Abstract
The present study has been conducted to evaluate the impact of silicon (Si) and salicylic acid (SA) in the regulation of Cd-induced toxicity in maize seedlings. Cadmium (Cd: 100 µM) significantly reduced root and shoot fresh weight and length, photosynthetic pigments, total soluble protein content and chlorophyll fluorescence parameters. Cadmium decreased root and shoot length by 23 and 19% and fresh weight by 27 and 24%, respectively when compared to their respective controls. Similarly, total chlorophyll, carotenoids and total soluble protein were decreased by 21, 18 and 28%, respectively by Cd. In contrast, the addition of SA (500 µM) and Si (10 µM), and their combination (SA + Si) together with Cd treatment successfully ameliorated Cd-induced harmful impacts on studied parameters as SA and Si alone and in combination reduced Cd accumulation and oxidative stresses and thus refurbish the damages. Cd significantly stimulated activity of superoxide dismutase while inhibited activities of ascorbate peroxidase (APX), glutathione reductase (GR) and dehydroascorbate reductase (DHAR), and declined total ascorbate and glutathione contents. In contrast, the addition of SA and Si alone and in combination stimulated the activities of APX, GR and DHAR and significantly increased levels of total ascorbate and glutathione. In conclusion, the present study suggested that although SA and Si both alone are able to alleviate Cd-induced toxicity in maize seedlings, but their combination was the most effective in nullifying Cd-induced toxicity in maize seedlings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd), a non-essential heavy metal, is one of the main pollutants of the environment and hazardous to all living organisms including plants and animals (Goncalves et al. 2009; Xu et al. 2011; Tripathi et al. 2012a). In plants, Cd causes serious harm to morphological, physiological, and biochemical processes (Bernard 2008; Tripathi et al. 2012a; Singh and Prasad 2013). Cd is liberated into soil and water through natural and anthropogenic activities (Friberg 2017). Absorbed Cd on plant roots can be loaded into the xylem through transport and reaches the leaves which then interferes with cellular redox homeostasis and thus leads to over production of reactive oxygen species (ROS) in plants (Gill et al. 2011). In addition, it reacts with pigments, nucleic acids, proteins, lipids and ultimately results in damage to cellular structures by their oxidation (Shah et al. 2001; Štork et al. 2013; He et al. 2013). Cd causes leaf chlorosis, reduced growth and disturbs several physiological processes such as photosynthesis and mineral regulation (Metwally et al. 2003; Nwugo and Huerta 2008; Popova et al. 2009; Sfaxi-Bousbih et al. 2010). Barceló and Poschenrieder (1990) showed that Cd negatively affected stomatal opening, transpiration, water balance and nutrient uptake in plants. In addition to this, some reports also advocated that Cd influences various photosynthetic enzymes, mainly those involved in the Calvin cycle (Vitória et al. 2001; Kulaeva and Tsyganov 2011; Song et al. 2017). Therefore, to counteract the lethal impact of Cd on plants, there are several techniques, chemical agents and plant growth regulators that are being used and of which silicon (Si) and salicylic acid (SA) are of major attention.
Silicon (Si) is the second most abundant element on the earth crust after oxygen, and is available in the form of silicic acid [Si(OH)4] to plants (Epstein 1999; Lux et al. 2002). Si offers extensive benefits to plant growth and development even under stress but has not yet been regarded as an essential element (Epstein 1999; Lux et al. 2002; Nwugo and Huerta 2008; Tripathi et al. 2012a, b; Mitani Ueno et al. 2016). Thus, it is known as a “quasi-essential element” for plant growth. It not only provides mechanical strength but also shows greater involvement in morpho-physiological to molecular traits of the plant system in normal as well as in stressed conditions (Epstein 1999; Tripathi et al. 2014, 2015). Thus, Si can be an efficient element for boosting growth and development in higher plants under stress (Guo et al. 2013; Muneer et al. 2017). Similarly, many studies have verified that Si is capable of enhancing the resistance of plants against the toxicity of metals including aluminum (Al), cadmium (Cd), chromium (Cr), iron (Fe), manganese (Mn), and zinc (Zn) (Horiguchi and Morita 1987; Shi et al. 2005; Singh et al. 2011; Tripathi et al. 2012a, b, 2015).
Additionally, salicylic acid (SA; 2-hydroxybenzoic acid) is one of the most dynamic plant hormones which naturally occurs in plants, quantitatively in lower amount-µg/g fresh weight or less (Raskin 1992; Rivas-San Vicente and Plasencia 2011). SA is usually available in a free form or in the form of glycosylated, methylated, glucoseester, or amino acid conjugates in the plant system (Raskin 1992; Lee et al. 1995). It is an important signal molecule in plants and is biosynthesized by two pathways. However, scientists using isotope feeding techniques have proposed that the phenylalanine ammonia lyase (PAL) pathway is the major SA biosynthetic pathway in which plants synthesize SA from cinnamate produced by PAL (Chen et al. 2009).
