Abstract
No information is available concerning the influence of dual application of 24-epibrassinolide (EBL) and spermine (Spm) on the nitrogen metabolism in plants subjected to drought conditions. As a first report, this investigation assesses the role of EBL, Spm, and their dual application on polyamine and protein pools in water-stressed plants. It explores the ameliorative effects of these foliar applications under water deficiency. Two maize hybrids (Giza 10 and Giza 129) were treated with or without EBL and/or Spm foliar applications under well-irrigated and drought-stressed conditions (75 and 50 % of field capacity). Dual application (25 mg l−1 Spm + 0.1 mg l−1 EBL) significantly relieved the drought-induced inhibition on the activities of ribulose-1,5-bisphosphate carboxylase and nitrate reductase and the contents of relative water, nitrate, and protein, particularly in hybrid Giza 129. Changes in the content of free polyamines and in the activity of polyamine biosynthetic and catabolic enzymes were detected when water-stressed plants were treated with EBL and/or Spm. Putrescine content and arginine decarboxylase activity were significantly increased in stressed hybrid Giza 10 plants treated by the dual application. However, spermidine and Spm levels as well as ornithine decarboxylase and S-adenosylmethionine decarboxylase activities were significantly increased in stressed hybrid Giza 129 plants treated with the dual application. Diamine oxidase, polyamine oxidase, protease activity, carbonyl content, and ethylene formation were increased in response to water stress and significantly decreased when stressed plants were treated by the dual application. Total free amino acids, phenols, and flavonoids concentration were increased with the increasing water stress level; moreover, they further increased in stressed plants treated with the dual application. Overall, the combined utilization of EBL and Spm serves as complementary tools to confer plant drought tolerance by altering polyamine, ethylene, and protein levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water stress is the major factor limiting crop production worldwide. It alters a series of physiological, biochemical, and molecular responses (Ribaut and others 2012). Enhancing plant drought tolerance is the major challenge faced by modern agriculture, where polyamines can play an important role (Gupta and others 2013). Plant polyamines are associated with the response of plants to diverse environmental stresses (Alcazar and others 2010; Talaat and Shawky 2012, 2013b; Gupta and others 2013). An increase in the endogenous polyamine content was reported in water-stressed plants confirming stress-specific roles of PAs. Moreover, those with a higher number of amino groups [spermidine (Spd) and spermine (Spm)] were more effective in scavenging of reactive oxygen species, than putrescine (Groppa and Benavides 2008; Kubis’ 2008; Zhou and Yu 2010). In addition, the fluctuations of polyamine levels vary among plant genotypes. A significant increase in the free Spd and Spm levels was observed in a drought-tolerant cultivar compared to increase of free putrescine levels in a drought-sensitive cultivar (Liu and others 2007; Zhang and Huang 2013). Furthermore, changes in the biosynthesis and catabolism of polyamines have occurred in plants under stress environments (Duan and others 2008; Groppa and Benavides 2008; Talaat and Shawky 2012, 2013b; Zhang and Huang 2013). Decreased protein content and enhanced protein carbonyl formation have been also detected in stressed plants (Bartoli and others 2004).
Brassinosteroids are a group of naturally occurring plant steroidal hormones with wide-ranging biological activities that offer the unique possibility of increasing crop yields through both changing plant metabolism and protecting plants from environmental stresses (Krishna 2003; Talaat and Shawky 2013a). Brassinosteroids have pleiotropic effects and can induce a broad spectrum of cellular responses including stem elongation, xylem differentiation, pollen tube growth, reproductive and vascular development, enzyme activation, membrane hyperpolarization, source/sink relationships, ethylene biosynthesis, proton pump activation, photosynthesis, nucleic acid and protein synthesis, and the regulation of gene expression (Bajguz 2000; Krishna 2003; Talaat and Shawky 2012). Previous studies have demonstrated that exogenous application of 24-epibrassinolide (EBL) influenced protein content, nitrate reductase activity, ethylene production, polyamines pool, and relative water content and enhanced plant drought tolerance (Arteca and Arteca 2008; Behnamnia and others 2009; Yuan and others 2010; Anjum and others 2011; Talaat and others 2015). However, the mechanism by which EBL induces protein and polyamine accumulation during water stress is not completely clear.
Polyamines are low molecular mass aliphatic amines found in all plant cells and can stabilize the membranes, scavenge free-radical, modulate the activities of certain ion channels, and control many aspects of DNA, RNA, and protein turnover under drought stress (Groppa and Benavides 2008; Alcazar and others 2010). Farooq and others (2009) have argued that among the polyamines, Spm is the most effective in improving drought tolerance. Moreover, Kasukabe and others (2004) proposed that Spm may play dual functions in stress-tolerance phenomena in plants, one as a direct stress-protecting compound and the other as a stress-signaling regulator. It was shown that exogenous application of Spm could alleviate the adverse effect of drought on plant development by altering polyamine levels, reducing ethylene production, enhancing protein content, and inducing organic solutes accumulation (Farooq and others 2009; Yiu and others 2009; Radhakrishnan and Lee 2013; Talaat and others 2015). However, how Spm regulates the content and biosynthesis of protein and polyamines under water deficiency is not fully known.
