby improving the scavenging capacity of O2·− and H2O2, and reducing oxidative stress (Cai et al. 2017; Jahan et al. 2019; Liang et al. 2018; Ni et al. 2018; Wang et al. 2012; Yin et al. 2019). By contrast, under low-temperature stress, melatonin-deficient tomatoes show reduced antioxidant capacity because of reduced ratios of GSH/GSSG and AsA/DHA (dehydroascorbic acid) (Wang et al. 2020a). Interestingly, Li et al. (2016b) found that exogenous melatonin enhanced the GSH/GSSG ratio in a H2O2-dependent manner and played a key role in inducing cucumber defense against photooxidative stress. Under postharvest refrigeration condition, exogenous melatonin can induce the transcription of AsA synthetic gene, increase the content of AsA, and improve the cold tolerance of peach fruit (Cao et al. 2018).
Melatonin Acts as an Enhancer or Protectant for Photosynthesis and Stomatal Conductance
Environmental stress usually causes stomatal closure and decreased photosynthesis in plants, leading to reduced productivity. In addition to its broad spectrum of antioxidant activity, melatonin is essential for the maintenance of photosynthetic capacity under various stress conditions. At low temperature, the inhibition of melatonin synthesis hinders the photosynthesis of tomatoes and delays the recovery of photosynthesis after the cessation of low-temperature stress, whereas the effect of exogenous melatonin application shows the opposite (Wang et al. 2020a). Similarly, exogenous melatonin reduces the photoinhibition caused by low-temperature stress by accelerating non-photochemical quenching (Ding et al. 2017). Under the combined stress of salinity and heat, exogenous melatonin treatment improves the performance of tomato photosynthesis and photosynthetic parameters (Martinez et al. 2018). Salt stress causes the reduction of D1 protein, and melatonin pretreatment promotes the repair of photosystem II by maintaining the synthesis of D1 protein and reduces the effect of salt stress on tomato photosynthesis (Zhou et al. 2016b). Melatonin can also reduce the damage of heavy metal toxicity to photosynthesis and alleviate the effects of aluminum and cadmium stress on the photosynthetic rate of rape seedlings (Sami et al. 2020). Comprehensive analysis of the current research results shows that melatonin can enhance the content of chlorophyll, improve the photosynthetic electron transport in photosynthesis, increase the photosynthetic rate, increase the stomatal conductance, and enhance tolerance of plants to stress (Cui et al. 2018; Li et al. 2015, 2017a, 2016c; Meng et al. 2014). The overexpression and silencing of melatonin synthetic genes also indicate the importance of melatonin in maintaining plant photosynthesis under stress conditions. Under cold stress, endogenous melatonin and chlorophyll content in the transgenic rice overexpressing human SNA (serotonin N-acetyltransferase) is significantly increased, showing strong resistance to low-temperature stress (Kang et al. 2010). In the melatonin production-deficient tomatoes produced by silencing the COMT1 gene, the photosynthesis photoreaction and carbon fixation reaction are suppressed; the energy absorption and distribution are reduced; the photosystem II activity and electron transmission efficiency are reduced, and the heat stress reaction is intensified (Ahammed et al. 2018). Transcriptome analysis also shows that melatonin upregulates the expression of genes related to photosynthesis (Arnao and Hernández-Ruiz 2015).
Melatonin Improves Plant Stress Resistance by Regulating Gene Expression
Considerable studies have shown that melatonin not only acts as an antioxidant, but also improves plant stress resistance by regulating gene expression. After melatonin treatment, the expression of chloride channel proteins (CLC1 and CLC2) is significantly upregulated, resulting in the activation of the SOS pathway and improving salt tolerance (Li et al. 2017c). Under different stresses, exogenous melatonin can upregulate the expression of antioxidant enzyme genes in the ascorbic acid–GSH cycle in apples to reduce H2O2 accumulation and regulate the expression of potassium channel protein genes to promote potassium ion absorption (Li et al. 2016a). In addition, melatonin enhances the resistance of cotton to Verticillium wilt by affecting lignin and gossypol synthetic gene expression (Li et al. 2019). Melatonin also induces gene expression involved in wax biosynthesis, promotes thickening of the cuticle of tomato leaves, and improves tolerance to water deficit (Ding et al. 2018). RNA-seq analysis shows that melatonin-induced plant cold tolerance is related to the enhanced expression of defense genes. These defense genes induced by melatonin are distributed in signal transmission, protection and detoxification, and transcriptional regulation (Li et al. 2017b).
Melatonin regulates the transcription of transcription factor genes, thereby activating stress-related gene expression and improving plant stress resistance. Under cold stress, melatonin upregulates the expression of the CBF/DREBs transcription factor, cold stress response gene COR15a, and transcriptional activators (CAMTA1, ZAT10, and ZAT12), thereby improving the cold tolerance of Arabidopsis (Bajwa et al. 2014). Exogenous melatonin induces the transcription of CBF, and the diurnal changes of endogenous melatonin can regulate AtCBF/DREB1 expression and improve the disease resistance of Arabidopsis (Shi et al. 2016). Under abiotic (salt, drought, and cold) and biological stresses (bacterial pathogen infection), endogenous melatonin increases significantly, resulting in increased transcription of CBF/DREB1s and multiple stress response genes (COR15A, RD22, and KIN1), and improves resistance to stress in Arabidopsis (Shi et al. 2015b). Melatonin induces heat shock factor HSFA1, which further upregulates the expression of heat response genes, thereby conferring heat tolerance and Cd tolerance on plants (Cai et al. 2017; Shi et al. 2015c). Under salt stress, the expression of SOS1 (salt overly sensitive 1), SOS2, and SOS3 genes is significantly induced by melatonin, leading to the activation of the SOS pathway and improvement of salt tolerance (Chen et al. 2017b). Similarly, melatonin upregulates the expression of ion transport proteins (MdNHX1 and MdAKT1) in Malus hupehensis and reduces salt stress damage by maintaining the ion homeostasis in the leaves (Li et al. 2012). Therefore, the signal pathways formed by plants in response to abiotic stress during long-term evolution, such as the DREB/CBF pathway in response to cold stress, HSF-HSP pathway in response to heat stress, and SOS3-SOS2-SOS1/NHX in response to salt stress, are all regulated by melatonin to varying degrees. The RNA-seq results also show that melatonin treatment can regulate the expression levels of multiple transcription factors (including MYB, WRKY, NAC, and ethylene-responsive transcription factors) in cucumber under NaCl stress (Zhang et al. 2014b). Similarly, RNA-seq shows that under abiotic stresses (including salt, drought, or low temperature), melatonin pretreatment upregulates the expression of various transcription factors (CBF/DREB, WRKY, MYB, bHLH, etc.) and improves stress resistance in Bermuda grass (Shi et al. 2015d).
