Abstract
A pot experiment was conducted to determine the effects of Glomus mosseae inoculation on growth and some biochemical activities in roots and shoots of pepper (Capsicum annuum L. cv. Zhongjiao 105) plants subjected to four levels of NaCl [0 (control), 25 (low), 50 (medium), and 100 (high) mM] for 30 days, after 30 days of establishment under non-saline conditions. In mycorrhizal (M) plants, root colonization varied from 48 to 16 %. M plants had higher root and shoot dry weight and leaf area compared with non-mycorrhizal (NM) plants. Under salinity stress, M plants accumulated higher amounts of leaf photosynthetic pigments as well as soluble sugar, soluble protein, and total free amino acids in roots and shoots than those of NM plants. In contrast, the accumulation of proline was less intense in M plants than NM plants. Salt stress induced oxidative stress by increasing malondialdehyde (MDA) content; however, the extent of oxidative damage in M plants was less compared with NM plants due to G. mosseae-enhanced activity of superoxide dismutase (SOD) and peroxidase (POD). We concluded that inoculation with G. mosseae improved growth performance and enhanced salt tolerance of pepper plants via improving photosynthetic pigments and the accumulation of organic solutes (except proline), reducing oxidative stress, and enhancing antioxidant activities of the SOD-POD system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In many arid and semi-arid regions, salinity is one of the most serious abiotic factors limiting the yield of agricultural crops resulting in a threat to food security (Evelin and others 2009; Porcel and others 2012; Evelin and Kapoor 2013). It is estimated that nearly 7 % of the world’s agricultural land is affected by salinity, and increased salinization of arable lands is expected to cause 30 % land loss within the next 25 years and up to 50 % by the middle of twenty-first century (Wang and others 2003; Evelin and others 2009; Porcel and others 2012; Ruiz-Lozano and others 2012; Kapoor and others 2013). Salt stress affects several important processes in plants such as growth, photosynthesis, protein synthesis, and energy and lipid metabolism (Parida and Das 2005; Abbaspour and others 2012; Rasool and others 2013). The earliest response of plants to salinity is a reduction in the rate of leaf surface expansion, and ultimately cessation of its expansion (Parida and Das 2005; Parvaiz and Satyawati 2008; Abdel Latef 2011).
Excess Na+ and Cl− in the environment caused osmotic stress that reduces the osmotic potential of the soil solution and hence water absorption by the plant roots (Rasool and others 2013). Osmotic adjustment (osmoregulation) is an important mechanism in salinity tolerance because it helps plants to maintain higher turgor under salt stress (Munns 1993; Zou and others 2013). One of the most important responses of plants to saline conditions is the synthesis of compatible organic solutes called osmolytes that are accumulated in the cytosol (Hamdia and Shaddad 2010). These organic solutes include soluble sugars, soluble protein, free amino acids, and proline. Generally, they protect plants from salt stress in different ways including (a) reducing entrance of salt into the plant, (b) regulating the concentration of salt in the cytoplasm (Munns and Tester 2008), (c) contributing to cellular osmotic adjustment, (d) detoxification of reactive oxygen species (ROS), and (e) protection enzymes in presence of high cytoplasmic electrolyte concentrations (Sheng and others 2011).
Salinity-induced osmotic stress is responsible for the oxidative stress caused by the production of ROS such as singlet oxygen (1O2), hydrogen peroxide (H2O2), superoxide anion radicle (\( {\text{O}}_{ 2}^{{ \bullet_{-} }} \)), and hydroxyl radicle (•OH) (Rasool and others 2013). ROS are toxic and cause damage to carbohydrates, proteins, lipids, and DNA, and may cause the death of the cell (Gill and Tuteja 2010). Plants possess ROS-scavenging enzymes such as superoxide dismutase (SOD) and peroxidase (POD) which keep ROS under a favorable level (Abdel Latef and Chaoxing 2011a).
Arbuscular mycorrhizal fungi (AMF) establish mutualistic associations with the root of 70–90 % of terrestrial plant species including halophytes, hydrophytes, and xerophytes (Smith and Read 2008; Abdel Latef and Chaoxing 2011b; Ruiz-Lozano and others 2012; Abdel Latef 2013; Yang and others 2014). Mycorrhizal association can improve the supply of water and nutrients to the host plant; in turn, up to 20 % of plant-fixed carbon is transferred to the AMF (Zou and others 2013). Of various biotechnological techniques being employed in recent years to alleviate the adverse effects of environmental stresses especially salinity, the application of various beneficial microbes including AMF as an inexpensive, eco-friendly, and sustainable alternative technique has acquired much importance for growing crops on salt-affected soils (Tian and others 2004; Evelin and others 2009; Porcel and others 2012; Ruiz-Lozano and others 2012; Kapoor and others 2013). Root colonization by AMF includes a series of morpho-physiological and biochemical events that are regulated by the interaction of plant and fungus, as well as by environmental factors (Kaya and others 2009; Selvakumar and Thamizhiniyan 2011). AMF usually live in saline soils and help plants to alleviate salinity stress by enhancing (1) nutrient uptake (Evelin and others 2012), (2) photosynthetic activity (Hajiboland and others 2010; Abdel Latef and Chaoxing 2011a), (3) water-use efficiency (Hajiboland and others 2010), (4) accumulation of compatible solutes (Talaat and Shawky 2011; Evelin and others 2013), and (5) enzymatic antioxidants (Abdel Latef and Chaoxing 2011a; Evelin and Kapoor 2013). These benefits promoted AMF to act as bio-ameliorators of saline soils (Evelin and others 2009).
