Abstract
FTL10 is an early-flowering mutant of rice (Oryza sativa L.) with a premature senescent phenotype. Early leaf senescence can cause negative effects on rice yield. Moreover, rice leaves are damaged under high-light conditions, which promote rice senescence. Artificial shading can reduce the amount of light absorbed by rice leaves. The aim of this study was to investigate the effects of shading at noon (11:30–14:00) on the senescence and yield of FTL10. The results showed that shading improved the total antioxidant capacity of rice leaves, reduced the accumulation of reactive oxygen species (ROS) and reduced the expression of genes related to senescence. In the shaded group, the degradation rate of chlorophyll and Rubisco proteins, which are related to photosynthesis, was relatively slow, and the photosynthetic rate was relatively high. Compared with those under the natural growth conditions, the proportion of photosynthetic electron allocated to photorespiration in the shaded group rice leaves was lower, and the proportion allocated to carbon fixation was higher. The yield data showed that the single-spike weight and yield per plant of rice significantly increased after shading. Therefore, our research shows that shading at noon could delay FTL10 senescence and increase yields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is the staple crop for approximately half of the global population (Iizumi and Ramankutty 2016). Rice yields are mainly provided by the photosynthesis of leaves after heading (Mae 1997). Leaf senescence is considered the final stage of leaf development and is vital for plant fitness (Lim et al. 2007), but it is an important factor that affects crop yield traits (Schippers et al. 2015). Leaf senescence can be triggered by many endogenous and external factors (Lee et al. 2015).
Reactive oxygen species (ROS), as an important factor, can not only stimulate the expression of senescence genes as signaling molecules (Juvany et al. 2013) but also accelerate senescence by destroying biological macromolecules as toxic substances (Taheri et al. 2014). A previous study showed that ROS play a dual role in the regulation of leaf senescence (Pintó-Marijuan and Munné-Bosch 2014). ROS are produced for many reasons, one of which is in response to excess light energy (Zhang et al. 2016). ROS can be produced by photosystem II (PSII) and photosystem I (PSI) when excess energy occurs within the electron transport chain (Asada 2006). During the growth of plants, especially at noon in the summer, excess light energy often occurs, which leads to high-light stress. It is well known that the components of the photosynthetic apparatus are damaged under high-light conditions (Kirchhoff 2014). High-light stress can reduce the net photosynthetic rate (Pn) and the amount of pigments (Stefanov et al. 2018). Studies have shown that ROS increase significantly and that Rubisco activity and the Pn decrease significantly with prolonged high-light stress (Lu et al. 2017; Serodio et al. 2017).
Plants have developed strategies to reduce high-light stress or ameliorate its effects. These strategies include reducing the absorption of light energy, the dissipation of chlorophyll (Chl) light energy and the removal of ROS produced by excess light energy (Takahashi and Badger 2011; Müller et al. 2001; Neill et al. 2002). The most basic strategy is to reduce the absorption of light energy. This feat can be achieved by chloroplast movement and leaf movement (Takahashi and Badger 2011), and anthocyanins can also form near the surface of some plant leaves to block light energy (Zhang et al. 2016). Studies have shown that shading can reduce the absorption of light by plant leaves, thereby altering plant growth and crop yields. At the heading stage, the duration of the seed-filling period and the seed-filling rate were increased by shading at all positions (Labra et al. 2017). In mango (Mangifera indica L.) trees, shading increased the photosynthetic activity by reducing stomatal limitations for carbon supply and was effective at alleviating photoinhibitory damage to PSII on sunny days (Jutamanee and Onnom 2016). The leaves of European beech (Fagus sylvatica L.) seedlings under low light had the ability to use light more efficiently (higher PSII efficiency) than did those under high light (Sevillano et al. 2018). However, in maize (Zea mays L.), shading reduces the Pn of leaves, leading to a decrease in yield (Ren et al. 2016).
The shading methods adopted by the above scholars mainly involve shading plants all day. In the present experiment, the shading treatment was carried out for a specific period of time, that is, from 11:30–14:00 every day. The rice material used in this paper was the OsFTL10 overexpression line FTL10, which exhibits premature flowering and senescence. Previous studies have shown that overexpression of the FT gene can promote early flowering in plants (Hayama et al. 2007). Overexpression of the FT homologous gene PdFT2 in poplar (Populus L.) can shorten its juvenile period, which therefore accelerates poplar breeding and research (Hsu et al. 2006). Overexpression of the OsFTL2 gene promotes an early-flowering phenotype in rice (Kojima et al. 2002). In this paper, FTL10 was shaded to explore the effects of reducing excess light on delaying the senescence of early-flowering rice.