Melatonin maintains chlorophyll content and increases photosynthetic rate by downregulating chlorophyll degradation-related gene expression and increasing photosynthesis-related gene expression. Chlorophyllase (CLH1) is a light-regulating enzyme related to chlorophyll degradation, and melatonin treatment results in significant downregulation of CLH1 expression in Arabidopsis (Weeda et al. 2014). Pheide a oxygenase (PAO) is another important enzyme that degrades the chlorophyll. Exogenous melatonin inhibits the expression of PAO in apples (Wang et al. 2012). Pretreatment of melatonin under salt stress can induce the expression of chlorophyll biosynthetic enzyme gene (ChlG) in salt-tolerant naked oats and increase the chlorophyll content of oat seedling leaves (Gao et al. 2019). Melatonin treatment significantly downregulates chlorophyll-degrading gene (Chlase, PPH and chl-prx) expression in creeping grass, which alleviates leaf aging caused by drought (Ma et al. 2018). Therefore, under the stress environment, the improvement of plant photosynthesis by melatonin is mainly achieved by regulating the expression of genes related to photosynthesis.
Melatonin improves the adaptability of plants to environmental stress by regulating other hormone signaling-related gene expression. In alleviating various stress injuries, a cross-talk is identified between melatonin and other plant hormones (Arnao and Hernández-Ruiz 2018; Weeda et al. 2014). Under drought stress, melatonin pretreatment induces abscisic acid (ABA) synthetic gene (MdNCED3) expression and inhibits ABA catabolism gene (MdCYP707A1 and MdCYP707A2) expression in apple leaves, thereby reducing the content of endogenous ABA (Li et al. 2015). Similarly, in cucumber seedlings, melatonin regulates the expression of ABA catabolism and biosynthetic genes to regulate ABA biosynthesis and catabolism, thereby reducing the inhibitory effect of NaCl stress on germination (Zhang et al. 2014a). Melatonin increases the ethylene content in tomatoes by upregulating the expression of ACS4 gene and enhances the expression of ethylene signal-related genes (NR, ETR4, EIL1, EIL3, and ERF2), thereby affecting the perception and sensitivity of ethylene and promoting fruit ripening and quality improvement (Sun et al. 2015). Melatonin can also regulate endogenous BR synthesis by inducing BR biosynthetic gene expression, thereby regulating the dark growth or shade growth of plants (Hwang and Back 2018). These results indicate that melatonin may be involved in the interaction with other plant hormones by improving plant stress tolerance. Environmental stress can also cause the expression of melatonin biosynthetic genes, thereby increasing endogenous melatonin (Arnao and Hernández-Ruiz 2019a). Melatonin plays an important role in regulating the expression of multiple genes, reflecting its multi-effect physiological role in plants (Zhang et al. 2015).
Melatonin Increases Plant Stress Resistance by Regulating Polyamine and Glucose Metabolism
Polyamines (PAs) are important plant growth regulators and are involved in regulating plant growth and development. PA metabolism is related to plant responses to various environmental stresses. Lei et al. (2004) found that putrescine and spermine were significantly increased in melatonin-treated carrot cells and confirmed that PAs participated in melatonin-mediated anti-apoptosis. Studies have shown that melatonin induces polyamine synthetic gene expression (Gong et al. 2017), regulates the synthesis of PAs (Jahan et al. 2019; Zhang et al. 2019b), reduces the degradation of PAs (Ke et al. 2018), and improves the tolerance of plants to environmental stress. The regulation of melatonin on polyamine metabolism provides a new idea for studying the mechanism of melatonin to alleviate the damage caused by environmental stress on plants. Exogenous melatonin can also regulate carbohydrate metabolism and significantly induce the production of sucrose, glucose, and fructose, and melatonin-mediated sugar metabolism may partially induce resistance to pathogens (Zhao et al. 2015).
Relationship Between Melatonin and Other Signaling Molecules and Signaling Pathways in Stress Response
Relationship Between Melatonin and Calcium Signal
Calcium signaling is an important regulator of various stress responses. The genes involved in calcium-dependent signal transduction in Arabidopsis are regulated by melatonin (Weeda et al. 2014). Under oxidative stress, the transcription of several calcium-related proteins such as calcium-dependent protein kinase (CDPK) in Bermuda grass is regulated by exogenous melatonin (Shi et al. 2015d). Wei et al. (2018a) discovered the first plant melatonin receptor (CAND2/PMTR1) in Arabidopsis and found that melatonin regulated stomatal closure through the CAND2/PMTR1-mediated H2O2 and Ca2+ signal transduction cascade pathways (Fig. 1).
Relationship of melatonin, NO, Ca2+, H2O2, and MAPK cascade in plant stress responses. GSNOR s-nitroglutathione reductase, NO nitric oxide, CAND2/PMTR1 phytomelatonin receptor, CDPK calcium-dependent protein kinase, OXI1 oxidative signal-inducible1, H2O2 hydrogen peroxide, Ca2+ calcium, MAPKKK3 MAPKK kinases 3, MKK4/5/7/9 MAPK kinases 4/5/7/9, MAPK3/6 mitogen-activated protein kinase 3/6, ICS1 isochorismate synthase 1, SA salicylic acid
Relationship Between Melatonin and NO Signal
Nitric oxide (NO), as an important signaling molecule, is involved in regulating a variety of physiological processes in plants. Melatonin plays an important role in plant stress response by interacting with NO (Fig. 1) (Arnao and Hernández-Ruiz 2019a). Exogenous melatonin improves the resistance of Arabidopsis to bacterial pathogen infection by inducing endogenous NO production (Shi et al. 2015a). Melatonin increases the tolerance of Arabidopsis to Fe deficiency by inducing polyamine-mediated NO accumulation (Zhou et al. 2016a). Exogenous melatonin treatment induces the production of NO in the roots of alkali-stressed tomatoes. NO, as a downstream signaling molecule, participates in melatonin-induced tomato tolerance to alkaline stress (Liu et al. 2015). Similarly, exogenous melatonin promotes the accumulation of endogenous NO by downregulating the expression of GSNOR (s-nitroglutathione reductase) in tomato seedlings, and NO, as a downstream signal, is involved in melatonin-induced adventitious root formation (Wen et al. 2016). Under salt stress, melatonin interacts with the nitric oxide signaling pathway in regulating sunflower growth and maintaining oxidative homeostasis, including the regulation of ONOO− (peroxynitrite anion) and O2·− accumulation (Arora and Bhatla 2017).