Little attention has been paid to the effect of AMF inoculation on the regulation of organic solute levels in pepper plants under salinity stress. Proline content decreased in leaves of mycorrhizal pepper compared to that in non-mycorrhizal pepper under salinity stress (Kaya and others 2009; Selvakumar and Thamizhiniyan 2011). (Beltrano and others 2013) reported that the shoot sugar content at high-salt stress in mycorrhizal pepper was considerably lower than that in non-stressed mycorrhizal pepper.
Pepper (Capsicum annuum L.) is one of the most important horticultural crops and widely cultivated for its fruits which have a high-nutritional value. The fruits of pepper are a rich source for vitamin C which protects the human body against oxidative damage (Zhani and others 2012). Pepper is not a salt tolerant vegetable; therefore, enhancing salt tolerance of pepper is an important task.
Thus, the question that arises here is what would happen if the pepper plants were treated with both salinity and AMF? Would inoculation with Glomus mosseae mitigate the adverse effect of salinity? Therefore, the aim of this work was to determine the changes in growth characteristics, root colonization, organic solutes (soluble sugar, soluble protein, total free amino acids, and proline), malondialdehyde (MDA), and activity of SOD and POD in roots and shoots as well as leaf photosynthetic pigments of pepper plants under salt stress and the possible role played by inoculation with G. mosseae in regulating salt-induced changes in these parameters.
Materials and Methods
Plant Culture, Mycorrhizal Inoculation, and Salt Treatments
Seeds of pepper (C. annuum L. cv. Zhongjiao 105) and mycorrhizal fungus inoculums of G. mosseae (Nicolson and Gerdemann) were obtained from the Institute of Vegetables and Flowers, CAAS, Beijing, P.R. China. Seeds were surface-sterilized with 2.5 % sodium hypochlorite solution for 10 min, rinsed four times with distilled water, and kept for germination on wet filter paper in Petri dishes at 25 °C. Seven-day-old seedlings were transferred to plastic pots (20 cm in depth and 20 cm in mouth diameter) containing 2 kg soil mixture (organic manure, soil and straw = 1:2:1). The soil mix was collected from the greenhouse of the Institute of Vegetables and Flowers. Soil properties were pH 7.12, 13.2 organic matter, 155 mg kg−1 available phosphorus, 460 mg kg−1 available nitrogen, and 500 mg kg−1 available potassium (Abdel Latef and Chaoxing 2011a). The soil was autoclaved for 30 min at 125 °C. At the same time, half of the pots (Mycorrhizal plants) were inoculated with 30 g G. mosseae. Non-mycorrhizal plants received the same weight of autoclaved inoculums. The inoculums were placed adjacent to each seeding root. Mycorrhizal fungus inoculums consisted of spores, soil, hyphae, and infected clove (Triffolium repens) root fragments from a stock culture of G. mosseae. The pots were irrigated with water to 80 % of their saturation capacity and supplied with half strength Hoagland nutrient solution once a week. The seedlings were grown for 30 days and then subjected to four levels of NaCl stress [0 (control), 25 (low), 50 (medium), and 100 (high) mM NaCl. The different saline solutions were added once, and the soil water saturation capacity of each pot was adjusted daily at 80 %. The experimental pots were placed in a greenhouse at an average temperature of 30/22 °C (day/night). The photon flux density ranged from 600 to 1,200 μmol m−2 s−2, and relative humidity was between 60 and 80 %.
Experimental Design
The experimental design consisted of eight treatments crossing two mycorrhizal inoculations levels (non-AMF and G. mosseae) with four soil salt levels (0, 25, 50, and 100 mM NaCl). Pots were arranged in a completely randomized block design. Six replicates of each treatment were applied leading to a total of 48 pots (2 seedlings/pot). The seedlings were harvested 30 days after NaCl treatments.
Root Colonization
A fraction of the roots were carefully washed, cut into 1 cm long segments, cleared in 10 % KOH at 90 °C for 20 min, acidified in 2 % HCl for 5 min, and stained with 0.01 % acid fuchsin (Kormanik and others 1980). Mycorrhizal colonization rate was measured using the grid-line intersection method described by Giovannetti and Mosse (1980).
Growth Measurements
Leaf area was measured by digital planimeter (Placom KP-90) to the nearest square centimeter. At harvest, roots and shoots were separately washed with tap water to remove any adhering debris. The root and shoot dry weights were determined after oven-drying at 70 °C for 48 h.