Among crop plants, maize (Zea mays L.) is one of the most essential food crops and is known to be susceptible to even moderate drought conditions (Ribaut and others 2012). EBL and Spm are actively involved in various physiological processes and are essential for plant growth, development, and stress tolerance. Although the effects of the exogenous application of EBL or Spm on plants have been extensively investigated in the last years, the mechanisms of their actions on ameliorating the deleterious effect generated by drought are still far from being completely understood. To clarify the mechanisms of their actions, the present study was undertaken to investigate the possible role of a single foliar application of EBL or Spm as well as their dual foliar application in improvement of drought tolerance, based upon modulation of polyamine and protein metabolism. The present investigation, as a first approach, hypothesizes that the dual application of EBL and Spm may give an advantage to overcome water stress problems through its positive effect on protein and polyamine pools. To investigate this hypothesis, two maize genotypes were subjected to well-watered conditions and water-stressed conditions (75 and 50 % of field capacity) with and without EBL and/or Spm foliar application. Results were quantified by measuring several physiological parameters, including contents of putrescine, Spd, Spm, nitrate, and protein, activities of arginine decarboxylase, ornithine decarboxylase, S-adenosylmethionine decarboxylase, diamine oxidase, polyamine oxidase, nitrate reductase, protease, and ribulose-1,5-bisphosphate carboxylase, and concentrations of total free amino acids, phenols, and flavonoids, ethylene production, carbonyl content as well as relative water content. The present study was an attempt to explore a clear pattern of drought-induced alteration on the above systems and the ameliorative effects of these foliar applications.
Materials and Methods
Plant Material
Grains of two maize hybrids (Giza 10 and Giza 129) were obtained from the Agriculture Research Center, Ministry of Agriculture, Giza. These two genotypes were selected based on their high yield productivity and we tried to increase their drought tolerance by using EBL and/or Spm foliar application.
Experimental Setup and Treatment Pattern
Two pot experiments were carried out in the greenhouse of the Department of Plant Physiology, Faculty of Agriculture, Cairo University at Giza, Egypt, during the two growing seasons of 2013 and 2014. Each experiment was performed in a completely randomized design with three factors: two maize hybrids (Giza 10 and Giza 129), three soil water conditions (well-watered condition and water deficit conditions at 75 and 50 % of field capacity), and four spraying treatments [0.00 (double-distilled water; DDW), 25 mg l−1 Spm, 0.1 mg l−1 EBL, and 25 mg l−1 Spm + 0.1 mg l−1 EBL]. Each experiment included 24 treatments and each treatment had nine replicates.
Plant Growth Conditions
The soil used was a clay loam (sand 37 %, silt 28 %, clay 35 %) (Inceptisols; FAO), collected from the Faculty of Agriculture, Cairo University Experimental Station, sieved (pore size, 2 mm), and diluted with quartz sand (particle diameter < 1 mm; 2:1, soil to sand, v/v). Before planting, soil chemical analysis was determined according to Cottenie and others (1982) and is presented in Table 1. Fertilization was carried out by adding ammonium nitrate (33.5 % N), calcium superphosphate (15.5 % P2O5), and potassium sulfate (48 % K2O) at the rate of 2.0, 2.0, and 0.5 g pot−1, respectively, before planting, as well as 2.0 g pot−1 ammonium nitrate 30 days after planting. For each plastic pot (30 cm diameter, 35 cm depth, filled with 15 kg of the soil mixture), four grains thinned to two after germination were planted on June 3 in both seasons. All pots were irrigated to soil saturation before planting. After planting, irrigation was applied at the appropriate times with tap water to maintain soil moisture near maximum water-holding capacity for 30 days. Water treatments were carried out 30 days after seeding.
Water Stress and Foliar Spraying Treatments
One month after sowing, the plants from each genotype were exposed to three soil water conditions: control condition (100 % field capacity; WW), water-stressed condition (75 % field capacity; WD1), and water-stressed condition (50 % field capacity; WD2). Soil water contents for treatments 100, 75, and 50 % field capacity were 15.5, 11.6, and 7.7 %, respectively. Soil water content (SWC) was calculated using the formula: SWC % = [(FW − DW)/DW] × 100, where FW was the fresh weight of a portion of the soil from the internal area of each pot and DW was the dry weight of the soil portion after oven drying at 85 °C for 4 days (Coombs and others 1987).
Plants at 60-days old from each water stress treatment were sprayed with 0.00 (double-distilled water; DDW), 0.1 mg l−1 EBL, 25 mg l−1 Spm, and 25 mg l−1 Spm + 0.1 mg l−1 EBL. EBL (C28H48O6, MW = 480.7) was purchased from Sigma (USA) and was dissolved in sufficient quantity of ethanol. Spm (C10H26N4, MW = 202.3) was also purchased from Sigma (USA) and was dissolved in sufficient quantity of autoclaved distilled water. Tween-20 (0.05 %) was added as surfactant at the time of treatment. Preliminary screening was performed for various concentrations of EBL and Spm to obtain the optimum responses, and the concentrations of 0.1 mg l−1 EBL and 25 mg l−1 Spm were selected. The spraying was done when the maize plants had 6–8 leaves fully developed (during pre-female inflorescence emerging stage).