Relationship Between Melatonin and the MAP Kinase Signaling Pathway
The MAP kinase cascade pathway is highly conserved in all eukaryotes, which plays an important role in transducing external signals into cells. Studies have shown that signals in melatonin-triggered Arabidopsis pathogen resistance can be transmitted through the MAPK signaling pathway (Lee et al. 2014a). By stimulating MAP kinase cascade reaction (MAPKKK3/OXI1-MAPKK4/5/7/9-MAPK3/6), melatonin indirectly induces the transcription of the ICS1 (isochorismate synthase 1) gene responsible for SA (salicylic acid) synthesis to improve the disease resistance of Arabidopsis. In addition, melatonin serves as a signaling molecule, downstream of NO and H2O2, and upstream of the MAPK cascade. 2-Hydroxymelatonin and N-acetyl serotonin can also activate MAPKs to confer biological stress, but to a lesser extent than melatonin (Lee and Back 2016b, 2017a). These results indicate that melatonin-induced pathogen resistance is related to the MAP kinase signaling cascade, and melatonin triggers plant defense responses through the MAPK signaling cascade (Fig. 1). Under drought and salt stress, melatonin pretreatment can enhance the expression of MAP kinase gene and transcription factor gene and regulate the expression of downstream stress response genes, thereby improving plant tolerance. The induction of melatonin on the MAPK cascade requires the regulation of H2O2 signaling molecules (Gao et al. 2019, 2018; Zhang et al. 2020).
Conclusions
Melatonin is an amphiphilic indole ring structure compound that can move easily through the cell membrane to the cytoplasm without difficulty by subcellular partition. As a growth-regulating substance, melatonin has the characteristics of high efficiency and environmental protection, which is known as an eco-friendly molecule (Galano et al. 2013). Melatonin plays an important role in regulating plant growth, development, and environmental adaptation, indicating that melatonin has a great potential in improving plant stress resistance and productivity. However, the research on the molecular mechanism of melatonin-mediated plant response to stress is not systematic, and the signal transduction pathways and signal molecules involved in melatonin-regulating plant stress remain relatively rare. The current research results indicate that Ca2+, H2O2, NO, and other signaling molecules and the MAP kinase cascade pathway are important participants in melatonin-induced plant tolerance to stress. However, the mutual regulation and cross-talk among these signaling molecules and signal transduction pathways are unclear. Therefore, the specific mechanism of melatonin-induced stress tolerance needs further study. In addition, melatonin and auxin have the same synthetic substrate, structure, and function, but the current understanding of the interaction between melatonin and auxin in the regulation of plant growth and development remains limited, which will be the focus of future research.
References
Aghdam M, Fard J (2017) Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria×anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem 221:1650–1657. https://doi.org/10.1016/j.foodchem.2016.10.123
Ahammed GJ, Xu W, Liu A, Chen S (2018) COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato. Front Plant Sci 9:998. https://doi.org/10.3389/fpls.2018.00998
Ahmad S, Su W, Kamran M, Ahmad I, Meng X, Wu X, Javed T, Han Q (2020) Foliar application of melatonin delay leaf senescence in maize by improving the antioxidant defense system and enhancing photosynthetic capacity under semi-arid regions. Protoplasma 257:1079–1092. https://doi.org/10.1007/s00709-020-01491-3
Alam MN, Wang Y, Chan Z (2018a) Physiological and biochemical analyses reveal drought tolerance in cool-season tall fescue (Festuca arundinacea) turf grass with the application of melatonin. Crop Pasture Sci 69(10):1041–1049. https://doi.org/10.1071/CP18394
Alam MN, Zhang L, Yang L, Islam MR, Liu Y, Luo H, Yang P, Wang Q, Chan Z (2018b) Transcriptomic profiling of tall fescue in response to heat stress and improved thermotolerance by melatonin and 24-epibrassinolide. BMC Genomics 19:224. https://doi.org/10.1186/s12864-018-4588-y
Arnao MB, Hernández-Ruiz J (2009) Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J Pineal Res 46:58–63. https://doi.org/10.1111/j.1600-079X.2008.00625.x
Arnao MB, Hernández-Ruiz J (2015) Functions of melatonin in plants: a review. J Pineal Res 59:133–150. https://doi.org/10.1111/jpi.12253
Arnao MB, Hernández-Ruiz J (2018) Melatonin and its relationship to plant hormones. Ann Bot 121:195–207. https://doi.org/10.1093/aob/mcx114
Arnao MB, Hernández-Ruiz J (2019a) Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci 24:38–48. https://doi.org/10.1016/j.tplants.2018.10.010
Arnao MB, Hernández-Ruiz J (2019b) Melatonin and reactive oxygen and nitrogen species: a model for the plant redox network. Melatonin Res 2:152–168
Arnao MB, Hernández-Ruiz J (2019c) Role of melatonin to enhance phytoremediation capacity. Appl Sci 9:5293. https://doi.org/10.3390/app9245293
Arora D, Bhatla SC (2017) Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic Bio Med 106:315–328. https://doi.org/10.1016/j.freeradbiomed.2017.02.042
Back K (2020) Melatonin metabolism, signaling and possible roles in plants. Plant J. https://doi.org/10.1111/tpj.14915
Back K, Tan DX, Reiter RJ (2016) Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pineal Res 61:426–437. https://doi.org/10.1111/jpi.12364