Photosynthetic Pigments
The concentrations of chlorophyll a, chlorophyll b, and carotenoids of the youngest fully-expanded leaf 1 week before harvest were assayed according to Arnon (1949) with some modifications by Zhang and Zhang (2006). The extraction was made from a 0.2 g fresh sample in 20 ml ethanol, acetone, and water (4.5: 4.5: 1, v/v/v) mixture and measured at 645, 663, and 470 nm with a UV–Vis spectrophotometer.
Organic Solutes
Soluble sugar was determined by the anthrone sulfuric acid method described by Badour (1959). The dried root and shoot tissue (0.1 g) was extracted by distilled water. One ml of the sugar extract was mixed with 9 ml of anthrone sulfuric acid reagent in a test tube and heated for 7 min at 100 °C. The absorbance was measured spectrophotometrically at 620 nm against a blank containing only distilled water and anthrone reagent.
Soluble protein of roots and shoots was determined according to the method described by Bradford (1976) using bovine serum albumin as a standard.
Total free amino acids of roots and shoots were extracted and estimated according to the method of Lee and Takanashi (1966). About 0.1 ml of the water extract containing free amino acids was mixed with 1.9 ml of ninhydrin-citrate-glycerol mixture in a test tube for 20 min at 100 °C. The absorbance was measured at 570 nm against a blank (only distilled water and the same reagent).
The proline concentration of roots and shoots was estimated according to Bates and others (1973). A known weight of dried tissue (0.2 g) was homogenized in 10 ml of 3 % sulfosalicylic acid and filtered. Two ml of the filtrate was reacted with 2 ml glacial acetic acid and 2 ml of acid-ninhydrin reagent in a test tube and heated for 1 h at 100 °C. The reaction mixture was extracted with 4 ml toluene. The chromophore was aspired from the aqueous phase and the absorbance was read at 520 nm using toluene as a blank.
Lipid Peroxidation
Lipid peroxidation was measured as the amount of MDA according to the thiobarbituric acid (TBA) method as described by Heath and Packer (1968) with some modifications by Zhang and Qu (2004). Fresh root and shoot samples (0.5 g) were homogenized with 5 % trichloroacetic acid and centrifuged at 4,000×g for 10 min. Two ml of extract was added to 2 ml of 0.6 % TBA and placed in a boiling water bath for 10 min, and absorbance was read at 532, 600, and 452 nm. The MDA concentration was calculated according to the formula 6.45 × (A 532–A 600) −0.56 × A 450.
Enzyme Extraction and Assay
Fresh roots and shoots (0.5 g) were homogenized in 5 ml phosphate buffer (0.1 mol/l and pH 7.8) and centrifuged at 10,000×g for 20 min at 4 °C, and the supernatant was used for SOD and POD assays. The activity of SOD (EC 1.15.1.1) was assayed with the nitro blue tetrazolium (NBT) method of Giannopolitis and Ries (1977). One unit of SOD was defined as the amount of enzyme required to cause 50 % inhibition of the reduction of NBT as monitored at 560 nm. POD (EC 1.11.1.7) activity was measured according to Hammerschmidt and others (1982) by monitoring the rate of guaiacol oxidation at 470 nm.
Statistical Analysis
The data were analyzed by analysis of variance (ANOVA) with SAS version 8.0 software, and treatment means were compared by Duncan’s test (p < 0.05).
Results
Root Colonization by G. mosseae Under Salinity Stress Conditions
Non-inoculated plants were not mycorrhized. Percentages of mycorrhizal colonization with G. mosseae ranged from 48 to 16 %. Mycorrhizal root colonization decreased with increasing NaCl concentration (Table 1).
Effect of Salinity and G. mosseae Inoculation on Growth Characteristics
The data in Table 1 showed that the dry weight of non-mycorrhizal (NM) roots was drastically reduced at all tested concentrations of NaCl. However, shoot dry weight and leaf area of NM did not significantly decrease up to the low level of NaCl (25 mM), beyond this concentration a significant reduction was observed compared to that of control plants. The maximum reduction in NM root and shoot dry weight as well as leaf area was recorded at the high-salt level (100 mM) and was approximately 66, 43, and 46 %, respectively, versus the control plants. Colonization with G. mosseae ameliorated growth parameters of mycorrhizal (M) plants versus NM plants regardless of the salt level (Table 1). This amelioration was more obvious in dry roots than that of dry shoot and leaf area. The maximum increase in M dry root, dry shoot as well as leaf area at the high-salt level was 81, 34, and 40 %, respectively, over NM plants (Table 1).
Effect of Salinity and G. mosseae Inoculation on Photosynthetic Pigments
In comparison with controls, photosynthetic pigments (chlorophyll a and b as well as carotenoids) of NM leaves did not decrease significantly up to the low level of NaCl, thereafter they were significantly decreased. The magnitude of decrease in chlorophyll b was higher than that of chlorophyll a and carotenoids (Fig. 1a–c). Inoculation with G. mosseae increased pigment concentrations at all salt levels even under the control level. At the high-NaCl level, M plants recorded about a 52, 109, and 56 % increase in chlorophyll a, chlorophyll b, and carotenoids, respectively, higher than NM plants (Fig. 1a–c).