Materials and Methods
Plant Materials and Growth Conditions
The rice material used in this paper was the OsFTL10 overexpression line FTL10. FTL10 has an early-flowering phenotype compared with wild type (ZH-11) plants (Fang et al. 2019). Sterilized seeds were planted in an artificial climate incubator and grown under controlled conditions, which consisted of an average temperature of 33 °C/28 °C (day/night), a relative humidity of 80%, and a photoperiod of 14/10 h (day/night). Once the seedlings had four leaves, they were taken out gently and transplanted to the biological garden of South China Normal University, Guangzhou, China. When the plants began to head, shading was carried out from 11:30–14:00 (noon) every day. For the shaded group, rice was planted in the field, and a 2.5 m high iron frame was fixed to the periphery. A shade net was hung on the iron frame through the bars to achieve the shade settings (Fig. 1). The photosynthetic photon flux density (PPFD) of the shade and without the shade was measured using an MQ-100 hand-held quantum meter (Apogee Instruments, Inc., USA), and the PPFD of the shade was 70% that without shade. The plants without shade were used as the control group (CK). Rice plants with different treatments in the field were randomly selected. The flag leaves of rice were cut every 10 days for the experiment after shading.
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted from flag leaves using an Omega R6827 kit (Omega) in accordance with the manufacturer’s instructions. First-strand cDNA was synthesized with oligo (dT) primers using an M-MLV reverse transcriptase kit (Takara), and the cDNA was stored at − 80 °C until further analysis. Quantitative real-time PCR (q-PCR) was performed using an Applied Biosystems platform. Each 10 μL PCR contained 0.5 μL of cDNA, 3.5 μL of RNAse-free water, 5.2 μL of SYBR® Premix Ex TaqTM II (Takara), and 0.4 μL of each primer, which were synthesized by Invitrogen. Rice OsUBQ was used as an internal control. Normalization and fold changes were calculated using the method as described by Livak and Schmittgen (2001). The primers for real-time PCR were designed as follows: 5′-CCAGGACAAGATGATCTGCC-3′ (forward) and 5′-AAGAAGCTGAAGCATCCAGC-3′ (reverse) for OsUBQ; 5′-ACCCGCAGCAGCTTCTCGCCAA-3′ (forward) and 5′-CAAGCACCAAAACCTCCTCCAT-3′ (reverse) for OsA12; 5′-AGGGGTGGTACAACAAGCTG-3′ (forward) and 5′-GCTCCTTGCGGAAGATGTAG-3′ (reverse) for OsSGR; 5′-TATCTCAGATCATACCGGATG-3′ (forward) and 5′-AAGTACTCCCTCAGCGATGG-3′ (reverse) for OsFTL10; and 5′-CAAGAAGCCGAACGGTTC-3′ (forward) and 5′-GTTAGAGTGGAGCAGCAT-3′ (reverse) for OsNAP.
Detection of H2O2 and O·−2 in Living Tissue
At 40 days after heading (DAH), H2O2 generation in the leaves was detected by an endogenous peroxidase-dependent in situ histochemical staining procedure using 3,3′-diaminobenzidine (DAB). The leaves were placed in a solution containing 1 mg mL−1 DAB (pH of 5.5) for vacuum infiltration three times (10 min per time) and then incubated at room temperature for 2 h. Oxidation of DAB leads to its polymerization and deposition at the site of H2O2 generation. The leaves were subsequently boiled in ethanol (95%, v/v) for 10 min and then stored in 60% glycerol. The H2O2 production was visualized as a reddish-brown color (Liu et al. 2007).
At 40 DAH, O·−2 production was monitored by nitroblue tetrazolium (NBT) staining. The leaves were placed in a solution containing NBT (1 mg mL−1) plus NaN3 (10 mM) in 10 mM potassium phosphate buffer (pH 7.8). After vacuum infiltration three times (10 min per time), the leaves were stained for 4 h at room temperature. The leaves were then boiled in ethanol (95%, v/v) for 10 min and subsequently stored in 60% glycerol. The NBT was visualized as a dark blue-colored formazan deposit (Liu et al. 2007).
Measurement of Chlorophyll Content
A total of 0.1 g of fresh leaves was extracted with 10 mL of 80% (v/v) acetone. The absorption of the extracts was measured at 663 and 645 nm using a UV–Vis 2450 spectrophotometer (Shimadzu, Tokyo, Japan). Chl a and Chl b were determined using the predetermined extinction coefficients and equations established by Lichtenthaler (1987): Chl a (mg L−1) = 12.21A663–2.81A645, and Chl b (mg L−1) = 20.13A645–5.03A663.