Salicylic acid is engaged in local as well as in systemic plant defense responses, performs several significant beneficial roles in growth, development, photosynthesis, transpiration, ion uptake and transport in the plant system (An and Mou 2011; Rivas-San Vicente and Plasencia 2011; Kawano and Bouteau 2013; et al. 2016). SA is reported to play a valuable role in improving tolerance against abiotic stresses in many plant species (Hayat et al. 2010; Khan et al. 2015). Further, studies related to Cd and SA interaction in various plant species demonstrated that SA significantly reduced Cd toxicity by regulating and decreasing the Cd uptake in plant organs, enhancing photosynthetic ability, defending membrane integrity and promoting heme oxygenase and ROS scavenging through improved antioxidant defense system (Shi et al. 2009; Cui et al. 2012; Guo et al. 2013; Wang et al. 2013; Asgher et al. 2014; Li et al. 2014; Janda et al. 2014; Belkadhi et al. 2015; Zhang et al. 2015).
Therefore, on the basis of the above facts, it can be seen that the beneficial role of Si and SA has been tested separately against stress responses in plants with much emphasis on Cd toxicity. However, the interactive impact of Si and SA against Cd or any other stress has not yet been investigated and thus needs research. Thus, the objective of the present study was to examine the interactive impact of SA and Si against Cd toxicity by analyzing growth, photosynthesis (as a chlorophyll a fluorescence), oxidative stress, antioxidant defense system and mineral element regulation in maize seedlings.
Materials and Methods
Plant Material and Growth Conditions
Maize seeds (Zea mays L. var. Super 20–20) were purchased from a certified supplier in a local market of Allahabad, India. The seeds were surface sterilized in 10% (v/v) sodium hypochlorite solution for 10 min and were washed with distilled water. After that, sterilized seeds were soaked for 4 h in distilled water and then healthy-looking uniformly sized seeds were kept in Petri plates (150 mm Rivera TM) lined with Whatman No-1 filter paper and moistened with half strength Hoagland solution (pH 6.5) (Arditti and Dunn 1969). Further, seeds were kept for germination in the dark at 28 ± 2 °C for 4 days and were grown under a photosynthetic photon flux density of 200 µmol photons m−2 s−1 and relative humidity of 60–70% with a light/dark cycle of 12/12 h at 28 ± 2 °C for 15 days in a growth chamber (Impact, Model IIC129). After this, uniform sized seedlings were collected and placed into half-strength Hoagland’s solution to acclimatize them for 7 days. After acclimatization, SA (500 µM), Si(10 µM), Si + SA (500 µM + 10 µM) and Cd (100 µM) treatments were given to the seedlings for 7 days. The concentrations of SA and Si were selected according to our previous studies, that is, Singh et al. (2014) and Tripathi et al. (2016). Sodium silicate (Na2SiO3), cadmium chloride (CdCl2) and salicylic acid (SA) (S.D. finechem limited Mumbai, India) were used to prepare the different concentrations in half strength Hoagland solution. After 7 days of treatments, root and shoot samples from the treated and untreated sets were harvested and different morphological, physiological and biochemical parameters were analyzed. During 7 days of treatments, the respective solution was changed twice. In addition, the untreated Zea mays L. seedlings were regarded as ‘control’.
Estimation of Growth
After 7 days of treatments, lengths and fresh weights of shoots and roots of control and treated plants were measured. Seedlings from each sample were randomly selected for this purpose. Shoot and root lengths were measured by using a centimeter scale. Root and shoot fresh weights were measured by using a digital balance.
Estimation of Chlorophyll and Carotenoids
Photosynthetic pigments (chlorophylls and carotenoids) were extracted and measured as per the method of Lichtenthaler (1987).
Determination of Total Soluble Protein
Total soluble protein contents of treated and untreated samples were measured by the method of Lowry et al. (1951) using bovine serum albumin as a standard.
Estimation of Cd and Mineral Contents (Ca and S)
Cadmium (Cd) and mineral (Ca and S) contents were estimated in treated and untreated maize seedlings. For determination of Cd, Ca and S contents in shoot and root, they were repeatedly washed with double-distilled water to remove absorbed culture medium and dried with clean tissue paper and then in an air-circulated oven. Dried samples (100 mg) of each treatment were digested in triacid mixture (HNO3, H2SO4 and HClO4 in 5:1:1 ratio) at 80 °C until a transparent solution is obtained. After cooling, the digested samples were maintained up to 30 ml with double-distilled water. Concentrations of Cd, Ca and S in the filtrate of digested samples were estimated using atomic absorption spectroscopy (AAS).
Chlorophyll a Fluorescence Measurements
For the assessment of photosynthetic performance, chlorophyll a fluorescence was recorded in the dark-adapted leaves of treated and untreated maize seedlings using a hand held leaf fluorometer (FluorPen FP 100, Photon System Instrument, Czech Republic) according to Strasser et al. (2000).