Effect of Salinity and G. mosseae Inoculation on Organic Solutes
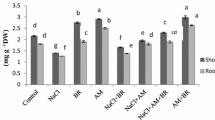
There is a marked decline in soluble sugar of NM root with increasing salinity levels and this decline reached to 61 % at the high level of salt compared with control. In NM shoot, soluble sugar increased up to the low level of NaCl, thereafter, nonsignificant and constant decrease was recorded at the medium and high level of salt (Table 2). Colonization with AMF stimulated the accumulation of soluble sugar in comparison with non-salinzed and salinized plants. The accumulation of soluble sugar in roots and shoots due to inoculation by G. mosseae was about 75 and 32 %, respectively, at the high-salt level over NM plants (Table 2).
NaCl treatment gradually lowered the soluble protein in NM roots, whereas the opposite trend was observed in NM shoots in comparison with control (Table 2). Mycorrhizal colonization progressively increased the soluble protein in M roots and shoots compared with NM organs. At the high level of NaCl, the increase in soluble protein in M roots and shoots was about 70 and 25 %, respectively, over NM plants (Table 2).
Salinity stress induced a drastic decrease in the total free amino acids of NM roots, whereas in shoots of NM plant, total free amino acids did not significantly change up to the low level of NaCl, whereas excess NaCl in the soil caused a significant accumulation in total free amino acids (Table 2). M plants had a higher concentration of total free amino acids than NM plants regardless of the salinity level. The accumulation of total free amino acids in roots and shoots due to AMF inoculation at the high-NaCl level was approximately 75 and 28 %, respectively, in comparison with NM plants (Table 2).
Salinity treatments exhibited a significant accumulation in proline concentration of NM roots, and the maximum accumulation was 2.5 times higher than those of controls at the high level of salinity. In NM shoots, all salinity levels caused a little decrease in proline concentration which did not exceed 10 % at all salinity levels compared with control (Table 2). Mycorrhization decreased the proline concentration in roots and shoots of M plants versus NM plants regardless of the salt level (Table 2). The maximum decreases in proline of M roots and shoots at the high level of salinity were 45 and 35 %, respectively, compared with NM plants. It is worthy to mention that, the decrease in proline shoots due to mycorrhization was mostly similar at all salinity levels.
Effect of Salinity and G. mosseae Inoculation on Lipid Peroxidation
The results showed that all salinity levels induced a sharp accumulation in MDA content of NM roots (Fig. 2a). In NM shoots, MDA content remained around the control value at the low-salinity level; beyond this level it markedly accumulated (Fig. 2b). At the high-salt concentration, the accumulation of MDA content in NM roots and shoots was about 108 and 61 %, respectively, over the control (Fig. 2a, b). Mycorrhizal plants maintained lower MDA content at all saline treatments in comparison with NM plants even under the control condition (Fig. 2a, b).
Effect of Salinity and G. mosseae Inoculation on SOD-POD System
Application of NaCl in the soil markedly increased the activities of SOD and POD in NM roots and shoots (Fig. 3a–d). This increase in the activity of SOD and POD was more obvious in shoots than roots at all salinity levels. At the high level of salinity, the activities of SOD and POD in NM roots were about 136 and 76 %, respectively, over the control, whereas in NM shoots the activities of SOD and POD were about 157 and 119 %, respectively, higher than that of control. Mycorrhizal colonization caused an increment of SOD-POD system production, which in turn may have improved salinity tolerance in M plants (Fig. 3a–d). In M roots, the activity of SOD was increased by 39, 18, 13, and 18 % and that of POD by 32, 71, 78, and 59 % at 0, 25, 50, and 100 mM NaCl, respectively. In M shoots, the SOD activity increment was 47, 31, 16, and 22 % and that of POD was 30, 18, 22, and 29 % at 0, 25, 50, and 100 mM NaCl, respectively, over NM plants.
Discussion
In this study, root colonization was negatively affected with increasing NaCl level, indicating that salt stress inhibited the growth of AMF. This inhibitory effect of salinity on root colonization may be due to the direct effect of NaCl on fungi (Zarei and Paymaneh 2013). This result is in harmony with previous studies on pepper plants reporting that addition of NaCl salt to the soil can hamper germination of spores, colonization capacity, and hyphal growth of fungus (Kaya and others 2009; Selvakumar and Thamizhiniyan 2011; Çekiç and others 2012; Beltrano and others 2013).