Sample Harvesting
Plant samples were collected after 25 days of EBL and Spm foliar application.
Quantification of Free Polyamines by High-Performance Liquid Chromatography (HPLC)
Free polyamines were extracted with 5 % perchloric acid containing 500 mg l−1 dithiothreitol according to Liu and Moriguchi (2007). After extraction, 200 µl of the solution was used for dansylation based on the method of Minocha and others (1991) with minor modification. At last 20 µl of the methanol-dissolved solution was loaded to an HPLC (Waters, USA) and detected via a fluorescence spectrophotometer with excitation and emission wavelengths of 365 and 510 nm, respectively. Quantification of free PAs was done as the average of the 4 independent replicates for each treatment. Results were compared with polyamine standards (Put, Spd and Spm; Sigma, USA). Polyamine content was expressed as nmol g−1 FW.
Assay of Polyamine Biosynthetic Enzyme (Arginine Decarboxylase, Ornithine Decarboxylase, and S-adenosylmethionine Decarboxylase) Activity
Leaf samples were homogenized in 100 mM K-phosphate buffer (pH 8.0) containing 0.1 mM phenylmethylsulfonyl fluoride, 1 mM pyridoxal phosphate (PLP), 5 mM dithiothreitol (DTT), 5 mM EDTA, 25 mM ascorbic acid, and 0.1 % polyvinylpyrrolidone. The homogenate was centrifuged at 12,000×g for 40 min at 4 °C, and the supernatant was dialyzed at 4 °C against 3 ml of 100 mM K-phosphate buffer (pH 8.0) containing 0.05 mM PLP, 1 mM DTT, and 0.1 mM EDTA for 24 h in darkness. The dialyzed extract was used for enzyme assay. Enzyme activity was determined according to Duan and others (2008). The reaction mixtures contained 100 mM Tris–HCl buffer (pH 8.5), 1 mM PLP, 5 mM DTT, 5 mM EDTA, 40 mM l-arginine, and the dialyzed enzyme extract for arginine decarboxylase (ADC; E.C. 4.1.1.19) determination; 100 mM Tris–HCl buffer (pH 8.0), 1 mM PLP, 5 mM DTT, 5 mM EDTA, 40 mM l-ornithine, and the dialyzed enzyme extract for ornithine decarboxylase (ODC; E.C. 4.1.1.17) determination; and 100 mM K-phosphate buffer (pH 7.5), 1 mM PLP, 5 mM DTT, 5 mM EDTA, 40 mM S-adenosylmethionine, and the dialyzed enzyme extract for S-adenosylmethionine decarboxylase (SAMDC; E.C. 4.1.1.50) determination. Carbon dioxide production from the different substrates was measured by the conventional Warburg technique at 37 °C for 30 min with gentle agitation. Enzyme activity was expressed in µl CO2 g−1 DW min−1.
Assay of Polyamine Catabolic Enzyme (Diamine Oxidase and Polyamine Oxidase) Activity
Leaf samples were homogenized in 100 mM K-phosphate buffer (pH 6.5) containing 5 mM dithiothreitol, and the extract was centrifuged at 16,000×g for 20 min at 4 °C. The supernatant was used for enzyme assay. Diamine oxidase (DAO; E.C. 1.4.3.6) and polyamine oxidase (PAO; E.C. 1.4.3.4) activities were assayed as per Asthir and others (2002) by using Put (for DAO) and Spd (for PAO) as substrates. The reaction mixture of 2.0 ml consisted of 0.1 ml of enzyme extract, 50 units of CAT, 0.1 % o-aminobenzaldehyde, and the reaction started with one of the two different buffer and substrate combinations, i.e., 10 mM Put in 50 mM K-phosphate buffer (pH 7.5) for DAO; 10 mM Spd in 50 mM K-phosphate buffer (pH 6.0) for PAO. The reaction was incubated at 30 °C for 3 h, and then stopped with 2.0 ml of 10 % (v/v) perchloric acid, and the tubes were centrifuged at 6500×g for 15 min. Formation of the Δ-pyrroline product was determined by reading the absorbance at 430 nm in spectrophotometer. Control reactions were carried out with inactivated enzyme prepared by heating for 20 min in a boiling water bath. Enzyme activity was expressed as nmol Δ-pyrroline g−1 FW h−1.
Determination of Carbonyl Content
Oxidative damage to proteins was estimated as the content of carbonyl groups (Reznick and Packer 1994). Leaf segments (0.5 g) were homogenized with 3 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 mM dithiothreitol (DTT), 5 µg ml−1 leupeptin, 5 µg ml−1 aprotinin, and 5 µg ml−1 antipain. The homogenate was centrifuged at 12,000×g for 10 min, and the pellet obtained was allowed to react with 500 µl of 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2 M HCl. These were allowed to stand in the dark at room temperature for 1 h, with vortexing every 10 min. After incubation, proteins were precipitated in 20 % TCA, centrifugation at 12,000×g for 5 min. The protein pellets were washed once more with TCA, and then washed three times with 1 ml portions of ethanol/ethyl acetate (1:1 v/v) to remove any free DNPH. The protein samples were resuspended in 1 ml of 6 M guanidine hydrochloride (dissolved in 20 mM phosphate buffer, pH 2.3) at 37 °C for 15 min with vortex mixing. Carbonyl contents were read at 370 nm (extinction coefficient, 22 mM−1 cm−1) against a blank treated with 2 M HCl instead of DNPH. Protein content was determined according to the method of Bradford (1976) with bovine serum albumin as standard.