Estimation of Superoxide Radical (SOR), Hydrogen Peroxide (H2O2)
For the estimation of superoxide radicals (SOR; O2·−) in maize seedlings, the procedure of Elstner and Heupel (1976) was adopted. This assay is based on the formation of NO2− from hydroxylamine in the presence of O2·−. A standard curve was prepared with NaNO2 and used to calculate the production rate of O2·−.
For the estimation of H2O2, fresh leaves of treated and untreated maize seedlings were homogenized in 0.1% (w/v) trichloroacetic acid (Velikova et al. 2000). The reaction mixture (2 ml) contained tissue extract (500 µl), 10 mM potassium phosphate buffer (pH 7.0) and 1M KI solution. Absorbance of the reaction mixture was read at 390 nm. The concentration of H2O2 was calculated using the standard curve prepared with H2O2.
Estimation of Lipid Peroxidation (MDA) and Membrane Stability
Lipid peroxidation (MDA; malondialdehyde) in maize seedlings was measured according to the method described by Heath and Packer (1968). The MDA concentration was calculated using the extinction coefficient 155 mM−1 cm−1.
Membrane Stability Index (MSI) in treated and untreated maize seedlings was determined as per the method of Sairam et al. (2002).
Antioxidant Enzymes Assays
Superoxide dismutase (SOD; EC 1.15.1.1) activity in treated and untreated maize seedlings was measured according to the method of Giannopolitis and Reis (1977). One unit (U) of SOD activity is defined as the amount of enzyme required to cause 50% inhibition in reduction of NBT. Ascorbate peroxidase (APX; EC 1.11.1.11) activity was determined according to the method of Nakano and Asada (1981). One unit of enzyme activity is defined as 1 nmol ascorbate oxidized min−1. Glutathione reductase (GR; EC 1.6.4.2) activity was assayed according to the method of Schaedle and Bassham (1977). One unit of enzyme activity is defined as 1 nmol NADPH oxidized min−1. Dehydroascorbate reductase (DHAR; EC 1.8.5.1) activity was assayed by the method of Nakano and Asada (1981). One unit of enzyme activity is defined as 1 nmol DHA reduced min−1.
Measurements of Total Ascorbate and Glutathione
The measurement of total ascorbate was performed according to the method of Gossett et al. (1994). Total glutathione was determined by the enzyme-recycling method of Brehe and Burch (1976).
Statistical Analysis
Results were statistically analyzed by analysis of variance (ANOVA). Duncan’s multiple range test was applied for mean separation for significant differences among treatments at p < 0.05 significance level. The results presented are the means ± standard error of three independent experiments with two replicates in each experiment (n = 6) to check the reproducibility of the results.
Results
Impact of SA, Si and SA + Si on Growth and Cd Accumulation Under Cd Stress
Exposure to Cd significantly caused visible toxicity symptoms in maize seedlings which were confirmed by the measurement of growth in terms of length and fresh weight of root and shoot which exhibited a significant decline under Cd treatment (Fig. 1; Table 1). Maize seedlings exposed to 100 µM Cd showed a decline of 24 and 27% in fresh weight and 19 and 23% in length of shoot and root, respectively, over the value of control. However, the addition of SA, Si and Si + SA along with Cd (100 µM) significantly (p < 0.05) alleviated Cd-induced reduction in fresh weight and length of maize seedlings as the percentage decline was then only 14, 9 and 6% in shoot fresh weight and 14, 10 and 9% in shoot length whereas in root fresh weight reductions were only 17, 11 and 9% and root length declined only by 17, 13 and 7%, respectively, over the value of controls (Table 1).
Further, in SA, Si and SA + Si treatments without Cd, fresh weights of shoot was significantly (p < 0.05) increased by 6, 8 and 15%, and lengths of shoot by 6, 12 and 18%, respectively. Similarly, root fresh weight increased by 8, 10 and 17%, whereas root lengths by 7, 15 and 23%, respectively, over the value of controls (Table 1).
Data related to the accumulation of Cd are depicted in Table 2. Data showed that Cd was significantly (p < 0.05) higher in Cd-treated maize seedlings as roots accumulated about 1266 ± 12.3 µg Cd g−1 dry weight and shoot about 380 ± 7.7 µg Cd g−1 dry weight (Table 2). However, upon addition of SA, Si and Si + SA Cd accumulation was significantly (p < 0.05) reduced and it was only 278 ± 9.3, 239 ± 10.6, 197 ± 9.6 in shoot and 1081 ± 10.1, 789 ± 6.8, 433 ± 5.9 µg Cd g−1 dry weight in roots, respectively (Table 1).