The inhibitory effects of salinity stress on the growth of the experimental pepper plants are in agreement with earlier reports on other plant species (Abdel Latef and Chaoxing 2011a; El-Amri and others 2013; Evelin and Kapoor 2013; Huang and others 2013). These inhibitory effects may be attributed to the fact that salt stress is known to retard growth as a result of osmotic stress, ionic toxicity, nutritional imbalance, and oxidative stress (Chen and others 2007; Abdel Latef 2010; Abdel Latef 2011; Oztekin and others 2013; Zou and others 2013; Hameed and others 2014). Interestingly, NM roots were more sensitive to salt stress than shoots. This may be a result of root vicinity to Na+ and Cl− ions. The decrease in leaf area by salt might be attributed to a decrease in leaf turgor or a decreased photosynthesis rate (Abdel Latef 2011). Colonization with AMF has been shown to improve the growth characteristics of salt-stressed pepper plants. The beneficial effects of AMF on pepper growth under saline conditions are in accordance with Demir (2004), Turkmen and others (2008), Kaya and others (2009), Çekiç and others (2012), and Beltrano and others (2013) working on pepper plants.
The retardation in photosynthetic pigments in NM pepper leaves under salinity stress coincided with findings of Kaya and others (2009) and Çekiç and others (2012). This result might be ascribed to the decrease in chloroplast number and disorganization of the thylakoid membrane structures of chloroplasts (Bruns and Hecht-Buchholz 1990; Parida and others 2003) or due to repression of specific enzymes that are responsible for the synthesis of photosynthetic pigments (Abdel Latef and Chaoxing 2011a). The decrease in chlorophyll b in NM leaves was higher than that of chlorophyll a indicating that chlorophyll a was more stable to salinity stress than chlorophyll b. M plants had higher pigment concentrations than NM plants. This may be the result of increased photosynthetic leaf area of M plants. Similar results on pepper plants were observed by various authors (Demir 2004; Kaya and others 2009; Çekiç and others 2012). They showed that the beneficial effects of mycorrhiza could be attributed to high-photosynthetic activity and then less prone to oxidative stress leading to the destruction of the photosynthetic apparatus (Evelin and others 2009; Yang and others 2014). Therefore, it could be suggested that the improvement in pigment concentration may be attributed to the adaptive defense system of M pepper plants against the toxic effect of salinity stress.
Osmolytes play a major role in the protection of plants from ultrastructure damage induced by salt stress. Osmolytes are water soluble small organic molecules that are non-toxic at high concentrations (Evelin and others 2013).
Soluble sugars are important osmolytes that contribute up to 50 % of the total osmotic potential in glycophytes subject to saline conditions (Parvaiz and Satyawati 2008). Their major functions are osmoprotection, osmotic adjustment, carbon storage, and radical scavenging under salinity stress (Koyro and others 2012; Rasool and others 2013). Protein accumulation under salt stress may provide a storage form of nitrogen that could be re-utilized later and may play a key role in osmoregulation (ZhongQun and others 2010; Rasool and others 2013). Total free amino acids are important organic osmotica contributing to osmotic adjustment in plants under saline conditions. Proline is an important compatible organic solute that plays a key role in osmoregulation in plants subjected to salt stress (Ahmad and others 2012). Increasing accumulation of proline has been reported in many plants subjected to saline conditions (Abdel Latef 2010, 2011; Zarei and Paymaneh 2013; Hameed and others 2014). It has been suggested that proline accumulation can serve as a symptom of salt-stress injury (Zhifang and Loescher 2003). On the contrary, it has been suggested that proline accumulation can serve as a sensor of salt tolerance (Parida and Das 2005; Hassanein and others 2009).
The relative ability of the plant or plant organ to stimulate the accumulation of cytosolutes in its tissues (osmotic regulation) will partially determine its tolerance to stress conditions (Abdel Latef 2010). In this study, the accumulation of soluble sugar, soluble protein as well as total free amino acids of NM shoots over NM roots alleviated the imposed salt stress via osmotic adjustment. Thus, the salt adaptation of NM shoots included soluble sugar, soluble protein, and total free amino acids involved in tissue growth and osmoregulation. However, the sensitivity of NM roots was associated with depletion in soluble sugar, soluble protein, and total free amino acids leading to a drastic reduction in growth even at the low level of salt. According to our results, proline remained constant in NM shoots and increased in NM roots even at the low level of salinity. Thus, proline was the only one among those measured organic solutes that was able to make a major contribution for osmotic adjustment in NM roots, whereas in shoots the contribution of proline in osmoregulation might be reduced.
Under salt stress, the results showed that improved salt tolerance of M plants compared with NM plants was closely associated with the increase in soluble sugar accumulation. The greater soluble sugar accumulation in M plants may be due to (1) colonization with AMF enhanced photosynthesis and allowed higher allocation of sugars from shoots to roots, (2) the sink effect of mycorrhizal fungus demanding sugars from shoot tissues, and (3) hydrolysis of starch to soluble sugar in the seedlings inoculated with mycorrhiza (Evelin and others 2009; ZhongQun and others 2010; Ruiz-Lozano and others 2012; Kapoor and others 2013). Our results are in a good agreement with previous studies on different plant species (Talaat and Shawky 2011; El-Amri and others 2013; Evelin and Kapoor 2013; Zou and others 2013). Conversely, our results are in disagreement with those of Beltrano and others (2013) who reported that the shoot sugar content at high-salt stress in mycorrhizal pepper was considerably lower than that in non-stressed mycorrhizal pepper.