Determination of Rubisco Protein
Rubisco protein determination was carried out according to the method as described by Liu et al. (2011). Fresh leaves (0.1 g) were homogenized in 2 mL of grinding medium composed of 50 mM Tris–HCl (pH of 7.8) buffer containing 5% (w/v) polyvinylpyrrolidone (PVP), 0.1% (v/v) β-mercaptoethanol, 0.1% (w/v) NaCl, 0.17% (w/v) phenylmethanesulfonyl fluoride (PMSF), and 2% (v/v) glycerol, and then the homogenate was centrifuged at 13,000×g for 10 min. The supernatant was subsequently collected for the determination of Rubisco and total soluble protein. The supernatant (50 μL) was added to an equal volume of sample buffer composed of 24% (v/v) glycerol, 2% (v/v) 2-mercaptoethanol, 10 mM Tris, 2% (w/v) sodium dodecyl sulfate (SDS), and 0.02% (w/v) bromophenol blue. The mixture was incubated in boiling water for 5 min. The samples (10 μL) were then loaded onto gels that consisted of 12.5% (w/v) resolving polyacrylamide gel and 4% (w/v) polyacrylamide gel stacker. The resolving gels consisted of 0.4% N,N’-methylene-bis-acrylamide, 12.1% acrylamide, 0.5 M Tris–HCl (pH 8.8), 0.08% ammonium persulfate, 0.08% N,N,N’,N’-tetramethylethylenediamine (TEMED), and 0.1% SDS. The stacking gels consisted of 0.1% N,N’-methylene-bis-acrylamide, 3.9% acrylamide, 0.125 M Tris–HCl (pH of 6.8), 0.05% ammonium persulfate, 0.1% SDS, and 0.08% TEMED. The total proteins were separated by SDS-PAGE with a Mini-PROTEAN 3 system (Bio-Rad, USA); a constant voltage of 80 V for 30 min was applied to concentrate the proteins, and then 110 V for 80 min was applied to separate the proteins. Afterward, the gels were stained with Coomassie Brilliant Blue R-250 (Sigma, USA) and destained overnight with gentle shaking to ensure that the background turned colorless. The molecular masses of the large and small subunits of Rubisco were 55 and 15 kDa, respectively. The SDS-PAGE gels were scanned using a GelDoc-It Imaging System (UVP, Upland, CA, USA), and the relative gray value of each band was detected by using TotalLab Quant software (TotalLab, Newcastle upon Tyne, UK).
The total soluble proteins in the leaves were measured using the supernatant after centrifugation. After the supernatant was diluted 50 times, 250 μL of the diluted supernatant was added to the same volume of Bradford solution. After 5 min, the absorbance was read at a wavelength of 595 nm (UV–Vis 2450 spectrophotometer, Shimadzu, Tokyo, Japan). The total soluble proteins were calculated via bovine serum albumen (BSA) as the standard.
Detection of the Total Oxidation Capacity of Leaves
Antioxidant capacity was evaluated by measuring the 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging rate. In brief, rice flag leaves (0.2 g) were ground with 2 mL of 95% methanol in an ice bath, and the homogenate was centrifuged at 13,000×g at 4 °C for 10 min. The supernatant was used to evaluate the DPPH scavenging rates according to the method as described by Saha et al. (2008). Afterward, 0.1 mL of the supernatant was added to 2.9 mL of 120 μM DPPH (dissolved in 95% methanol) for 5 min in the dark. The mixture was subsequently measured at 517 nm (UV-2450, Shimadzu, Kyoto, Japan). Different concentrations of DPPH were used as standards.