Ethylene Analysis
Ethylene production was determined by enclosing 0.5 g plant material in a 3-ml glass tube. After 30 min at 20 °C under natural light conditions, 100 ml air was taken through the septa and shaken with 0.25 mol dm−3 mercuric perchlorate in 2 mol dm−3 PCA for 5 min according to Mol and others (2004). The released ethylene was determined by gas chromatography (Hewlett Packard, 5890) equipped with a flame ionization detector and a column of activated alumina (30 m length × 0.53 mm inner diameter). Column temperature was 60 °C, and injector and detector temperature were 80 and 120 °C, respectively; carrier gas flow rate, N2 at 50 ml min−1.
Assay of Ribulose-1,5-bisphosphate Carboxylase Activity
Extraction procedures followed those of Makino and others (1988). Ribulose-1,5-bisphosphate carboxylase (RuBPC; E.C. 4.1.1.39) was activated for 20 min at 0 °C after preparation of the supernatant in the activation medium that contained 75 mM Hepes–KOH at pH 7.5, 10 mM MgCl2, and 10 mM NaHCO3. RuBPC was assayed at 25 °C in a medium that contained 100 mM bicine at pH 8.2, 5 mM MgCl2, 10 mM NaHCO3, 5 mM creatine phosphate, 1 mM ATP-2 Na, 0.1 mM NADH, 0.3 mM RuBP, 10 units of phosphocreatine kinase, 10 units of glyceraldehydes-3-phosphate dehydrogenase, and 10 units of phosphoglycerate kinase, as described by Sawada and others (1990). The enzymatic activities were corrected for the decrease in absorbance at 340 nm in a control assay medium prepared without ribulose bisphosphate.
Estimation of Nitrate Content
The nitrate content was determined by the method described by Singh (1988). One hundred mg of dried leaf powder was digested with 5 ml of 2 % acetic acid for 20 min and was filtered through Whatman filter paper. The extract was diluted to 10 ml, to which 500 mg of powder mixture, consisting of citric acid, manganese sulfate monohydrate, sulfanilamide, N-1-naphthylethylenediamine dihydrochloride, and powdered zinc, was added. The reaction mixture was centrifuged, and the color of the supernatant was read at 540 nm. Nitrate content was calculated by comparing the value of the sample with the calibration curve, drawn by using standard nitrate solution.
Assay of Nitrate Reductase Activity
The activity of nitrate reductase (NR; E.C. 1.6.6.1) was measured following the method adopted by Jaworski (1971). The fresh leaf samples were cut into small pieces and transferred to plastic vials containing phosphate buffer (pH 7.5) followed by the addition of potassium nitrate and isopropanol solutions. The reaction mixture was incubated at 30 °C for 2 h followed with the addition of N-1- naphthylethylenediamine dihydrochloride and sulfanilamide. The absorbance of the color was read at 540 nm and was compared with the calibration curve. The activity of NR (nmol NO2 g−1 leaf FM h−1) was computed on fresh mass basis.
Assay of Protease Activity
Fresh leaves were homogenized in a medium composed of 50 mM potassium phosphate buffer (pH 7.8). Protease activity (E.C. 3.4.22.44) was determined by the casein digestion assay described by Drapeau (1974). By this method, one unit is that amount of enzyme which releases acid soluble fragments equivalent to 0.001 A280 per minute at 37 °C and pH 7.8.
Estimation of Total Protein Content
Grain protein content was determined by the method of Lowry and others (1951). The samples were homogenized in DDW, and 5 % trichloroacetic acid was added to precipitate the proteins. The precipitate was dissolved in 1 % NaOH solution. Blue color was developed using Folin phenol reagent, and the absorbance was read at 660 nm, using spectrophotometer.
Determination of Total Free Amino Acids
Dried ground leaves (0.5 g) were homogenized in 80 % ethanol for 10 min and centrifuged at 600×g. The supernatant was collected. The extraction procedure was repeated twice to ensure the complete extraction of amino acids. Total free amino acids were determined with the ninhydrin reagent method (Moore and Stein 1954). One ml acetate buffer (pH 5.4) and l ml chromogenic agent were added to 1 ml free amino acids extraction. The mixture was heated in boiling water bath for 15 min. After cooling in tap water, 3 ml ethanol (60 %, v/v) was added. The absorbance at 570 nm was then monitored.