The accumulation of soluble sugar induced by AMF symbiosis is a positive response to salt stress because sugars can maintain the osmotic equilibrium in plant cells, prevent structural changes in soluble proteins, and protect membrane integrity (Sheng and others 2011).
The present study showed that AMF colonization increased soluble protein accumulation of M plants compared with NM plants throughout the period of stress. This increase in soluble protein by AMF inoculation might be attributed to AM-mediated activation of certain plant genes (Sheng and others 2011). Our results suggested that the increase in soluble protein contributed to the enhanced tolerance of M plants.
The higher concentration of total free amino acids in M pepper plants under salt stress over NM plants may be attributed to improved N uptake in M plants (Sheng and others 2011; Evelin and others 2012; Evelin and Kapoor 2013).
When salt-stressed pepper plants were inoculated with G. mossaea, proline concentration declined compared with non-mycorrhizal plants. This result was in accordance with the results of Kaya and others (2009) in pepper, Sheng and others (2011) in maize and Evelin, and Kapoor (2013) in fenugreek. This reduction in proline concentration in M plants was associated with a noticeable accumulation of other organic solutes (soluble sugar, soluble protein, and total free amino acids). Thus, proline may not be the main compatible solute participating in osmoregulation of mycorrhizal plants, and the lower accumulation of proline in M plants suggests that the degree of injury was slighter so that there was no need to synthesize more proline for osmotic adjustment protection. Therefore, we can suggest that mycorrhization with G. mossaea may protect pepper plants against the detrimental effects of salt stress by the accumulation of organic solutes including soluble sugar, soluble protein, and total free amino acids (except proline).
Salinity stress can lead to membrane lipid peroxidation thereby disrupting membrane integrity (Evelin and Kapoor 2013). MDA is the indicator of damage to membranes by excessive ROS, or indirectly it is the measure of membrane stability. Increased MDA content in NM and M plants with increasing salt stress could be the result of oxidative damage to membranes due to excessive generation of ROS. However, lower values of MDA in M plants compared with NM plants suggest that the presence of AM fungi can be a strategy for alleviating the deleterious effects of salt stress in pepper plants. This confirms the results of Çekiç and others (2012).
It is well known that plants can induce effective antioxidant systems to protect themselves against oxidation damage. The antioxidant defense systems include enzymatic and non-enzymatic systems in which SOD and POD are the important antioxidant enzymes because they can efficiently prevent the accumulation of \( {\text{O}}_{ 2}^{{ \bullet_{-} }} \) and H2O2 and minimize the deleterious effect of ROS (Jaleel and others 2009; Abdel Latef and Chaoxing 2011b). Within a cell, SOD constitutes the first line of defense against ROS (Kang and others 2012) and it converts \( {\text{O}}_{ 2}^{{ \bullet_{ - } }} \) to O2 and H2O2. POD catalyzes the reduction of H2O2 by various molecules such as phenolic compounds and is directly involved in ROS scavenging (Kravic and others 2013). In this work, M plants had higher SOD and POD activities in roots and shoots compared to that of NM plants under salinity stress. These results are consistent with previous reports of higher activity of SOD and POD in M plants under salt stress (He and others 2007; Hajiboland and others 2010; Abdel Latef and Chaoxing 2011a; Evelin and Kapoor 2013). This implies that colonization by G. mossae could alleviate the damage of ROS, protect the pepper plants against the oxidative stress, and finally improve salt tolerance of pepper plants.
In conclusion, G. mosseae can protect pepper plants against salt stress by enhancing a series of morpho-physiological parameters such as plant growth, photosynthetic pigments, organic solutes including soluble sugar, soluble protein, and total free amino acids (except proline) and antioxidant activities of the SOD-POD system. Therefore, the encouragement of this symbiotic association between AMF and pepper plants is of great interest in its commercial production especially in many arid and semi-arid regions. Also, this allows for a more environmentally friendly type of agriculture, which may become an issue in the twenty-first century.