Distribution of Photosynthetic Electron
The Pn and dark respiration rate (Rd) were measured by using an LI-6400 portable photosynthesis system (LI-COR, Inc., USA) on sunny days from 8:30–11:00 a.m. The operating parameters in the leaf chamber included a flow velocity of 400 μmol mol−1, a humidity of 55 ± 5%, and a leaf mean temperature of 31 °C. The PPFD was set at 800 μmol m−2 s−1 (Pn) and 0 μmol m−2 s−1 (Rd). Chlorophyll fluorescence parameters were measured with a PAM-2100 portable pulse-modulated fluorimeter (Walz, Efeltrich, Germany). The chlorophyll fluorescence (F) and maximum fluorescence (Fm′) were recorded under actinic light at 800 μmol m−2 s−1 under steady-state conditions from the leaves in which the Pn was measured. The maximum chemical efficiency of PSII (ΦPSII) was calculated as ΦPSII = (Fm′ − Fs)/Fm′ (Genty et al. 1989). Furthermore, the JT was calculated as JT = ΦPSII × PPFD × 0.85 × 0.5, in which the PPFD referred to the intensity of actinic light used to measure ΦPSII, 0.85 indicated the absorptivity of the blade, and 0.5 referred to the assumption that the light energy absorbed by the leaves was equally distributed between PSI and PSII (Valentini et al. 1995). The JC and JO were calculated according to the formulas JC = 1/3(JT + 8(Pn + Rd)) and JO = 2/3(JT − 4(Pn + Rd)), respectively (Melis et al. 1987).
Measurement of Rubisco Carboxylation Efficiency
Leaf photosynthetic CO2-response curves were generated by using an LI-6400 portable photosynthesis system (LI-COR, Inc., USA) on sunny days from 8:30–11:00 a.m. The CO2 concentration gradients (400, 200, 150, 100, 50, 20, 400, 400, 600, 800, 1000, and 1300 μmol mol−1) were set up for the CO2-response curves, and the waiting times were 90–180 s for each gradient. The humidity was 55 ± 5%, and the leaf mean temperature was 31 °C. The data were automatically recorded by the LI-6400. Rubisco carboxylation efficiency (CE) was represented by the slope of the linear regression equation of the Pn and CO2 concentration (20, 50, 100, 150, and 200 μmol mol−1).
Analysis of Yield and Yield Characteristics in Rice
Yield and its component traits were measured, including single-spike weight, grain yield per plant, thousand-grain weight and seed set. Ten plants in the middle of the inner two rows of each field plot were evaluated to score the following traits for each plant: single-spike weight (weight in grams per panicle at 12–14% moisture), yield per plant (weight in grams of grains per plant at 12–14% moisture), thousand-grain weight (weight in grams of 1000 grains from bulk-homogenized seeds at 12–14% moisture), and seed set (the number of filled grains per main panicle at maturity).
Statistical Analysis
The values are expressed as the means ± standard errors (SEs). Statistical significance was determined via Student’s t-test with SPSS Statistics 20.0 (IBM, NY, USA). The means were considered significantly different at P < 0.05. SigmaPlot 13.0 (Systat Software Inc., USA) was used to conduct the linear regression analysis and plot the data.
Results
Leaf Senescence and Antioxidant Capacity
The relative expression of senescence-related genes in FTL10 leaves was detected at 40 DAH. The expression was higher in the leaves of the CK; specifically, the expression of the genes OsA12 and OsSGR was significantly higher in the CK than in the shade treatment, and there was no significant difference regarding the genes OsFTL10 and OsNAP (Fig. 2a). Moreover, the accumulation of O·−2 and H2O2 was lower in the leaves in the shade treatment but higher in the leaves of the CK at 40 DAH (Fig. 2b). The total antioxidant capacity (TAC) of the leaves was measured and was found to increase during the senescence process of rice. The TAC in response to shading was significantly higher than that of the CK at 20 DAH. In addition, the TAC was higher under shade at 10, 30 and 40 DAH, but these differences were not significant (Fig. 2c). The phenotypes leaves at 40 DAH are shown in Fig. 2d.
Relative expression of senescence-related genes, accumulation of ROS, total oxidation capacity and the phenotype of rice leaves. a Relative expression of the OsFTL10, OsA12, OsNAP and OsSGR genes. b Accumulation of O·−2 (stained by nitroblue tetrazolium, NBT) and H2O2 (stained by 3,3′-diaminobenzidine, DAB) in rice leaves. c Changes in the total antioxidant capacity (TAC) after heading. d The phenotype of rice leaves at 40 days after heading (DAH). The data are presented as the means ± SEs (n = 4). The error bars represent the standard errors of four biological replicates, and the asterisks indicate significant differences (two-sided Student’s t-test, *P < 0.05, **P < 0.01, ns no significance)
Changes in Photosynthetic Capability
During the process of rice senescence, the total Chl content decreased gradually, and it was significantly higher in the shaded group than in the CK at 20 and 40 DAH; however, there was no significant difference at 10 or at 30 DAH (Fig. 3a). The contents of Chl a and Chl b also decreased gradually, and there was no significant difference between the shaded and CK treatments, except for Chl a at 40 DAH (Fig. 3b, c). The soluble protein contents decreased gradually during the process of rice senescence (Fig. 3d). The Rubisco content and CE in the shaded leaves were significantly higher than those in the leaves of the CK at 40 DAH (Fig. 3f). The Pn decreased rapidly after heading and was significantly higher in the shaded rice leaves than in the leaves of the CK at 10, 20 and 40 DAH (Fig. 3e).