Bajwa V, Shukla M, Sherif S, Murch S, Saxena P (2014) Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J Pineal Res 56:238–245. https://doi.org/10.1111/jpi.12115
Byeon Y, Back K (2014) An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J Pineal Res 56:408–414. https://doi.org/10.1111/jpi.12129
Byeon Y, Back K (2016) Low melatonin production by suppression of either serotonin N-acetyltransferase or N-acetylserotonin methyltransferase in rice causes seedling growth retardation with yield penalty, abiotic stress susceptibility, and enhanced coleoptile growth under anoxic conditions. J Pineal Res 60:348–359. https://doi.org/10.1111/jpi.12317
Byeon Y, Lee HY, Hwang OJ, Lee HJ, Lee K, Back K (2015) Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J Pineal Res 58:470–478. https://doi.org/10.1111/jpi.12232
Byeon Y, Lee HY, Back K (2016) Cloning and characterization of the serotonin N-acetyltransferase-2 gene (SNAT2) in rice (Oryza sativa). J Pineal Res 61:198–207. https://doi.org/10.1111/jpi.12339
Cai SY, Zhang Y, Xu YP et al (2017) HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J Pineal Res. https://doi.org/10.1111/jpi.12387
Cao S, Shao J, Shi L, Xu L, Shen Z, Chen W, Yang Z (2018) Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Sci Rep 8:806. https://doi.org/10.1038/s41598-018-19363-5
Cao Y, Qi C, Li S, Wang Z, Wang X, Wang J, Ren S, Li X, Zhang N, Guo Y (2019) Melatonin alleviates copper toxicity via improving copper sequestration and ROS scavenging in cucumber. Plant Cell Physiol 60:562–574. https://doi.org/10.1093/pcp/pcy226
Chen G, Huo Y, Tan DX, Liang Z, Zhang W, Zhang Y (2003) Melatonin in Chinese medicinal herbs. Life Sci 73:19–26. https://doi.org/10.1016/s0024-3205(03)00252-2
Chen Q, Qi WB, Reiter RJ, Wei W, Wang BM (2009) Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J Plant Physiol 166:324–328. https://doi.org/10.1016/j.jplph.2008.06.002
Chen L, Fan J, Hu Z, Huang X, Amombo E, Liu A, Bi A, Chen K, Xie Y, Fu J (2017a) Melatonin is involved in regulation of bermudagrass growth and development and response to low K+ stress. Front Plant Sci 8:2038. https://doi.org/10.3389/fpls.2017.02038
Chen Z, Xie Y, Gu Q, Zhao G, Zhang Y, Cui W, Xu S, Wang R, Shen W (2017b) The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radical Bio Med 108:465–477. https://doi.org/10.1016/j.freeradbiomed.2017.04.009
Chen YE, Mao JJ, Sun LQ, Huang B, Ding CB, Gu Y, Liao JQ, Hu C, Zhang ZW, Yuan S, Yuan M (2018a) Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol Plantarum 164:349–363. https://doi.org/10.1111/ppl.12737
Chen Z, Gu Q, Yu X, Huang L, Xu S, Wang R, Shen W, Shen W (2018b) Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann Bot 121:1127–1136. https://doi.org/10.1093/aob/mcx207
Chen J, Li H, Yang K, Wang Y, Yang L, Hu L, Liu R, Shi Z (2019) Melatonin facilitates lateral root development by coordinating PAO-derived hydrogen peroxide and Rboh-derived superoxide radical. Free Radical Bio Med 143:534–544. https://doi.org/10.1016/j.freeradbiomed.2019.09.011
Cui G, Sun F, Gao X, Xie K, Zhang C, Liu S, Xi Y (2018) Proteomic analysis of melatonin-mediated osmotic tolerance by improving energy metabolism and autophagy in wheat (Triticum aestivum L.). Planta 248:69–87. https://doi.org/10.1007/s00425-018-2881-2
Debnath B, Islam W, Li M, Sun Y, Lu X, Mitra S, Hussain M, Liu S, Qiu D (2019) Melatonin mediates enhancement of stress tolerance in plants. Int J Mol Sci. https://doi.org/10.3390/ijms20051040
Ding F, Wang M, Liu B, Zhang S (2017) Exogenous melatonin mitigates photoinhibition by accelerating non-photochemical quenching in tomato seedlings exposed to moderate light during chilling. Front Plant Sci 8:244. https://doi.org/10.3389/fpls.2017.00244
Ding F, Wang G, Wang M, Zhang S (2018) Exogenous melatonin improves tolerance to water deficit by promoting cuticle formation in tomato plants. Molecules. https://doi.org/10.3390/ijms20051040
Dubbels R, Reiter R, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara H, Schloot W (1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res 18:28–31. https://doi.org/10.1111/j.1600-079x.1995.tb00136.x
Erdal S (2019) Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep 38:1001–1012. https://doi.org/10.1007/s00299-019-02423-z
Fan J, Xie Y, Zhang Z, Chen L (2018) Melatonin: a multifunctional factor in plants. Int J Mol Sci. https://doi.org/10.3390/ijms19051528
Galano A, Tan D, Reiter R (2013) On the free radical scavenging activities of melatonin’s metabolites, Afmk and Amk. J Pineal Res 54:245–257. https://doi.org/10.1111/jpi.12010
Gao H, Zhang Z, Chai H, Cheng N, Yang Y, Wang D, Yang T, Cao W (2016) Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol Tec 118:103–110. https://doi.org/10.1016/j.postharvbio.2016.03.006
Gao W, Zhang Y, Feng Z, Bai Q, He J, Wang Y (2018) Effects of melatonin on antioxidant capacity in naked oat seedlings under drought stress. Molecules. https://doi.org/10.3390/molecules23071580
Gao W, Feng Z, Bai Q, He J, Wang Y (2019) Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int J Mol Sci. https://doi.org/10.3390/ijms20051176
Gong X, Shi S, Dou F, Song Y, Ma F (2017) Exogenous melatonin alleviates alkaline stress in Malus hupehensis Rehd. by regulating the biosynthesis of polyamines. Molecules. https://doi.org/10.3390/molecules22091542
Hasan MK, Ahammed GJ, Yin L, Shi K, Xia X, Zhou Y, Yu J, Zhou J (2015) Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front Plant Sci 6:601. https://doi.org/10.3389/fpls.2015.00601