Impact of SA, Si and SA + Si on Photosynthetic Pigments and Total Soluble Protein Under Cd Stress
Photosynthetic pigments including total chlorophyll and carotenoids, and protein of treated maize seedlings were also analyzed and the results are depicted in Table 1. Data reveal that Cd (100 µM) significantly (p < 0.05) reduced chlorophyll, carotenoids, and protein contents of maize seedlings by 21, 18 and 28%, respectively, over the values of control (Table 1). In contrast, the addition of SA, Si and SA + Si along with Cd (100 µM) in the nutrient medium significantly (p < 0.05) alleviated the Cd-induced toxic impact and thus reductions were only 13, 10 and 7% in total chlorophyll, 15, 9 and 6% in carotenoids, and 18, 11 and 5% in protein in comparison to the Cd treatment alone (Table 1). In addition to this, separate treatments of SA, Si and SA + Si had significantly increased chlorophyll, carotenoids, and protein contents of maize seedlings over the value of control (Table 1).
Impact of SA, Si and SA + Si on Chlorophyll a Fluorescence Characteristics Under Cd Stress
The chlorophyll a fluorescence (JIP test) is a rapid and sensitive technique in assessing photosynthetic performance in plants, and thus in the present study a JIP test was conducted to measure the impact of Cd treatment on Chl a fluorescence features in maize seedlings also exposed to SA, Si and SA + Si (Fig. 2). The data suggested that treatments of SA, Si and SA + Si alone positively influenced characteristics OJIP transient as compared to values of controls. Due to Cd treatment, the quantum yield of primary photochemistry (φP0 or Phi_P0), yield of electron transport per trapped exciton (W0 or Psi_0), the quantum yield of electron transport (φE0) activity and performance index of PS II were significantly reduced over the values of respective controls (Fig. 2). Conversely, addition of SA, Si and SA + Si together with Cd significantly mitigated ΨP0, W0 and PIABS except φE0 over the values of Cd alone-treated seedlings. Further, addition of SA, Si and SA + Si treatments alone significantly increased φE0 and PIABS except φP0 and W0, as compared to respective values of controls (Fig. 2).
In addition, Cd treatment significantly reduced the ratio of active RC which was pointed out by enhanced energy flux parameters including ABS, TR0, ET0 and DI0 per RC (Fig. 2). However, addition of SA, Si and SA + Si together with Cd treatment significantly alleviated the toxic impacts of Cd on energy flux parameters (Fig. 2).
Impact of SA, Si and SA + Si on Reactive Oxygen Species, Lipid Peroxidation and Membrane Stability Under Cd Stress
It is well known that reactive oxygen species (ROS) are key signatures of stress signaling and stress caused by heavy metals. Hence, to examine the oxidative stress status, we measured levels of SOR and H2O2 in maize seedlings and data suggested that in Cd-treated plants SOR and H2O2 contents were significantly enhanced by 90 and 54%, respectively over the value of controls whereas in SA, Si and SA + Si treated plants the levels of both stress markers (SOR and H2O2) were not influenced significantly (Fig. 3a). Beside this, upon addition of SA, Si and SA + Si together with Cd, the level of both the stress markers (SOR and H2O2) was significantly (p < 0.05) reduced as compared to the Cd-alone-treated plants.
Interactive effect of SA and Si on superoxide radicals (SOR) (a), hydrogen peroxide (H2O2) (a), lipid peroxidation (as MDA, malondialdehyde) (b) and membrane stability (b) in maize seedlings exposed to Cd stress. Bars followed by different letter(s) show significant difference at p < 0.05 significance level according to the Duncan’s multiple range test
Lipid peroxidation is also one of the major stress markers, indicating the impact of various stresses on lipids. Similarly, the data of the present study clearly indicated that due to the exposure to Cd the level of lipid peroxidation (MDA content) was enhanced by 59% in maize seedlings (Fig. 3b). However, the level of MDA was not influenced significantly under the treatments of SA, Si and SA + Si alone in maize seedlings. In contrast to this, in the addition of SA, Si and SA + Si together with Cd the level of MDA was lowered significantly (p < 0.05) as compared to Cd-alone-treated maize seedlings (Fig. 3b).
Impact of SA, Si and SA + Si on Activities of Enzymatic Antioxidants Under Cd Stress
The results show that Cd treatment (100 µM) significantly (p < 0.05) increased SOD activity in maize seedlings (Fig. 4a), whereas addition of SA, Si and SA + Si alone and in combination with Cd significantly (p < 0.05) maintained higher SOD levels in maize seedlings.
Interactive impact of SA and Si on the activities of superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), dehydroascorbate reductase (DHAR) and total ascorbate and glutathione in maize seedlings exposed to Cd stress. Bars followed by different letter(s) show significant difference at p < 0.05 significance level according to the Duncan’s multiple range test
Further in the case of APX, GR and DHAR, results demonstrated that Cd treatment significantly inhibited activities of all these enzymes by 21, 32 and 21%, respectively, over the values of controls in maize seedlings (Fig. 4a, b). In contrast, addition of SA, Si and SA + Si together with the Cd treatments alleviated Cd-induced reduction in APX, GR and MDHAR as compared to the value of Cd-treated maize seedlings (Fig. 4a, b).