References
Abbaspour H, Afshari H, Abdel-Wahhab A (2012) Influence of salt stress on growth, pigments, soluble sugars and ion accumulation in three pistachio cultivars. J Medicinal Plants Res 6:2468–2473
Abdel Latef AA (2010) Changes of antioxidative enzymes in salinity tolerance among different wheat cultivars. Cereal Res Comm 38:43–55
Abdel Latef AA (2011) Ameliorative effect of calcium chloride on growth, antioxidant enzymes, protein patterns and some metabolic activities of canola (Brassica napus L.) under seawater stress. J Plant Nutr 34:1303–1320
Abdel Latef AA (2013) Growth and some physiological activities of pepper (Capsicum annuum L.) in response to cadmium stress and mycorrhizal symbiosis. J Agr Sci Tech 15:1437–1448
Abdel Latef AA, Chaoxing H (2011a) Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci Hort 127:228–233
Abdel Latef AA, Chaoxing H (2011b) Arbuscular mycorrhizal influence on growth, photosynthetic pigments, osmotic adjustment and oxidative stress in tomato plants subjected to low temperature stress. Acta Physiol Plant 33:1217–1225
Ahmad P, Hakeem KR, Kumar A, Ashraf M, Akram NA (2012) Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr J Biotechnol 11:2694–2703
Arnon DT (1949) Copper enzymes in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Badour SSA (1959) Analytisch–chemische Untersuchung des Kaliummangels bei Chlorella im Vergleich mit anderen Mangelzuständen. Ph.D dissertation Göttingen. [Analytical-chemical investigation of potassium deficiency in Chlorella in comparison with other deficiencies]. Ph.D dissertation, Göttingen University, Göttingen, Germany
Bates LS, Wladren PR, Tear DT (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beltrano J, Ruscitti M, Arango MC, Ronco M (2013) Effects of arbuscular mycorrhiza inoculation on plant growth, biological and physiological parameters and mineral nutrition in pepper grown under different salinity and p levels. J Soil Sci Plant Nutr 13:123–141
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem 72:248–254
Bruns S, Hecht-Buchholz C (1990) Light and electron microscope studies on the leaves of several potato cultivars after application of salt at various developmental stages. Potato Res 33:33–41
Çekiç FÖ, Ünyayar S, Ortas I (2012) Effects of arbuscular mycorrhizal inoculation on biochemical parameters in Capsicum annuum grown under long term salt Stress. Turk J Bot 36:63–72
Chen Z, Zhou M, Newman I, Mendham N, Zhang G, Shabala S (2007) Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct Plant Biol 34:150–162
Demir S (2004) Influence of arbuscular mycorrhiza on some physiological growth parameters of pepper. Turk J Biol 28:85–90
El-Amri SM, Al-Whaibi MH, Abdel-Fattah GM, Siddiqui MH (2013) Role of mycorrhizal fungi in tolerance of wheat genotypes to salt stress. Afr J Microbiol Res 7:1286–1295
Evelin H, Kapoor R (2013) Arbuscular mycorrhizal symbiosis modulates antioxidant response in salt-stressed Trigonella foenum-graecum plants. Mycorrhiza. doi:10.1007/s00572-013-0529-4
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Annals Bot 104:1263–1280
Evelin H, Giri B, Kapoor R (2012) Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigat ion of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 22:203–217
Evelin H, Giri B, Kapoor R (2013) Ultrastructural evidence for AMF mediated salt stress mitigation in Trigonella foenum-graecum. Mycorrhiza 23:71–86
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular infection in roots. New Phytol 84:489–500
Hajiboland R, Aliasgharzadeh N, Laiegh SF, Poschenreider C (2010) Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 331:313–327
Hamdia MA, Shaddad MAK (2010) Salt tolerance of crop plants. a review. J Stress Physiol Biochem 6:64–90
Hameed A, Dilfuza E, Abd-Allah EF, Hashem A, Kumar A, Ahmad P (2014) Salinity stress and arbuscular mycorrhizal symbiosis in Plants. In: Miransari M (ed) Use of microbes for the alleviation of soil stresses, vol 1. Springer Science + Business Media, New York, pp 139–159
Hammerschmidt R, Nuckles EM, Kuc J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrchum lagenarium. Physiol Plant Pathol 20:73–82
Hassanein RA, Hassanein AA, Haider AS, Hashem HA (2009) Improving salt tolerance of Zea Mays L. plants by presoaking their grains in glycine betaine. Aust J Basic Appl Sci 3:928–942
He Z, He C, Zhang Z, Zou Z, Wang H (2007) Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress. Colloids Surf B 59:128–133
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Huang JC, Lai WA, Singh S, Hameed A, Young CC (2013) Response of mycorrhizal hybrid tomato cultivars under saline stress. J Soil Sci Plant Nutr 13:469–484
Jaleel CA, Riadh K, Gopi R, Manivannan P, Inès J, AI-Juburi HJ, Zhao CX, Shao HB, Panneerselvam R (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436
Kang HM, Wang G, Chen K, Bai J (2012) Antioxidative system’s responses in the leaves of six Caragana species during drought stress and recovery. Acta Physiol Plant 34:2145–2154
Kapoor R, Evelin H, Mathur P, Giri B (2013) Arbuscular mycorrhiza: Approaches for abiotic stress tolerance in crop plants for sustainable agriculture. In: Tuteja N, Gill SS (eds) Plant acclimation to environmental stress. Springer Science + Business Media, LLC, Dordrecht, pp 359–401. doi:10.1007/978-1-4614-5001-6_14