Hernández-Ruiz J, Arnao M (2018) Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agronomy 8:33. https://doi.org/10.3390/agronomy8040033
Huang YH, Liu SJ, Yuan S, Guan C, Tian DY, Cui X, Zhang YW, Yang FY (2017) Overexpression of ovine AANAT and HIOMT genes in switchgrass leads to improved growth performance and salt-tolerance. Sci Rep-uk. https://doi.org/10.1038/s41598-017-12566-2
Huang B, Chen YE, Zhao YQ, Ding CB, Liao JQ, Hu C, Zhou LJ, Zhang ZW, Yuan S, Yuan M (2019) Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front Plant Sci 10:677. https://doi.org/10.3389/fpls.2019.00677
Hwang OJ, Back K (2018) Melatonin is involved in skotomorphogenesis by regulating brassinosteroid biosynthesis in rice plants. J Pineal Res 65:e12495. https://doi.org/10.1111/jpi.12495
Jahan MS, Shu S, Wang Y, Chen Z, He M, Tao M, Sun J, Guo S (2019) Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol 19:414. https://doi.org/10.1186/s12870-019-1992-7
Janice L, Tebello N, Santy D (1998) The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: an adsorptive voltammetric study. J Pineal Res 24:15–21. https://doi.org/10.1111/j.1600-079x.1998.tb00361.x
Kang K, Kim Y, Park S, Back K (2009) Senescence-induced serotonin biosynthesis and its role in delaying senescence in rice leaves. Plant Physiol 150:1380–1393. https://doi.org/10.1104/pp.109.138552
Kang K, Lee K, Park S, Kim Y, Back K (2010) Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J Pineal Res 49:176–182. https://doi.org/10.1111/j.1600-079X.2010.00783.x
Kang K, Kong K, Park S, Natsagdor U, Kim YS, Back K (2011) Molecular cloning of a plant N-acetylserotonin methyltransferase and its expression characteristics in rice. J Pineal Res 50:304–309. https://doi.org/10.1111/j.1600-079X.2010.00841.x
Ke Q, Ye J, Wang B, Ren J, Yin L, Deng X, Wang S (2018) Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front Plant Sci 9:914. https://doi.org/10.3389/fpls.2018.00914
Lee HY, Back K (2016a) 2-Hydroxymelatonin promotes the resistance of rice plant to multiple simultaneous abiotic stresses (combined cold and drought). J Pineal Res 61:303–316. https://doi.org/10.1111/jpi.12347
Lee HY, Back K (2016b) Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J Pineal Res 60:327–335. https://doi.org/10.1111/jpi.12314
Lee HY, Back K (2017a) Melatonin is required for H2O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. J Pineal Res. https://doi.org/10.1111/jpi.12379
Lee K, Back K (2017b) Overexpression of rice serotonin N-acetyltransferase 1 in transgenic rice plants confers resistance to cadmium and senescence and increases grain yield. J Pineal Res. https://doi.org/10.1111/jpi.12392
Lee HJ, Back K (2019a) 2-Hydroxymelatonin confers tolerance against combined cold and drought stress in tobacco, tomato, and cucumber as a potent anti-stress compound in the evolution of land plants. Melatonin Res 2:35–46
Lee K, Back K (2019b) Melatonin-deficient rice plants show a common semidwarf phenotype either dependent or independent of brassinosteroid biosynthesis. J Pineal Res 66:e12537. https://doi.org/10.1111/jpi.12537
Lee H, Byeno Y, Back K (2014a) Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J Pineal Res 57:262–268. https://doi.org/10.1111/jpi.12165
Lee H, Byeon Y, Lee K, Lee H, Back K (2014b) Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localization. J Pineal Res 75:418–426. https://doi.org/10.1111/jpi.12181
Lee K, Zawadzka A, Czarnocki Z, Reiter RJ, Back K (2016) Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). J Pineal Res 61:470–480. https://doi.org/10.1111/jpi.12361
Lei X, Zhu R, Zhang G, Dai Y (2004) Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: the possible involvement of polyamines. J Pineal Res 36:126–131. https://doi.org/10.1046/j.1600-079x.2003.00106.x
Lerner A, Case J, Takahashi Y, Lee T, Mori W (1958) Isolation of melatonin, a pineal factor that lightens melanocytes. J Am Chem Soc 80:2587. https://doi.org/10.1021/ja01543a060
Li C, Wang P, Wei Z, Liang D, Liu C, Yin L, Jia D, Fu M, Ma F (2012) The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res 53:298–306. https://doi.org/10.1111/j.1600-079X.2012.00999.x
Li C, Tan DX, Liang D, Chang C, Jia D, Ma F (2015) Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J Exp Bot 66:669–680. https://doi.org/10.1093/jxb/eru476
Li C, Liang B, Chang C, Wei Z, Zhou S, Ma F (2016a) Exogenous melatonin improved potassium content in Malus under different stress conditions. J Pineal Res 61:218–229. https://doi.org/10.1111/jpi.12342
Li H, He J, Yang X, Li X, Luo D, Wei C, Ma J, Zhang Y, Yang J, Zhang X (2016b) Glutathione-dependent induction of local and systemic defense against oxidative stress by exogenous melatonin in cucumber (Cucumis sativus L.). J Pineal Res 60:206–216. https://doi.org/10.1111/jpi.12304
Li X, Tan DX, Jiang D, Liu F (2016c) Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. J Pineal Res 61:328–339. https://doi.org/10.1111/jpi.12350
Li H, Chang J, Chen H, Wang Z, Gu X, Wei C, Zhang Y, Ma J, Yang J, Zhang X (2017a) Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front Plant Sci 8:295. https://doi.org/10.3389/fpls.2017.00295
Li H, Chang J, Zheng J, Dong Y, Liu Q, Yang X, Wei C, Zhang Y, Ma J, Zhang X (2017b) Local melatonin application induces cold tolerance in distant organs of Citrullus lanatus L. via long distance transport. Sci Rep 7:40858. https://doi.org/10.1038/srep40858
Li X, Yu B, Cui Y, Yin Y (2017c) Melatonin application confers enhanced salt tolerance by regulating Na+ and Cl- accumulation in rice. Plant Growth Regul 83:441–454. https://doi.org/10.1007/s10725-017-0310-3