Impact of SA, Si and SA + Si on Ascorbate and Glutathione Levels Under Cd Stress
Cd (100 µM) treatment significantly reduced the level of ASC and GSH by 32 and 24%, respectively, over the value of controls (Fig. 4c). However, addition of SA, Si and SA + Si together with the Cd treatment significantly alleviated the toxic impact of Cd and managed the levels of ASC and GSH as their contents showed a decrease of only 18,12 and 8% in ASC and 16,11 and 7% in GSH, respectively, over the values of Cd-alone-treated maize seedlings (Fig. 4c). Further, application of SA, Si and SA + Si without Cd also showed stimulation in ASC and GSH.
Impact of SA, Si and SA + Si on Ca and S Under Cd Stress
The levels of Ca and S were tested and data suggested that SA, Si and SA + Si treatments alone positively influenced the Ca level in maize seedlings. However, Cd treatment significantly reduced levels of Ca by 25%. However, interestingly the addition of SA, Si and SA + Si along with Cd-improved levels of Ca through mitigating Cd toxicity as reductions were only 18, 11 and 4%, respectively (Table 2).
On the other hand, treatments of SA, Si and SA + Si alone did not influence the level of S in maize seedlings. However, Cd treatment enhanced S levels by 22% over the value of controls (Table 2). The addition of SA, Si and SA + Si together with Cd balanced the S content in maize seedlings as it was then 17, 11 and 7%, respectively, over the value of respective Cd treatment (Table 2).
Discussion
The results of this study clearly revealed that singly and in combination SA and Si significantly modulated Cd-induced toxicity in maize seedlings. Data of the study revealed that growth, photosynthetic pigments and chlorophyll a fluorescence parameters of maize seedlings were significantly altered by Cd which could be associated with the excess Cd accumulation in plants (Table 1; Fig. 2; Table 2). In the same way, Cd-induced toxic impacts on growth have been observed in a variety of plant species (Wu et al. 2004; Drazic and Mihailovic 2005; Singh and Prasad 2013) which could be attributed primarily to repress cell division and/or cell growth and cell elongation which mostly occurs by an irreparable silencing of the proton pump (Liu et al. 2004). However, application of SA, Si and SA + Si significantly improved growth, photosynthetic pigments and chlorophyll a fluorescence parameters of maize seedlings which were because of a significant decrease in Cd accumulation (Tables 1, 2; Fig. 2), as protective effects of Si and SA were reported in other plants (Arberg 1981; Tripathi et al. 2012a, b; Singh and Prasad 2013). Impacts of SA and Si alone have been well determined such as Khan et al. (2003) and Khodary (2004) have shown impacts of SA on corn and soybean and found that SA as an endogenous regulator is capable of increasing growth characteristics including dry mass, leaf area, level of pigments and photosynthetic rate. For Si, Tripathi et al. (2012a) and Vaculík et al. (2012) have observed a growth-promoting role of Si in plants. However, the combined effect of SA and Si has not yet been explored in plants under both normal and stress conditions. Our results showed that though both SA and Si alone are able to reduce Cd toxicity, their combination was more effective in this assignment.
One of the adverse outputs of metal stress is the generation of ROS in excess amounts like superoxide radical (O2·−), hydrogen peroxide (H2O2), hydroxyl radical (•OH) etc. which subsequently cause oxidative damage in plants (Vaculík et al. 2015; Tripathi et al. 2017). The results show that Cd significantly stimulated ROS generation in maize seedlings which coincided with the enhanced damage to lipids as indicated by enhanced lipid peroxidation and decreased membrane stability (Fig. 3a, b). However, treatment of maize seedlings with Si and SA alone and in combination significantly ameliorated Cd-induced toxic effects by lowering ROS and damage to lipids and thus maintaining membrane stability (Fig. 3a, b). These results imply that Si and SA both were able to mitigate Cd-induced negative consequences in maize seedlings.