Kaya C, Ashraf M, Sonmez O, Aydemir S, Tuna AL, Cullu MA (2009) The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci Hort 121:1–6
Kormanik PP, Bryan WC, Schultz RC (1980) Procedure and equipment for staining large number of plant roots for endomycorrhizal assay. Can J Microbiol 26:536–538
Koyro HW, Ahmad P, Geissler N (2012) Abiotic stress responses in plants: an overview. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer Science + Business Media, New York, pp 1–28
Kravić N, Marković K, Anđelković V, Hadži-Tašković V, Babić V, Vuletić M (2013) Growth, proline accumulation and peroxidase activity in maize seedlings under osmotic stress. Acta Physiol Plant 35:233–239
Lee YP, Takanashi T (1966) An improved colorimetric determination of amino acids with the use of ninhydrin. Anal Biochem 14:71–77
Munns R (1993) Physiological process limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ 16:15–24
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Oztekin GB, Tuzel Y, Tuzel IH (2013) Does mycorrhiza improve salinity tolerance in grafted plants? Sci Hort 149:55–60
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants. Ecotoxicol Environ Safety 60:324–349
Parida AK, Das AB, Mittra B (2003) Effects of NaCl stress on the structure, pigment complex composition and photosynthetic activity of mangrove Bruguiera parviflora chloroplasts. Photosynthetica 41:191–200
Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants—a review. Plant Soil Environ 54:89–99
Porcel R, Aroca R, Ruiz-Lozano JM (2012) Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron Sustain Dev 32:181–200
Rasool S, Hameed A, Azooz MM, Muneeb-u-Rehman, Siddiqi TO, Ahmad P (2013) Salt stress: causes, types and responses of plants. In: P Ahmad and others (eds.) Ecophysiology and responses of plants under salt stress. Springer Science + Business Media, LLC, New York, pp 1–24. doi:10.1007/978-1-4614-4747-4_1
Ruiz-Lozano JM, Porcel R, Azcόn C, Aroca R (2012) Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J Exp Bot. doi:10.1093/jxb/ers126
Selvakumar G, Thamizhiniyan P (2011) The effect of the arbuscular mycorrhizal (AM) fungus Glomus intraradices on the growth and yield of Chilli (Capsicum annuum L.) under salinity stress. World Appl Sci J 14:1209–1214
Sheng M, Tang M, Zhang F, Huang Y (2011) Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 21:423–430
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, San Diego
Talaat NB, Shawky BT (2011) Influence of arbuscular mycorrhizae on yield, nutrients, organic solutes, and antioxidant enzymes of two wheat cultivars under salt stress. J Plant Nutr Soil Sci 174:283–291
Tian CY, Feng G, Li XL, Zhang FS (2004) Different effects of arbuscular mycorrhizal fungal isolates from saline or nonsaline soil on salinity tolerance of plants. Appl Soil Ecol 26:143–148
Turkmen O, Sensoy S, Demir S, Erdinc C (2008) Effects of two different AMF species on growth and nutrient content of pepper seedlings grown under moderate salt stress. Afr J Biotechnol 7:392–396
Wang WX, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Yang Y, Tang M, Sulpice R, Chen H, Tian S, Ban Y (2014) Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J Plant Growth. doi:10.1007/s00344-013-9410-0
Zarei M, Paymaneh Z (2013) Effect of salinity and arbuscular mycorrhizal fungi on growth and some physiological parameters of Citrus jambheri. Arch Agron Soil Sci. doi:10.1080/03650340.2013.853289
Zhang ZL, Qu W (2004) Experimental guidance of plant physiology. High Education, Beijing
Zhang ZA, Zhang MS (2006) Experimental guide for plant physiology. High education, Beijing
Zhani K, Elouer MA, Aloui H, Hannachi C (2012) Selection of a salt tolerant Tunisian cultivar of chili pepper (Capsicum frutescens). Eurasia J Biosci 6:47–59
Zhifang G, Loescher WH (2003) Expression of a celery mannose 6-phosphate reductase in Arabidopsis thaliana enhances salt tolerance and induces biosynthesis of both mannitol and glucosyl-mannitol dimmer. Plant Cell Environ 26:275–283
ZhongQun H, HaoRu T, HuanXiu L, ChaoXing H, ZhiBin Z, HuaiSong W (2010) Arbuscular mycorrhizal alleviated ion toxicity, oxidative damage and enhanced osmotic adjustment in tomato subjected to NaCl stress. American-Eurasian J Agric Environ Sci 7:676–683
Zou YN, Liang YC, Wu QS (2013) Mycorrhizal and non-mycorrhizal responses to salt stress in trifoliate orange: plant growth, root architecture and soluble sugar accumulation. Int J Agric Biol 15:565–569
Acknowledgments
This work was supported by the Egyptian Ministry of Higher Education and Scientific Research (ParOwn 1207) grant and the Chinese National Science and Technology (Project 2011BAD12B03). The authors would like to thank Dr. Ahmad Hassan for revision of English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel Latef, A.A.H., Chaoxing, H. Does Inoculation with Glomus mosseae Improve Salt Tolerance in Pepper Plants?. J Plant Growth Regul 33, 644–653 (2014). https://doi.org/10.1007/s00344-014-9414-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-014-9414-4