Li C, Zhao Q, Gao T, Wang H, Zhang Z, Liang B, Wei Z, Liu C, Ma F (2018a) The mitigation effects of exogenous melatonin on replant disease in apple. J Pineal Res 65:e12523. https://doi.org/10.1111/jpi.12523
Li J, Arkorful E, Cheng S, Zhou Q, Li H, Chen X, Sun K, Li X (2018b) Alleviation of cold damage by exogenous application of melatonin in vegetatively propagated tea plant (Camellia sinensis (L.) O. Kuntze). Sci Hortic-amsterdam 238:356–362. https://doi.org/10.1016/j.scienta.2018.04.068
Li C, He Q, Zhang F, Yu J, Li C, Zhao T, Zhang Y, Xie Q, Su B, Mei L, Zhu S, Chen J (2019) Melatonin enhances cotton immunity to Verticillium wilt via manipulating lignin and gossypol biosynthesis. Plant J 100:784–800. https://doi.org/10.1111/tpj.14477
Liang C, Zheng G, Li W, Wang Y, Hu B, Wang H, Wu H, Qian Y, Zhu X, Tan D, Chen S, Chu C (2015) Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J Pineal Res 59:91–101. https://doi.org/10.1111/jpi.12243
Liang C, Li A, Yu H, Li W, Liang C, Guo S, Zhang R, Chu C (2017) Melatonin regulates root architecture by modulating auxin response in rice. Front Plant Sci 8:134. https://doi.org/10.3389/fpls.2017.00134
Liang D, Gao F, Ni Z, Lin L, Deng Q, Tang Y, Wang X, Luo X, Xia H (2018) Melatonin improves heat tolerance in kiwifruit seedlings through promoting antioxidant enzymatic activity and glutathione s-transferase transcription. Molecules 23:584. https://doi.org/10.3390/molecules23030584
Liu N, Gong B, Jin Z, Wang X, Wei M, Yang F, Li Y, Shi Q (2015) Sodic alkaline stress mitigation by exogenous melatonin in tomato needs nitric oxide as a downstream signal. J Plant Physiol 186:68–77. https://doi.org/10.1016/j.jplph.2015.07.012
Ma X, Zhang J, Burgess P, Rossi S, Huang B (2018) Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ Exp Bot 145:1–11. https://doi.org/10.1016/j.envexpbot.2017.10.010
Martinez V, Nieves-Cordones M, Lopez-Delacalle M, Rodenas R, Mestre TC, Garcia-Sanchez F, Rubio F, Nortes PA, Mittler R, Rivero RM (2018) Tolerance to stress combination in tomato plants: new insights in the protective role of melatonin. Molecules. https://doi.org/10.3390/molecules23030535
Mauriz J, Collado P, Veneroso C, Reiter R, González-Gallego J (2013) A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J Pineal Res 54:1–14. https://doi.org/10.1111/j.1600-079X.2012.01014.x
Meng J, Xu T, Wang Z, Fang Y, Xi Z, Zhang Z (2014) The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J Pineal Res 57:200–212. https://doi.org/10.1111/jpi.12159
Moustafa-Farag M, Almoneafy A, Mahmoud A, Elkelish A, Arnao MB, Li L, Ai S (2020) Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 10:54. https://doi.org/10.3390/biom10010054
Mukherjee S, David A, Yadav S, Baluška F, Bhatla S (2014) Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol Plantarum 152:714–728. https://doi.org/10.1111/ppl.12218
Nawaz MA, Jiao Y, Chen C, Shireen F, Zheng Z, Imtiaz M, Bie Z, Huang Y (2018) Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J Plant Physiol 220:115–127. https://doi.org/10.1016/j.jplph.2017.11.003
Ni J, Wang Q, Shah FA, Liu W, Wang D, Huang S, Fu S, Wu L (2018) Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 23:799. https://doi.org/10.3390/molecules23040799
Park S, Back K (2012) Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J Pineal Res 53:385–389. https://doi.org/10.1111/j.1600-079X.2012.01008.x
Park S, Lee D, Jang H, Byeon Y, Kim Y, Back K (2013) Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J Pineal Res 54:258–263. https://doi.org/10.1111/j.1600-079x.2012.01029.x
Pelagio-Flores R, Muñoz-Parra E, Ortiz-Castro R, López-Bucio J (2012) Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J Pineal Res 53:279–288. https://doi.org/10.1111/j.1600-079X.2012.00996.x
Pickles V, Sutcliffe J (1955) The effects of 5-hydroxytryptamine, indole-3-acetic acid, and some other substances, on pigment effusion, sodium uptake, and potassium efflux, by slices of red beetroot in vitro. Biochim Biophys Acta 17:244–251. https://doi.org/10.1016/0006-3002(55)90356-5
Qi ZY, Wang KX, Yan MY, Kanwar MK, Li DY, Wijaya L, Alyemeni MN, Ahmad P, Zhou J (2018) Melatonin alleviates high temperature-induced pollen abortion in solanum lycopersicum. Molecules 23:386. https://doi.org/10.3390/molecules23020386
Qiao Y, Yin L, Wang B, Ke Q, Deng X, Wang S (2019) Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiol Biochem 139:342–349. https://doi.org/10.1016/j.plaphy.2019.03.037
Ren S, Rutto L, Katuuramu D (2019) Melatonin acts synergistically with auxin to promote lateral root development through fine tuning auxin transport in Arabidopsis thaliana. PLoS ONE. https://doi.org/10.1371/journal.pone.0221687
Sami A, Shah FA, Abdullah M, Zhou X, Zhou K (2020) Melatonin mitigates cadmium and aluminium toxicity through modulation of antioxidant potential in Brassica napus L. Plant Biol. https://doi.org/10.1111/plb.13093
Sarropoulou V, Therios I, Dimassi-Theriou K (2012) Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus × P. canescens), and MxM 60 (P. avium × P. mahaleb). J Pineal Res 52:38–46. https://doi.org/10.1111/j.1600-079X.2011.00914.x
Shah AA, Ahmed S, Yasin NA (2020) 2-Hydroxymelatonin induced nutritional orchestration in Cucumis sativus under cadmium toxicity: modulation of non-enzymatic antioxidants and gene expression. Int J Phytoremediation 22:497–570. https://doi.org/10.1080/15226514.2019.1683715
Shi H, Chen Y, Tan D, Reiter R, Chan Z, He C (2015a) Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J Pineal Res 59:102–108. https://doi.org/10.1111/jpi.12244