To combat abiotic stress like Cd-mediated excess ROS accumulation, plants are naturally equipped with a well-coordinated antioxidant defense strategy. Antioxidant defense methods consist of two components, that is, enzymatic antioxidants like SOD, APX, GR, DHAR, etc. and non-enzymatic antioxidants such as ascorbate, glutathione, etc. (Mittler et al. 2002; Tripathi et al. 2016, 2017). SOD is the first line of security against ROS and dismutases highly toxic O2·− into comparatively less toxic H2O2 (Mittler 2002). The important enzymes of the ascorbate–glutathione cycle are APX, GR, MDHAR and DHAR participating in managing H2O2 by producing ascorbate and glutathione (Tripathi et al. 2017). SOD activity was stimulated by Cd treatment (Fig. 4a) indicating that under such conditions maize seedlings were under severe oxidative stress as evidenced from enhanced ROS levels and lipid peroxidation (Figs. 3, 4), and significant enhancement in SOD activity was for regulating the ROS level. However, upon either single or combined treatment of Si and SA, SOD activity showed downregulation (Fig. 3a) suggesting that its activity is not required in larger extent as indicators of oxidative stress (ROS and lipid peroxidation) also exhibited significant decline. In contrast to the SOD activity, activities of APX, GR and DHAR, and contents of ascorbate and glutathione were significantly reduced by Cd treatment (Fig. 3a, b). APX is considered as a chief enzyme of H2O2 metabolism and responsible for fine tuning of the H2O2 levels, therefore, it could not damage the cell on one side and on another side it could act as a signaling molecule during developmental processes of plants (Singh et al. 2017). Under Cd stress, inhibition in activity of APX resulted in buildup of H2O2 in maize seedlings which caused significant lipid damage as evidenced from enhanced lipid peroxidation (Figs. 3, 4).Though, upon Si or SA treatment singly as well as in combination, APX activity increased in comparison to the Cd treatment alone indicating a significant role of APX in managing Cd-induced oxidative stress as this fact is supported by reduction in ROS level and lipid peroxidation. Under Cd stress, decline in ascorbate and glutathione contents could be correlated with inhibition in DHAR and GR activity (Fig. 4b). On one hand, ascorbate and glutathione are the main buffering agents of the cell, maintaining its reducing environment by regulating levels of ROS and on another hand they contribute in several developmental processes of the cell for instance in cell division, maintenance of cytoskeleton, etc. (Foyer and Noctor 2011). Therefore, under Cd stress, decline in ascorbate and glutathione contents may be linked with hampered growth of maize seedlings by reason of enhanced occurrence of oxidative damage. However, upon Si and SA addition alone, as well as in combination, GR and DHAR activity was significantly increased resulting in increased pools of ascorbate and glutathione for regulating ROS as evidenced from greater decline in lipid peroxidation. Under such conditions, improvement in growth of maize seedlings may be the second reason for Si and SA mediated amelioration of Cd toxicity.
Conclusions
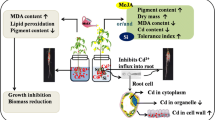
The outcome of the current study showed that Si and SA both were able to attenuate Cd toxicity in maize seedlings. Among Si and SA, Si was more effective whereas the combination of Si and SA was more effective than their single treatments in alleviating Cd toxicity in maize seedlings. Si and SA-alleviated Cd toxicity was linked with downregulation of Cd accumulation and up-regulation of antioxidants like APX, GR, DHAR, ascorbate and glutathione which resulted in better growth of maize seedlings by restoring photosynthesis. These results are agronomically significant as both Si and SA are cost effective and easily available, so can be recommended for managing metal stress in crop plants. The proposed model of Si and SA-mediated alleviation of Cd toxicity in maize seedlings is given in Fig. 5.
Abbreviations
- SA:
-
Salicylic acid
- AsA:
-
Reduced ascorbate
- APX:
-
Ascorbate peroxidase
- DHA:
-
Dehydroascorbate
- DHAR:
-
Dehydroascorbate reductase
- Fv/Fm:
-
Maximum photochemical efficiency of PS II
- GR:
-
Glutathione reductase
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- NPQ:
-
Non-photochemical quenching
- qP:
-
Photochemical quenching
- ROS:
-
Reactive oxygen species
- Si:
-
Silicon
- SOD:
-
Superoxide dismutase
- SOR:
-
Superoxide radical
References
An C, Mou Z (2011) Salicylic acid and its function in plant immunity. J Integer Plant Biol 53:412–428. https://doi.org/10.1111/j.1744-7909.2011.01043.x
Arberg B (1981) Plant growth regulators. XLI. Monosubstituted benzoic acid. Swed J Agric Res 11:93–105
Arditti J, Dunn A (1969) Environmental plant physiology—experiments in cellular and plant physiology. Rinehart and Winston Inc, Holt
Asgher M, Khan MIR, Anjum NA, Khan NA (2014) Minimising toxicity of cadmium in plants—role of plant growth regulators. Protoplasma 252:399–413
Barceló JUAN, Poschenrieder C (1990) Plant water relations as affected by heavy metal stress: a review. J Plant Nutr 13(1):1–37