Changes in the contents of chlorophyll, soluble proteins and Rubisco; the Pn; and the CE at 40 days after heading (DAH). a Changes in total chlorophyll (Chl). b Changes in chlorophyll a (Chl a). c Changes in chlorophyll b (Chl b). d Changes in soluble proteins. e Changes in the Pn. f The Rubisco content and Rubisco carboxylation efficiency (CE). The data are presented as the means ± SEs (n = 4). The error bars represent the standard errors of four biological replicates, and the asterisks indicate significant differences (two-sided Student’s t-test, *P < 0.05, ns no significance)
Changes in the Distribution of Photosynthetic Electron
During the process of rice senescence, the total electron transport (JT) of leaves gradually declined (Fig. 4a). Moreover, the electron transport to carboxylation ratio (JC) decreased gradually, and the trend of variation was consistent with that of JT (Fig. 4b). However, the electron transport to oxygenation ratio (JO) increased gradually, and the trend corresponded to that of JO/JT (Fig. 4c, d). During the aging process of rice, the JO and JO/JT of the CK leaves were significantly higher than those of the shaded leaves at 30 and 40 DAH.
Distribution of photosynthetic electron flow in flag leaves at 0, 10, 20, 30, and 40 days after heading (DAH). a Changes in the total photosynthetic electron flow (JT). b Changes in electron transport to Rubisco-dependent carboxylation and oxygenation (c). d Fractions of total electron flow partitioned to photorespiration (JO/JT) in flag leaves. The data are presented as the means ± SEs (n = 4). The error bars represent the standard errors of four biological replicates, and the asterisks indicate significant differences (two-sided Student’s t-test, *P < 0.05, ns no significance)
Yield and Grain Phenotypes
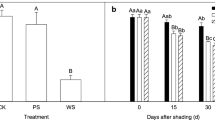
The grain phenotypes are shown in Fig. 5a. The results showed that the seed setting rate of the shaded group was lower than that of the CK group, but there was no significant difference. The single-spike weight and yield per plant were significantly higher in the shaded treatment than in the CK. Moreover, the thousand-grain weight was higher in the shaded group than in the CK, but there was no significant difference (Fig. 5b).
Comparison of yields and grain phenotypes. a Grain phenotypes. b Single-spike weight, yield per plant, thousand-grain weight and seed setting rate of the shaded and CK groups. The data are presented as the means ± SEs (n = 4). The error bars represent the standard errors of four biological replicates, and the asterisks indicate significant differences (two-sided Student’s t-test, *P < 0.05, ns no significance)
Discussion
Leaf senescence is a normal physiological phenomenon in rice, has a very positive effect on rice itself and is necessary for the transport of organic matter from sources to sinks (Lim et al. 2007). However, premature senescence is unfavorable. In agricultural production, premature senescence will shorten the functional period of leaves and can reduce rice yields. The rice material FTL10 in this paper has the characteristics of premature senescence (Fang et al. 2019). Leaf senescence can be triggered by many endogenous and external factors (Lee et al. 2015). Light intensity is one of the most important factors for plant growth, affecting growth, survival, development, and crop productivity (Wang et al. 2013). However, too high of light intensity will cause damage to plants and accelerate plant senescence. A previous report showed that light intensity is an important limiting factor influencing photosynthetic electron transport and carbon assimilation in woody plants (Lin et al. 2000). ROS are produced by PSII or PSI when excess energy occurs within the electron transport chain (Asada 2006). The results of the present study showed that shade can reduce the accumulation of ROS and can improve the TAC of FTL10 (Fig. 2b, c). The increased accumulation of ROS, which can act as signals, can promote the senescence of plant leaves (Sedigheh et al. 2011). In our study, shading significantly reduced the relative expression of the OsA12 and OsSGR genes, and the expression of the OsFTL10 and OsNAP genes was also reduced slightly in the shade group. Overexpression of the OsFTL10 gene induces early flowering in rice (Fang et al. 2019). It has been demonstrated that OsA12 is a gene involved in rice leaf senescence (Sun et al. 2013). The OsSGR gene can promote the disaggregation of the light-harvesting complex and can promote the subsequent breakdown of apo-protein and Chl during senescence (Jiang et al. 2011). It has been reported that up-regulation of the OsNAP gene significantly promotes senescence, and the knockdown of OsNAP results in delayed senescence (Liang et al. 2014). The above results showed that shading could reduce the accumulation of ROS, which led to delayed senescence of FTL10 leaves.