Shi H, Qian Y, Tan D, Reiter R, He C (2015b) Melatonin induces the transcripts of CBF/DREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis. J Pineal Res 59:334–342. https://doi.org/10.1111/jpi.12262
Shi H, Tan D, Reiter R, Ye T, Yang F, Chan Z (2015c) Melatonin induces class A1 heat-shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J Pineal Res 58:335–342. https://doi.org/10.1111/jpi.12219
Shi H, Wang X, Tan D, Reiter R, Chan Z (2015d) Comparative physiological and proteomic analyses reveal the actions of melatonin in the reduction of oxidative stress in Bermuda grass (Cynodon dactylon (L). Pers.). J Pineal Res 59:120–131. https://doi.org/10.1111/jpi.12246
Shi H, Wei Y, He C (2016) Melatonin-induced CBF/DREB1s are essential for diurnal change of disease resistance and CCA1 expression in Arabidopsis. Plant Physiol Biochem 100:150–155. https://doi.org/10.1016/j.plaphy.2016.01.018
Simlat M, Ptak A, Skrzypek E, Warchoł M, Morańska E, Piórkowska E (2018) Melatonin significantly influences seed germination and seedling growth of Stevia rebaudiana Bertoni. PeerJ 6:e5009. https://doi.org/10.7717/peerj.5009
Su X, Xin L, Li Z, Zheng H, Mao J, Yang Q (2018) Physiology and transcriptome analyses reveal a protective effect of the radical scavenger melatonin in aging maize seeds. Free Radic Rese 52:1094–1109. https://doi.org/10.1080/10715762.2018.1472378
Sun Q, Zhang N, Wang J, Zhang H, Li D, Shi J, Li R, Weeda S, Zhao B, Ren S, Guo Y (2015) Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J Exp Bot 66:657–668. https://doi.org/10.1093/jxb/eru332
Sun Q, Zhang N, Wang J, Cao Y, Li X, Zhang H, Zhang L, Tan D, Guo Y (2016) A label-free differential proteomics analysis reveals the effect of melatonin on promoting fruit ripening and anthocyanin accumulation upon postharvest in tomato. J Pineal Res 61:138–153. https://doi.org/10.1111/jpi.12315
Sun C, Lv T, Huang L, Liu X, Jin C, Lin X (2020a) Melatonin ameliorates aluminum toxicity through enhancing aluminum exclusion and reestablishing redox homeostasis in roots of wheat. J Pineal Res. https://doi.org/10.1111/jpi.12642
Sun S, Wen D, Yang W, Meng Q, Shi Q, Gong B (2020b) Overexpression of caffeic acid o-methyltransferase 1 (COMT1) increases melatonin level and salt stress tolerance in tomato plant. J Plant Growth Regul 39:1221–1235. https://doi.org/10.1007/s00344-019-10058-3
Tan D (2015) Melatonin and plants. J Exp Bot 66:625–626. https://doi.org/10.1093/jxb/eru523
Tan DX, Reiter RJ (2020) An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J Exp Bot 71(16):4677–4689. https://doi.org/10.1093/jxb/eraa235
Tan DX, Manchester LC, Mascio P, Martinez GR, Prado FM, Reiter RJ (2007) Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phytoremediation. FASEB J 21:1724–1729. https://doi.org/10.1096/fj.06-7745com
Tan XL, Fan ZQ, Kuang JF, Lu WJ, Reiter RJ, Lakshmanan P, Su XG, Zhou J, Chen JY, Shan W (2019) Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J Pineal Res 67:e12570. https://doi.org/10.1111/jpi.12570
Wang P, Yin L, Liang D, Li C, Ma F, Yue Z (2012) Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J Pineal Res 53:11–20. https://doi.org/10.1111/j.1600-079X.2011.00966.x
Wang L, Zhao Y, Reiter R, He C, Liu G, Lei Q, Zuo B, Zheng X, Li Q, Kong J (2014) Changes in melatonin levels in transgenic ‘Micro-Tom’ tomato overexpressing ovine AANAT and ovine HIOMT genes. J Pineal Res 56:134–142. https://doi.org/10.1111/jpi.12105
Wang P, Sun X, Wang N, Tan D, Ma F (2015) Melatonin enhances the occurrence of autophagy induced by oxidative stress in Arabidopsis seedlings. J Pineal Res 58:479–489. https://doi.org/10.1111/jpi.12233
Wang Q, An B, Wei Y, Reiter RJ, Shi H, Luo H, He C (2016) Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis. Front Plant Sci 7:1882. https://doi.org/10.3389/fpls.2016.01882
Wang L, Feng C, Zheng X, Guo Y, Zhou F, Shan D, Liu X, Kong J (2017) Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J Pineal Res 63:e12429. https://doi.org/10.1111/jpi.12429
Wang M, Zhang T, Ding F (2019) Exogenous melatonin delays methyl jasmonate-triggered senescence in tomato leaves. Agronomy 9:795. https://doi.org/10.3390/agronomy9120795
Wang M, Zhang S, Ding F (2020a) Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants. Antioxidants 9:218. https://doi.org/10.3390/antiox9030218
Wang X, Zhang H, Xie Q, Liu Y, Lv H, Bai R, Ma R, Li X, Zhang X, Guo YD, Zhang N (2020b) SlSNAT interacts with HSP40, a molecular chaperone, to regulate melatonin biosynthesis and promote thermotolerance in tomato. Plant Cell Physiol 61:909–921. https://doi.org/10.1093/pcp/pcaa018
Weeda S, Zhang N, Zhao X, Ndip G, Guo Y, Buck G, Fu C, Ren S (2014) Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 9:e93462. https://doi.org/10.1371/journal.pone.0093462
Wei W, Li Q, Chu Y, Reiter R, Yu X, Zhu D, Zhang W, Ma B, Lin Q, Zhang J, Chen S (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 66:695–707. https://doi.org/10.1093/jxb/eru392
Wei J, Li DX, Zhang JR, Shan C, Rengel Z, Song ZB, Chen Q (2018a) Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J Pineal Res 65:e12500. https://doi.org/10.1111/jpi.12500
Wei Z, Gao T, Liang B, Zhao Q, Ma F, Li C (2018b) Effects of exogenous melatonin on methyl viologen-mediated oxidative stress in apple leaf. Int J Mol Sci 19:316. https://doi.org/10.3390/ijms19010316
Wen D, Gong B, Sun S, Liu S, Wang X, Wei M, Yang F, Li Y, Shi Q (2016) Promoting roles of melatonin in adventitious root development of solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front Plant Sci 7:718. https://doi.org/10.3389/fpls.2016.00718