Belkadhi A, De Haro A, Obregon S, Chaı¨bi W, Djebali W (2015) Positive effects of salicylic acid pretreatment on the composition of flax plastidial membrane lipids under cadmium stress. Environ Sci Pollut R 22(2):1457–1467
Bernard A (2008) Cadmium and its adverse effects on human health. Indian J Med Res 128(4):557
Brehe JE, Burch HB (1976) Enzymatic assay for glutathione. Anal Biochem 74:189–197
Chen Z, Zheng Z, Huang J, Lai Z, Fan B (2009) Biosynthesis of salicylic acid in plants. Plant Signal Behav 4(6):493–496
Cui W, Li L, Gao Z, Wu H, Xie Y, Shen W (2012) Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J Exp Bot 63:5521–5534
Drazic G, Mihailovic N (2005) Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant sci 168(2):511–517
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxyl ammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Epstein E (1999) Annual review of plant physiollogy and plant molecular biology. Silicon 50:641–664
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155(1):2–18
Friberg L (2017) Cadmium in the environment. CRC Press, Boca Raton
Giannopolitis CN, Reis SK (1977) Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Gill SS, Khan NA, Anjum NA, Tuteja N (2011) Amelioration of cadmium stress in crop plants by nutrients management: morphological, physiological and biochemical aspects. Plant Stress 5(1):1–23
Gonçalves JF, Antes FG, Maldaner J, Pereira LB, Tabaldi LA, Rauber R, Rossato LV, Bisognin DA, Dressler VL, Flores EM, Nicoloso FT (2009) Cadmium and mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Physiol Biochem 47:814–821
Gossett DR, Millhollon EP, Cran LM (1994) Antioxidant response to NaCl stress in salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Guo Q, Meng L, Mao P-C, Jia Y-Q, Shi Y-J (2013) Role of exogenous salicylic acid in alleviating cadmium-induced toxicity in Kentucky bluegrass. Biochem Syst Ecol 50:269–276
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68(1):14–25
He JL, Li H, Luo J, Ma CF, Li SJ, Qu L, Gai Y, Jiang X, Janz D, Polle A, Tyree M (2013) A transcriptomic network underlies microstructure and physiological responses to cadmium in Populus canescens. Plant Physiol 162:424–439
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Horiguchi T, Morita S (1987) Mechanism of manganese toxicity and tolerance of plants VI. Effect of silicon on alleviation of manganese toxicity of barley. J Plant Nutr 10:2299–2310. https://doi.org/10.1080/01904168709363778
Janda T, Gondor OK, Yordanova R, Szalai G, Pa´l M (2014) Salicylic acid and photosynthesis: signalling and effects. Acta Physiol Plant 36:2537–2546
Kawano T, Bouteau F (2013) Crosstalk between intracellular and extracellular salicylic acid signaling events leading to long-distance spread of signals. Plant Cell Rep 32:1125–1138. https://doi.org/10.1007/s00299-013-1451-0
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462
Kulaeva OA, Tsyganov VE (2011) Molecular-genetic basis of cadmium tolerance and accumulation in higher plants. Russian J Genet 1(5):.349
Li X, Ma L, Bu N, Li Y, Zhang L (2014) Effects of salicylic acid pretreatment on cadmium and/or UV-B stress in soybean seedlings. Biol Plant 58:195–199
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu J, Zhu Q, Zhang Z, Xu J, Yang J, Wong MH (2004) Variations in cadmium accumulation among rice cultivars and types and the selection of cultivars for reducing cadmium in the diet. J Sci Food Agric 85:147–153
Liu Y, Xiao T, Baveye PC, Zhu J, Ning Z, Li H (2015) Potential health risk in areas with high naturally-occurring cadmium background in southwestern China. Ecotoxicol Environ Saf 112:122–131
Liu Z, Ding Y, Wang F, Ye Y, Zhu C (2016) Role of salicylic acid in resistance to cadmium stress in plants. Plant Cell Rep 35(4):719–731
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Lux A, Luxova´ M, Hattori T, Inanaga S, Sugimoto Y (2002) Silicification in sorghum (Sorghum bicolor) cultivars with different drought tolerance. Physiol Plant 115:87–92
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132(1):272–281
Mitani-Ueno N, Yamaji N, Ma F (2016) High silicon accumulation in the shoot is required for down-regulating the expression of Si transporter genes in rice. Plant Cell Physiol 57:2510–2518
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Muneer S, Park YG, Kim S, Jeong BR (2017) Foliar or Sub irrigation silicon supply mitigates high temperature stress in strawberry by maintaining photosynthetic and stress-responsive proteins. J Plant Growth Regul 36(4):836–845
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nwugo CC, Huerta AJ (2008) Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice plants exposed to low-level cadmium. Plant Soil 311:73–86
Popova LP, Maslenkova LT, Yordanova RY, Ivanova AP, Krantev AP, Szalai G, Janda T (2009) Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol Biochem 47(3):224–231
Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Biol 43(1):439–463
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62(10):3321–3338
Sairam RK, Rao KV, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163(5):1037–1046
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1022
Sfaxi-Bousbih A, Chaoui A, El Ferjani E (2010) Cadmium impairs mineral and carbohydrate mobilization during the germination of bean seeds. Ecotox Environ Safe 73(6):1123–1129