Determination of Relative Water Content (RWC)
RWC was determined in fresh leaf disks of 2 cm2 diameter. Disks were weighed quickly and immediately floated on DDW in Petri dishes to saturate them with water for the next 24 h in dark. The adhering water of the disks was blotted, and turgor mass was noted. Dry mass of the disks was recorded after dehydrating them at 70 °C for 48 h. RWC was calculated (Hayat and others 2007) by placing the values in the following formula:
Determination of Phenolic Compounds and Flavonoids
Total phenolic compounds and total flavonoids were extracted with a mixture of methanol, chloroform, and 1 % (w/v) NaCl (2:2:1). Total phenolic compounds were assayed quantitatively by A765 with Folin–Ciocalteu reagent (Rivero and others 2001). The total flavonoids content was measured by a colorimetric method (Kim and others 2003). Aliquots of phenolic extracts were mixed with 2 ml of double-distilled H2O and 0.15 ml of 5 % (w/v) NaNO2. After 5 min, 0.15 ml of 10 % (w/v) AlCl3·6H2O solution was added; the mixture was allowed to stand for another 5 min, and then, 1 ml of 1 M NaOH was added. The reaction solution was mixed and kept for 15 min, and absorbance was determined at 415 nm. The results of phenols and flavonoids concentration were expressed as mg caffeic acid g−1 DW and mg rutin g−1 DW, respectively.
Statistical Analysis
Data were statistically analyzed on the basis of a completely randomized design, with a three-way factorial arrangement (Snedecor and Cochran 1980). Combined analysis was made for the two growing seasons, because the results of the two seasons followed a similar trend. Data were analyzed by three-way ANOVA for main effects (G, genotype; D, drought level; T, foliar application treatments) and three first-order interactions (G × D, G × T, D × T) and one second-order interaction (G × D × T). All values are means of four replicates. Significant differences were calculated using the least-significant-difference (LSD) test at p < 5 % level.
Results
Effects of genotypes, drought levels, foliar application treatments, and interactions were tested on the contents of Put, Spd, Spm, carbonyl, nitrate, protein, and relative water, activities of ADC, ODC, SAMDC, DAO, PAO, RuBPC, NR, and protease, and concentrations of total free amino acids, total phenols, total flavonoids, and ethylene level. Data were analyzed by three-way ANOVA. All the measured parameters were significantly affected by the genotypes, drought levels, and foliar application treatments, except the Put content. In particular, the effect of the variables’ interactions produced significant changes in the contents of Spd, Spm, activities of ADC, ODC, SAMDC, and concentration of total flavonoids (Table 2).
Water deficiency enhanced Put content in both genotypes; the increment was more significant in hybrid Giza 10. Furthermore, under drought conditions, levels of Spd and Spm were increased in hybrid Giza 129 and decreased in hybrid Giza 10 (Fig. 1). Foliar treatments did not significantly alter the contents of Put, Spd, and Spm under well-watered condition. However, when the exogenous applications were combined with the drought treatments, they modified the PAs pool. Moreover, the accumulation pattern of PAs in water-stressed plants was not consistent in the two genotypes due to these foliar applications. Stressed hybrid Giza 129 plants treated with EBL and/or Spm showed higher levels of Spd and Spm and lower levels of Put as compared to untreated plants. However, stressed hybrid Giza 10 plants showed higher Put and lower Spd and Spm levels than the corresponding untreated ones. The maximum increase was detected in drought-stressed (50 % of field capacity) hybrid Giza 129 plants sprayed with the dual application that had 47.1 and 50.8 % higher levels of Spd and Spm, respectively, over unsprayed ones.
Changes in the endogenous polyamine contents [putrescine (Put), spermidine (Spd), and spermine (Spm)] (nmol g−1 FW) in leaves of two maize genotypes (Giza 129 and Giza 10) when 60-day-old plants were subjected for different foliar application treatments [control (double-distilled water), 25 mg l−1 spermine, 0.1 mg l−1 24-epibrassinolide, and 25 mg l−1 spermine + 0.1 mg l−1 24-epibrassinolide] under different water stress treatments [well-watered (WW), drought stress (WD1; 75 % of field capacity) and drought stress (WD2; 50 % of field capacity)]. Every column in each graph represents the mean (±SE) of four replicates. Asterisks indicate significant differences at the 0.05 level compared with the unsprayed plant
ADC activity was increased in response to drought and/or foliar applications, which was greater in hybrid Giza 10 than in hybrid Giza 129 (Fig. 2). Water stress increased ODC and SAMDC activities in both genotypes; the increment was more significant in hybrid Giza 129 (Fig. 2). Moreover, when water stress treatments were associated with the exogenous treatments, particularly with the dual application, ODC and SAMDC activities were increased significantly (p < 0.05) in both genotypes, but more greatly in hybrid Giza 129. Dual application, the most effective treatment, enhanced the activities of ADC in hybrid Giza 10 leaves by 12.6, 46.1, and 68.0 %, ODC in hybrid Giza 129 leaves by 16.3, 52.7, and 83.3 %, and SAMDC in hybrid Giza 129 leaves by 13.3, 48.6, and 63.3 % compared to values of untreated plants under well-watered and water-stressed (75 and 50 % of field capacity) conditions, respectively.