ROS, as highly toxic substances, constantly attack photosynthetic organs in plants (Hideg and Schreiber 2007), degrade biological macromolecules, and disrupt the integrity of membranes (Taheri et al. 2014). Our previous report showed that the accumulation of ROS leads to the rapid degradation of Chl and protein (Zhang et al. 2018). The results of the present study show that the total Chl content was significantly higher in the shade group than in the CK group at 20 and 40 DAH and that the soluble protein content in the shade group was significantly higher than that in the CK group at 10, 30, and 40 DAH (Fig. 3). Shading can increase leaf Chl and decrease Chl a/b ratios to alter light-use efficiency (Gregoriou et al. 2007). Chl and protein are the most important physiological indicators of plant senescence. A decrease in Chl and protein content can directly reflect plant senescence. Moreover, as an important component of photosynthesis, the degradation of Chl and Rubisco severely affects the Pn. The results showed that the trend of the Pn was consistent with that of Chl and Rubisco. The Pn of the shade group was significantly higher than that of the CK group (Fig. 3). Such effects accelerate the senescence of leaves and reduce the Pn (Fig. 3e). The yield of rice mainly originates from the photosynthesis of leaves. Therefore, the photosynthetic capability of leaves is crucial to rice yields (Ainsworth and Long 2005). In this paper, the excess light energy was reduced by shading at noon, which maintained a high Pn in the rice leaves.
Our previous study showed that the excitation pressure of PSII increased significantly during the aging process in rice leaves and that the transmission of photosynthetic electron flow was hindered (Ou et al. 2003). The photosynthetic electron flow allocated to JC declined gradually in the rice leaves after heading (Fig. 4b). This phenomenon may be due to the decrease in Rubisco content and CE, which leads to a decrease in the use efficiency of photosynthetic electron flow for Rubisco. However, the low Rubisco content and CE led to an increased photosynthetic electron flow being allocated to JO (Fig. 4c) and formed increased amounts of ROS (Asada 2006). Studies have shown that increasing the JO/JF ratio and it was considered to constitute an efficient photoprotective mechanism in woody plants under strong light (Lin et al. 2000). The results showed that the JF and JO in the CK were significantly higher than those in the shade group at the late stage of FTL10 senescence (Fig. 4). This finding showed that shading can reduce the generation of excess photosynthetic electron and can improve the use efficiency of light energy; shading can also reduce the damage to leaves caused by ROS produced by excess light energy. Previous studies reported that shading affects not only the carbohydrate accumulation in grain and the filling rate but also the yield and quality traits of rice (Wang et al. 2013; Mo et al. 2015). In our study, increased single-spike weight and yield per plant after shading were also observed (Fig. 5). The above results showed that shading at noon could reduce excess photosynthetic electron, improve the use efficiency of light energy and increase FTL10 yields.
In summary, the experimental results show that shading can reduce the production of ROS and slow the senescence process of FTL10 leaves from 11:30–14:00 (noon) every day. In addition, shading can improve the use efficiency of light energy and can maintain a relatively high Pn, thereby increasing the yield of FTL10.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–371. https://doi.org/10.1111/j.1469-8137.2004.01224.x
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396. https://doi.org/10.1104/pp.106.082040
Fang M, Zhou Z, Zhou X, Yang H, Li M, Li H (2019) Overexpression of OsFTL10 induces early flowering and improves drought tolerance in Oryza sativa L. Peer J 7:e6422. https://doi.org/10.7717/peerj.6422
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92. https://doi.org/10.1016/S0304-4165(89)80016-9
Gregoriou K, Pontikis K, Vemmos S (2007) Effects of reduced irradiance on leaf morphology, photosynthetic capacity, and fruit yield in olive (Olea europaea L.). Photosynthetica 45:172–181. https://doi.org/10.1007/s11099-007-0029-x