Xiao S, Liu L, Wang H, Li D, Bai Z, Zhang Y, Sun H, Zhang K, Li C (2019) Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 14:e0216575. https://doi.org/10.1101/618959
Xu W, Cai S, Zhang Y, Wang Y, Ahammed G, Xia X, Shi K, Zhou Y, Yu J, Reiter R, Zhou J (2016) Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J Pineal Res 61:457–469. https://doi.org/10.1111/jpi.12359
Xu L, Yue Q, Xiang G, Bian F, Yao Y (2018) Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hortic Res 5:41. https://doi.org/10.1038/s41438-018-0045-y
Yang WJ, Du YT, Zhou YB, Chen J, Xu ZS, Ma YZ, Chen M, Min DH (2019) Overexpression of TaCOMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis. Int J Mol Sci 20:652. https://doi.org/10.3390/ijms20030652
Ye T, Yin X, Yu L, Zheng SJ, Cai WJ, Wu Y, Feng YQ (2019) Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-mass spectrometry. J Pineal Res 66:e12531. https://doi.org/10.1111/jpi.12531
Ye J, Yang W, Li Y, Wang S, Yin L, Deng X (2020) Seed pre-soaking with melatonin improves wheat yield by delaying leaf senescence and promoting root development. Agronomy 10:84. https://doi.org/10.3390/agronomy10010084
Yin L, Wang P, Li M, Ke X, Li C, Liang D, Wu S, Ma X, Li C, Zou Y, Ma F (2013) Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J Pineal Res 54:426–434. https://doi.org/10.1111/jpi.12038
Yin Z, Lu J, Meng S, Liu Y, Mostafa I, Qi M, Li T (2019) Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. J Plant Interact 14:453–463. https://doi.org/10.1080/17429145.2019.1645895
Zeng L, Cai J, Li J, Lu G, Li C, Fu G, Zhang X, Ma H, Liu Q, Zou X, Cheng Y (2018) Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J Integr Agr 17:328–335. https://doi.org/10.1016/S2095-3119(17)61757-X
Zhang N, Zhao B, Zhang H, Weeda S, Yang C, Yang Z, Ren S, Guo Y (2013) Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J Pineal Res 54:15–23. https://doi.org/10.1111/j.1600-079X.2012.01015.x
Zhang H, Zhang N, Yang R, Wang L, Sun Q, Li D, Cao Y, Weeda S, Zhao B, Ren S, Guo Y (2014a) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res 57:269–279. https://doi.org/10.1111/jpi.12167
Zhang N, Zhang H, Zhao B, Sun Q, Cao Y, Li R, Wu X, Weeda S, Li L, Ren S, Reiter R, Guo Y (2014b) The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J Pineal Res 56:39–50. https://doi.org/10.1111/jpi.12095
Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S, Guo Y (2015) Roles of melatonin in abiotic stress resistance in plants. J Exp Bot 66:647–656. https://doi.org/10.1093/jxb/eru336
Zhang J, Shi Y, Zhang X, Du H, Xu B, Huang B (2017a) Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ Exp Bot 138:36–45. https://doi.org/10.1016/j.envexpbot.2017.02.012
Zhang N, Zhang H, Sun Q, Cao Y, Li X, Zhao B, Wu P, Guo Y (2017b) Proteomic analysis reveals a role of melatonin in promoting cucumber seed germination under high salinity by regulating energy production. Sci Rep 7:503. https://doi.org/10.1038/s41598-017-00566-1
Zhang H, Wang L, Shi K, Shan D, Zhu Y, Wang C, Bai Y, Yan T, Zheng X, Kong J (2019a) Apple tree flowering is mediated by low level of melatonin under the regulation of seasonal light signal. J Pineal Res 66:e12551. https://doi.org/10.1111/jpi.12551
Zhang Q, Liu X, Zhang Z, Liu N, Li D, Hu L (2019b) Melatonin improved waterlogging tolerance in alfalfa (Medicago sativa) by reprogramming polyamine and ethylene metabolism. Front Plant Sci 10:44. https://doi.org/10.3389/fpls.2019.00044
Zhang T, Shi Z, Zhang X, Zheng S, Wang J, Mo J (2020) Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci Hortic 262:109070. https://doi.org/10.1016/j.scienta.2019.109070
Zhao H, Xu L, Su T, Jiang Y, Hu L, Ma F (2015) Melatonin regulates carbohydrate metabolism and defenses against Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis thaliana. J Pineal Res 59:109–119. https://doi.org/10.1111/jpi.12245
Zheng X, Tan DX, Allan AC, Zuo B, Zhao Y, Reiter RJ, Wang L, Wang Z, Guo Y, Zhou J, Shan D, Li Q, Han Z, Kong J (2017) Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci Rep 7:41236. https://doi.org/10.1038/srep41236
Zhou C, Liu Z, Zhu L, Ma Z, Wang J, Zhu J (2016a) Exogenous melatonin improves plant iron deficiency tolerance via increased accumulation of polyamine-mediated nitric oxide. Int J Mol Sci 17:1777. https://doi.org/10.3390/ijms17111777
Zhou X, Zhao H, Cao K, Hu L, Du T, Baluska F, Zou Z (2016b) Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front Plant Sci 7:1823. https://doi.org/10.3389/fpls.2016.01823
Zia SF, Berkowitz O, Bedon F, Whelan J, Franks AE, Plummer KM (2019) Direct comparison of Arabidopsis gene expression reveals different responses to melatonin versus auxin. BMC Plant Biol 19:567. https://doi.org/10.1186/s12870-019-2158-3
Zuo B, Zheng X, He P, Wang L, Lei Q, Feng C, Zhou J, Li Q, Kong J (2014) Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J Pineal Res 57:408–417. https://doi.org/10.1111/jpi.12180
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31860054, 32060711, 31460099).
Author information
Authors and Affiliations
Contributions
TZ and SZ wrote manuscript. JW, YS and LZ collected literature and wrote manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Handling editor: Rhonda Peavy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, T., Wang, J., Sun, Y. et al. Versatile Roles of Melatonin in Growth and Stress Tolerance in Plants. J Plant Growth Regul 41, 507–523 (2022). https://doi.org/10.1007/s00344-021-10317-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10317-2