Changes in the activities of arginine decarboxylase (ADC), ornithine decarboxylase (ODC), and S-adenosylmethionine decarboxylase (SAMDC) (µl CO2 g−1 DW min−1) in leaves of two maize genotypes (Giza 129 and Giza 10) when 60-day-old plants were subjected for different foliar application treatments [control (double-distilled water), 25 mg l−1 spermine, 0.1 mg l−1 24-epibrassinolide, and 25 mg l−1 spermine + 0.1 mg l−1 24-epibrassinolide] under different water stress treatments [well-watered (WW), drought stress (WD1; 75 % of field capacity), and drought stress (WD2; 50 % of field capacity)]. Every column in each graph represents the mean (±SE) of four replicates. Asterisks indicate significant differences at the 0.05 level compared with the unsprayed plant
Drought significantly (p < 0.05) increased DAO and PAO activities, the effect was more pronounced in hybrid Giza 129 (for DAO) and in hybrid Giza 10 (for PAO) (Fig. 3). EBL and/or Spm treatments under water stress conditions considerably decreased their activities, particularly in hybrid Giza 10 (for DAO) and in hybrid Giza 129 (for PAO). Dual application, the most effective treatment, reduced the values of DAO in hybrid Giza 10 leaves by 6.7, 13.3, and 16.0 % and PAO in hybrid Giza 129 leaves by 10.0, 15.7, and 22.1 % under well-watered and water-stressed (75 % and 50 % of field capacity) conditions, respectively, when compared with untreated plants.
Changes in the activities of diamine oxidase (DAO) and polyamine oxidase (PAO) (nmol Δ-pyrroline g−1 FW h−1) in leaves of two maize genotypes (Giza 129 and Giza 10) when 60-day-old plants were subjected for different foliar application treatments [control (double-distilled water), 25 mg l−1 spermine, 0.1 mg l−1 24-epibrassinolide, and 25 mg l−1 spermine + 0.1 mg l−1 24-epibrassinolide] under different water stress treatments [well-watered (WW), drought stress (WD1; 75 % of field capacity) and drought stress (WD2; 50 % of field capacity)]. Every column in each graph represents the mean (±SE) of four replicates. Asterisks indicate significant differences at the 0.05 level compared with the unsprayed plant
Water-stressed plants showed enhanced protein carbonyl formation (Fig. 4), especially in hybrid Giza 10. However, EBL and/or Spm treatments decreased its content under both well-watered and water-stressed conditions, particularly in hybrid Giza 129. The best result was obtained by the dual application treatment, which significantly (p < 0.05) reduced carbonyl content in hybrid Giza 129 leaves by 12.3, 21.7, and 31.8 % compared to values of untreated plants under well-watered and water-stressed (75 % and 50 % of field capacity) conditions, respectively.
Changes in the carbonyl content (nmol mg−1 protein) in leaves of two maize genotypes (Giza 129 and Giza 10) when 60-day-old plants were subjected for different foliar application treatments [control (double-distilled water), 25 mg l−1 spermine, 0.1 mg l−1 24-epibrassinolide, and 25 mg l−1 spermine + 0.1 mg l−1 24-epibrassinolide] under different water stress treatments [well-watered (WW), drought stress (WD1; 75 % of field capacity) and drought stress (WD2; 50 % of field capacity)]. Every column in each graph represents the mean (±SE) of four replicates. Asterisks indicate significant differences at the 0.05 level compared with the unsprayed plant
Water stress significantly (p < 0.05) increased ethylene production, especially in hybrid Giza 10 (Fig. 5). By contrast, the single foliar application of Spm and the dual foliar application of Spm plus EBL significantly (p < 0.05) reduced the drought-induced ethylene production compared with untreated stressed plants, particularly in hybrid Giza 129. Dual application, the most effective treatment, alleviated the adverse effect of water deficit and decreased the ethylene production in hybrid Giza 129 leaves by 7.7, 12.5, and 21.0 % under well-watered and water deficit (75 and 50 % of field capacity) conditions, respectively, when compared with untreated plants.
Changes in the ethylene level (nl g−1 DW) in leaves of two maize genotypes (Giza 129 and Giza 10) when 60-day-old plants were subjected for different foliar application treatments [control (double-distilled water), 25 mg l−1 spermine, 0.1 mg l−1 24-epibrassinolide, and 25 mg l−1 spermine + 0.1 mg l−1 24-epibrassinolide] under different water stress treatments [well-watered (WW), drought stress (WD1; 75 % of field capacity) and drought stress (WD2; 50 % of field capacity)]. Every column in each graph represents the mean (±SE) of four replicates. Asterisks indicate significant differences at the 0.05 level compared with the unsprayed plant
Drought significantly (p < 0.05) decreased RuBPC activity, especially in hybrid Giza 10 (Fig. 6). Nevertheless, treated stressed plants with foliar applications, particularly with the dual application, overcame the toxic effect generated by water stress and significantly (p < 0.05) enhanced the RuBPC activity in hybrid Giza 129 leaves by 28.6, 54.6, and 70.6 % compared to values of untreated plants under well-watered and water deficit (75 and 50 % of field capacity) conditions, respectively.