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation superoxide anion generation and activities of antioxidant enzymes in growing rice seedling. Plant Sci 161:1135–1144
Shi Q, Bao Z, Zhu Z, He Y, Qian Q, Yu J (2005) Silicon-mediated alleviation of Mn toxicity in Cucumis sativus in relation to activities of superoxide dismutase and ascorbate peroxidase. Phytochemistry 66:1551–1559. https://doi.org/10.1016/j.phytochem.2005.05.006
Shi G, Cai Q, Liu Q, Wu L (2009) Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol Plant 31:969–977
Shi X, Zhang Ch, Wang H, Zhang F (2005) Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 272:53–60
Singh S, Prasad SM (2013) Foliar application of salicylic acid minimizes cadmium-induced toxicity in Solanum melongena L. seedlings through an improved antioxidant system. Biochem Cell Arch 13:383–393
Singh VP, Kumar J, Singh S, Prasad SM (2014) Dimethoate modifies enhanced UV-B effects on growth, photosynthesis and oxidative stress in mung bean (Vigna radiata L.) seedlings: Implication of salicylic acid. Pestic Biochem Physiol 116:13–23
Singh VP, Singh S, Tripathi DK, Prasad SM, Chauhan DK (eds) (2017) Reactive oxygen species in plants: boon or bane-revisiting the role of ROS. John Wiley & Sons, Hoboken
Singh VP, Tripathi DK, Kumar D, Chauhan DK (2011) Influence of exogenous silicon addition on aluminium tolerance in rice seedlings. Biol Trace Elem Res 144(1–3):1260–1274
Song J, Feng SJ, Chen J, Zhao WT, Yang ZM (2017) A cadmium stress-responsive gene AtFC1 confers plant tolerance to cadmium toxicity. BMC Plant Biol 17(1):187
Stork F, Backor M, Klejdus B, Hedbavny J, Kovacik J (2013) Changes of metalinduced toxicity by H2O2/NO modulators in Scenedesmus quadricauda (Chlorophyceae). Environ Sci Pollut Res 20:5502–5511
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanisms, regulation and adaptation. Taylor & Francis, London, pp 445–483
Tripathi DK, Mishra RK, Singh S, Singh S, Singh VP, Singh PK, Chauhan DK, Prasad SM, Dubey NK, Pandey AC (2017) Nitric oxide ameliorates zinc oxide nanoparticles phytotoxicity in wheat seedlings: implication of the ascorbate-glutathione cycle. Front Plant Sci 8:1
Tripathi DK, Singh VP, Kumar D, Chauhan DK (2012a) Rice seedlings under cadmium stress: effect of silicon on growth, cadmium uptake, oxidative stress, antioxidant capacity and root and leaf structures. Chem Ecol 28(3):281–291
Tripathi DK, Singh VP, Kumar D, Chauhan DK (2012b) Impact of exogenous silicon addition on chromium uptake, growth, mineral elements, oxidative stress, antioxidant capacity, and leaf and root structures in rice seedlings exposed to hexavalent chromium. Acta Physiol Plant 34(1):279–289
Tripathi DK, Singh VP, Gangwar S, Prasad SM, Maurya JN, Chauhan DK (2014) Role of silicon in enrichment of plant nutrients and protection from biotic and abiotic stresses. In: Improvement of crops in the Era of climatic changes. Springer, New York, pp 39–56
Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK, Rai AK (2015) Silicon-mediated alleviation of Cr (VI) toxicity in wheat seedlings as evidenced by chlorophyll florescence, laser induced breakdown spectroscopy and anatomical changes. Ecotoxicol Environ Saf 113:133–144
Tripathi DK, Singh S, Singh VP, Prasad SM, Chauhan DK, Dubey NK (2016) Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front Environ Sci 4:46
Vaculík M, Landberg T, Greger M, Luxová M, Stoláriková M, Lux A (2012) Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Ann Bot-Londonn 110(2):433–443
Vaculík M, Pavlovič A, Lux A (2015) Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxicol Environ Saf 120:66–73
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain-treated bean plants. Plant Sci 151:59–66
Vitória AP, Lea PJ, Azevedo RA (2001) Antioxidant enzymes responses to cadmium in radish tissues. Phytochemistry 57(5):701–710
Wang Q, Liang X, Dong Y, Xu L, Zhang X, Kong J, Liu S (2013) Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J Plant Growth Regul 32:721–731
Wu FB, Chen FK, Wei KG, Zhang P (2004) Effects of cadmium on free amino acids, glutathione, and ascorbic acid concentration in two barley genotypes (Hordeum vulgare L.) differing in cadmium tolerance. Chemosphere 57:447–454
Xu WF, Shi WM, Yan F, Zhang B, Liang JS (2011) Mechanisms of cadmium detoxification in cattail (Typha angustifolia L.). Aqua Bot 94:37–43
Zhang Y, Xu S, Yang S, Chen Y (2015) Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo L.). Protoplasma 252:911–924
Acknowledgements
Swati Singh is thankful to the University Grants Commission, New Delhi for providing D. Phil Fellowship. The authors are thankful to UGC for providing FIST Grant to the Department of Botany University of Allahabad.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declared that they do not have any conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, S., Singh, V.P., Prasad, S.M. et al. Interactive Effect of Silicon (Si) and Salicylic Acid (SA) in Maize Seedlings and Their Mechanisms of Cadmium (Cd) Toxicity Alleviation. J Plant Growth Regul 38, 1587–1597 (2019). https://doi.org/10.1007/s00344-019-09958-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09958-1