Hayama R, Agashe B, Luley E, King R, Coupland G (2007) A circadian rhythm set by dusk determines the expression of FT homologs and the short-day photoperiodic flowering response in pharbitis. Plant Cell 19:2988–3000. https://doi.org/10.1105/tpc.107.052480
Hideg E, Schreiber U (2007) Parallel assessment of ROS formation and photosynthesis in leaves by fluorescence imaging. Photosynth Res 92:103–108. https://doi.org/10.1007/s11120-007-9146-4
Hsu CY, Liu Y, Luthe DS, Yuceer C (2006) Poplar FT2 Shortens the juvenile phase and promotes seasonal flowering. Plant Cell 18:1846–1861. https://doi.org/10.1105/tpc.106.041038
Iizumi T, Ramankutty N (2016) Changes in yield variability of major crops for 1981–2010 explained by climate change. Environ Res Lett 11:34003. https://doi.org/10.1088/1748-9326/11/3/034003
Jiang H, Chen Y, Li M, Xu X, Wu G (2011) Overexpression of SGR results in oxidative stress and lesion-mimic cell death in rice seedlings. J Integr Plant Biol 53:375–387. https://doi.org/10.1111/j.1744-7909.2011.01037.x
Jutamanee K, Onnom S (2016) Improving photosynthetic performance and some fruit quality traits in mango trees by shading. Photosynthetica 54:542–550. https://doi.org/10.1007/s11099-016-0210-1
Juvany M, Müller M, Munné-Bosch S (2013) Photo-oxidative stress in emerging and senescing leaves: a mirror image? J Exp Bot 64:3087–3098. https://doi.org/10.1093/jxb/ert174
Kirchhoff H (2014) Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Philos T R Soc B 369:20130225. https://doi.org/10.1098/rstb.2013.0225
Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the arabidopsis FT Gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43:1096–1105. https://doi.org/10.1093/pcp/pcf156
Labra MH, Struik PC, Evers JB, Calderini DF (2017) Plasticity of seed weight compensates reductions in seed number of oilseed rape in response to shading at flowering. Eur J Agron 84:113–124. https://doi.org/10.1016/j.eja.2016.12.011
Lee SH, Sakuraba Y, Lee T, Kim KW, An G, Lee HY, Paek NC (2015) Mutation of Oryza sativa CORONATINE INSENSITIVE 1b (OsCOI1b) delays leaf senescence. J Integr Plant Biol 57:562–576. https://doi.org/10.1105/tpc.15.00110
Liang CZ, Wang YQ, Zhu YN, Tang JY, Hu B, Liu LC, Ou SJ, Wu HK, Sun XH, Chu JF, Chu CC (2014) OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc Natl Acad Sci USA 111:10013–10018. https://doi.org/10.1073/pnas.1321568111
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58:115–136. https://doi.org/10.1146/annurev.arplant.57.032905.105316
Lin ZF, Peng CL, Sun ZJ (2000) Effect of light intensity on partitioning of photosynthetic electron transport to photorespiration in four subtropical forest plants. Sci China Life Sci 43:347–354. https://doi.org/10.1007/BF02879298
Liu Y, Ren D, Pike S, Pallardy S, Gassmann W, Zhang S (2007) Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J 51:941–954. https://doi.org/10.1111/j.1365-313X.2007.03191.x
Liu Y, Yu L, Wang R (2011) Level of ascorbic acid in transgenic rice for L-galactono-1, 4-lactone dehydrogenase overexpressing or suppressed is associated with plant growth and seed set. Acta Physiol Plant 33:1353–1363. https://doi.org/10.1007/s11738-010-0669-5
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lu T, Meng Z, Zhang G, Qi M, Sun Z, Liu Y, Li T (2017) Sub-high temperature and high light intensity induced irreversible inhibition on photosynthesis system of tomato plant (Solanum lycopersicum L.). Front Plant Sci 8:365
Mae T (1997) Physiological nitrogen efficiency in rice: nitrogen utilization, photosynthesis and yield potential. Plant Soil 196:201–210. https://doi.org/10.1023/a:1004293706242
Melis A, Spangfort M, Andersson B (1987) Light-absorption and electron-transport balance between photosystem II and photosystem I in spinach chloroplasts. Photochem Photobiol 45:129–136. https://doi.org/10.1111/j.1751-1097.1987.tb08413.x
Mo Z, Li W, Pan S, Fitzgerald TL, Xiao F, Tang Y, Wang Y, Duan M, Tian H, Tang X (2015) Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 8:9. https://doi.org/10.1186/s12284-015-0040-y
Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566. https://doi.org/10.2307/4279788
Neill S, Gould K, Kilmartin P, Mitchell K, Markham K (2002) Antioxidant activities of red versus green leaves in Elatostema rugosum. Plant, Cell Environ 25:539–547. https://doi.org/10.1046/j.1365-3040.2002.00837.x