Changes in the activity of ribulose-1,5-bisphosphate carboxylase (RuBPC) (µmol CO2 mg−1 protein min−1) in leaves of two maize genotypes (Giza 129 and Giza 10) when 60-day-old plants were subjected for different foliar application treatments [control (double-distilled water), 25 mg l−1 spermine, 0.1 mg l−1 24-epibrassinolide, and 25 mg l−1 spermine + 0.1 mg l−1 24-epibrassinolide] under different water stress treatments [well-watered (WW), drought stress (WD1; 75 % of field capacity), and drought stress (WD2; 50 % of field capacity)]. Every column in each graph represents the mean (±SE) of four replicates. Asterisks indicate significant differences at the 0.05 level compared with the unsprayed plant
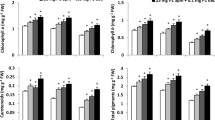
Values of nitrate content, NR activity, and grains total protein content were significantly (p < 0.05) decreased in water-stressed plants, particularly in hybrid Giza 10 (Fig. 7). However, EBL and/or Spm treatments overcame the drought injuries. Hybrid Giza 129 exhibited higher values of these parameters over hybrid Giza 10, especially in dual application-treated plants. Leaf nitrate content was significantly (p < 0.05) improved in hybrid Giza 129 treated with the dual application by 15.0, 22.9, and 39.3 %, while leaf NR activity improved by 19.8, 30.0, and 42.6 % and grains total protein content improved by 14.5, 31.4, and 50.2 % under well-watered and water deficit (75 and 50 % of field capacity) conditions, respectively, when compared with untreated plants. On the other hand, plants subjected to drought conditions exhibited a significant increase in the protease activity, particularly in hybrid Giza 10 (Fig. 7). EBL and/or Spm foliar applications under well-watered and water-stressed conditions modified its values. When these treatments were associated with water stress, low activity of protease was observed, particularly in hybrid Giza 129 plants treated with the dual application. Dual application reduced protease activity in hybrid Giza 129 leaves by 9.8, 21.5, and 29.6 % compared to values of untreated plants under well-watered and water-stressed (75 and 50 % of field capacity) conditions, respectively.
Changes in the nitrate content (nmol g−1 leaf DW), nitrate reductase (NR) activity (nmol NO2 g−1 leaf FM h−1), protease activity (Units g−1 leaf FW), and grains total protein content (mg g−1 DW) in two maize genotypes (Giza 129 and Giza 10) when 60-day-old plants were subjected for different foliar application treatments [control (double-distilled water), 25 mg l−1 spermine, 0.1 mg l−1 24-epibrassinolide, and 25 mg l−1 spermine + 0.1 mg l−1 24-epibrassinolide] under different water stress treatments [well-watered (WW), drought stress (WD1; 75 % of field capacity), and drought stress (WD2; 50 % of field capacity)]. Every column in each graph represents the mean (±SE) of four replicates. Asterisks indicate significant differences at the 0.05 level compared with the unsprayed plant
Concentrations of total free amino acids were increased under drought conditions, and this increase was more significant in water-stressed plants treated by EBL and/or Spm, particularly in hybrid Giza 129 (Fig. 8). Dual application gave the best results and significantly (p < 0.05) increased total free amino acid concentrations in hybrid Giza 129 leaves by 16.0, 25.8, and 37.5 % under well-watered and water deficit (75 and 50 % of field capacity) conditions, respectively, when compared with untreated plants.
Changes in the concentrations of total free amino acids (mg g−1 DW) in leaves of two maize genotypes (Giza 129 and Giza 10) when 60-day-old plants were subjected for different foliar application treatments [control (double-distilled water), 25 mg l−1 spermine, 0.1 mg l−1 24-epibrassinolide, and 25 mg l−1 spermine + 0.1 mg l−1 24-epibrassinolide] under different water stress treatments [well-watered (WW), drought stress (WD1; 75 % of field capacity), and drought stress (WD2; 50 % of field capacity)]. Every column in each graph represents the mean (±SE) of four replicates. Asterisks indicate significant differences at the 0.05 level compared with the unsprayed plant
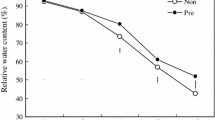
Leaf RWC of the water-stressed plants showed significantly (p < 0.05) lower values than in non-stressed ones, particularly in hybrid Giza 10 and that was 25.4 and 39.7 % in response to drought stress conditions at 75 and 50 % of field capacity, respectively (Fig. 9). However, the dual application significantly (p < 0.05) overcame the damage caused by water stress and enhanced its value in hybrid Giza 129 leaves by 29.1 and 36.2 % under drought stress conditions at 75 and 50 % of field capacity, respectively, when compared with untreated plants.
Changes in the relative water content (%) in leaves of two maize genotypes (Giza 129 and Giza 10) when 60-day-old plants were subjected for different foliar application treatments [control (double-distilled water), 25 mg l−1 spermine, 0.1 mg l−1 24-epibrassinolide, and 25 mg l−1 spermine + 0.1 mg l−1 24-epibrassinolide] under different water stress treatments [well-watered (WW), drought stress (WD1; 75 % of field capacity), and drought stress (WD2; 50 % of field capacity)]. Every column in each graph represents the mean (±SE) of four replicates. Asterisks indicate significant differences at the 0.05 level compared with the unsprayed plant
The accumulation of phenolic and flavonoid compounds has also been detected in response to drought and/or exogenous treatments. Both genotypes accumulated phenols and flavonoids in response to water stress conditions and/or foliar applications; however, hybrid Giza 129 showed the higher content of these compounds under the different conditions tested (Fig. 10). Dual application, the most effective treatment, enhanced phenolic compounds in hybrid Giza 129 plants by 15.1, 26.4, and 50.4 % and enhanced flavonoids by 12.5, 27.3, and 58.0 % compared to values of untreated plants under well-watered and water-stressed (75 and 50 % of field capacity) conditions, respectively.