Ou Z, Peng C, Lin G, Yang C (2003) Relationship between PSII excitation pressure and content of Rubisco large subunit or small subunit in flag leaf of super high-yielding hybrid rice. Acta Botanica Sinica 45:929–935
Pintó-Marijuan B, Munné-Bosch S (2014) Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: advantages and limitations. J Exp Bot 65:3845–3857. https://doi.org/10.1093/jxb/eru086
Ren B, Cui H, Camberato JJ, Dong S, Liu P, Zhao B, Zhang J (2016) Effects of shading on the photosynthetic characteristics and mesophyll cell ultrastructure of summer maize. Sci Nat 103:67. https://doi.org/10.1007/s00114-016-1392-x
Saha MR, Hasan SMR, Akter R, Hossaina MM, Alamb MS, Alam MA, Mazumderc MEH (2008) In vitro free radical scavenging activity of methanol extract of the leaves of Mimusops Elengi Linn. Bangladesh J Vet Med 6:197–202. https://doi.org/10.3329/bjvm.v6i2.2336
Schippers JH, Schmidt R, Wagstaff C, Jing HC (2015) Living to die and dying to live: the survival strategy behind leaf senescence. Plant Physiol 169:914–930. https://doi.org/10.1104/pp.15.00498
Sedigheh HG, Mortazavian M, Norouzian D, Atyabi M, Akbarzadeh A, Hasanpoor K, Ghorbani M (2011) Oxidative stress and leaf senescence. BMC Res Notes 4:477. https://doi.org/10.1186/1756-0500-4-477
Serodio J, Schmidt W, Frankenbach S (2017) A chlorophyll fluorescence-based method for the integrated characterization of the photophysiological response to light stress. J Exp Bot 68:1123–1135. https://doi.org/10.1093/jxb/erw492
Sevillano I, Short I, Campion J, Grant OM, Grant J, O’Reilly C (2018) Comparison of photosynthetic performance of Fagus sylvatica seedling sunder natural and artificial shading. Environ Exp Bot 152:90–96. https://doi.org/10.1016/j.envexpbot.2018.03.015
Stefanov M, Yotsova E, Markovska Y, Apostolova EL (2018) Effect of high light intensity on the photosynthetic apparatus of two hybrid lines of Paulownia grown on soils with different salinity. Photosynthetica 56:832–840. https://doi.org/10.1007/s11099-017-0735-y
Sun B, Zhou Y, Lin Y (2013) Preliminary functional analysis of a rice leaf senescence up-regulated gene. Acta Agron Sini 38:1988–1996. https://doi.org/10.3724/SP.J.1006.2012.01988
Taheri P, Irannejad A, Goldani M, Tarighi S (2014) Oxidative burst and enzymatic antioxidant systems in rice plants during interaction with Alternaria alternate. Eur J Plant Pathol 140:829–839. https://doi.org/10.1007/s10658-014-0512-8
Takahashi S, Badger MR (2011) Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16:53–60. https://doi.org/10.1016/j.tplants.2010.10.001
Valentini R, Epron D, Angelis PD, Matteucci G, Dreyer E (1995) In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L.) leaves: diural cycle under different levels of water supply. Plant, Cell Environ 18:631–640. https://doi.org/10.1111/j.1365-3040.1995.tb00564.x
Wang L, Deng F, Ren WJ, Yang WY (2013) Effects of shading on starch pasting characteristics of Indica hybrid rice (Oryza sativa L.). Plos ONE 8(7):e68220
Zhang TJ, Chow WS, Liu XT, Zhang N P, Liu N, Peng CL (2016) A magic red coat on the surface of young leaves: anthocyanins distributed in trichome layer protect Castanopsis fissa leaves from photoinhibition. Tree Physiol 36:1296–1306. https://doi.org/10.1093/treephys/tpw080
Zhang QL, Wei YX, Peng CL (2018) Effects of endogenous ascorbic acid on resistance to high-temperature stress in excised rice leaves. Photosynthetica 56:1453–1458. https://doi.org/10.1007/s11099-018-0836-2
Acknowledgments
The authors are very grateful to Professor Hongqing Li (South China Normal University, China) for the generous gift of rice seeds. This work was funded by Guangdong Province Natural Science Foundation (2015A030311023, 2017A030313167). The study was also supported by the National Natural Science Foundation of China (31870374, 31570398). The study was also supported by the Innovation Project of Graduate School of South China Normal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Q., Zhai, J., Wei, Y. et al. Effects of Shading on the Senescence and Photosynthetic Physiology of the Early-Flowering Rice Mutant FTL10 at Noon. J Plant Growth Regul 39, 776–784 (2020). https://doi.org/10.1007/s00344-019-10021-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-10021-2