Changes in the concentrations of total phenols (mg caffeic acid g−1 DW) and total flavonoids (mg rutin g−1 DW) in leaves of two maize genotypes (Giza 129 and Giza 10) when 60-day-old plants were subjected for different foliar application treatments [control (double-distilled water), 25 mg l−1 spermine, 0.1 mg l−1 24-epibrassinolide, and 25 mg l−1 spermine + 0.1 mg l−1 24-epibrassinolide] under different water stress treatments [well-watered (WW), drought stress (WD1; 75 % of field capacity), and drought stress (WD2; 50 % of field capacity)]. Every column in each graph represents the mean (±SE) of four replicates. Asterisks indicate significant differences at the 0.05 level compared with the unsprayed plant
Discussion
Maize, being third most important crop worldwide, is highly sensitive to drought stress (Ribaut and others 2012). Brassinosteroids and polyamines have been reported to counteract water stress in several plants (Behnamnia and others 2009; Farooq and others 2009; Yuan and others 2010; Anjum and others 2011; Radhakrishnan and Lee 2013). Understanding the possible ameliorative action of EBL and/or Spm in water stress mitigation is of great importance. Our previous study has shown that foliar application of EBL and/or Spm significantly improved the plant drought tolerance and decreased the accumulation of ROS by enhancing their scavenging through elevation of antioxidant enzyme activity and improving the redox state of ascorbate and glutathione (Talaat and others 2015). In this study, we investigated whether exogenous EBL and/or Spm had other roles in the nitrogen metabolism and how these hormones could influence polyamine and protein pools. This article exemplifies a new area of research which has the potential for exciting and important developments. To the best of our knowledge, this is the first study to investigate the effect of the dual application of EBL and Spm on the polyamine and protein metabolism in plants subjected to drought conditions.
Results of this study clearly demonstrated that drought conditions impaired nitrogen metabolism mainly by altering levels and forms of endogenous polyamines (Figs. 1, 2, and 3; Table 2), increasing carbonyl content (Fig. 4), enhancing ethylene production (Fig. 5), and disrupting protein synthesis (Fig. 7; Table 2). Drought stress induces high levels of ROS in plant cells, which can disrupt normal metabolism through membrane lipid peroxidation, DNA/RNA nicking, protein oxidation, enzyme inhibition, and eventually cell death (Mittler 2002). However, exogenous application of EBL and/or Spm protected plants against the detrimental effect of salinity and significantly improved the nitrogen pool by their involvement in the modification of PAs pool (Figs. 1, 2, and 3), the inhibition of ethylene synthesis (Fig. 5), the protection of protein synthesis (Figs. 4 and 7), and the increasing of leaf relative water content (Fig. 9). Furthermore, some protective substances such as free amino acids, phenols, and flavonoids (Figs. 8 and 10) also participated in enhancing the drought tolerance capacity in these plants.
Water deficiency alters the endogenous PAs concentration (Liu and others 2007; Groppa and Benavides 2008). As shown in Fig. 1, the leaf PA (Put, Spm, Spd) profile was correlated with tissue water stress level and varied markedly depending on plant genotype. The rise in PAs, particularly Spd and Spm, was detected in water-stressed hybrid Giza 129 plants. Liu and others (2007), and Zhang and Huang (2013) suggested a special role for Spd and Spm under water deficit conditions. The synthesis of Spm and Spd was more effective in the tolerant genotype. Data in Fig. 1 also revealed that EBL and/or Spm foliar applications under drought conditions caused excessive accumulation of Put, with a decrease in Spd and Spm content in hybrid Giza 10 plants; whereas in hybrid Giza 129, the same treatment under the same stress condition induced a significant increase in Spd and Spm content and a decrease in Put level, suggesting that the endogenous level of PAs may be a limiting factor for drought tolerance. In hybrid Giza 129 plants, exogenous applications reduced the accumulation of Put induced by drought conditions, and promoted the conversion of Put into Spd and Spm. Obviously, foliar treatments could protect plants against drought damage by altering PA levels and forms.
In view of the above results, differential accumulation of PAs during water stress has been reported and seems to have potential for counteracting drought injury. In parallel, changes in the activities of PA biosynthetic and catabolic enzymes were also observed (Figs. 1, 2, and 3), which regulated the level of PA in plant tissues. In this respect, Liu and others (2007) stated that the ability of plants to control stress can be correlated to their ability to synthesize PAs. Data in Figs. 1, 2, and 3 revealed that drought conditions caused an increase in the free fraction of Put in hybrid Giza 10 plants, mainly due to an increase in ADC activity, together with an apparent increase in PAO activity. Further analysis of PA catabolic and biosynthetic enzyme activity in hybrid Giza 129 indicated that ODC, SAMDC, and DAO enzymatic activity increased significantly in correlation with Spd and Spm accumulation. Regulation of PA biosynthesis and catabolism in response to abiotic stresses has been also reported by Duan and others (2008), Groppa and Benavides (2008), Talaat and Shawky (2012, 2013b), and Zhang and Huang (2013). Alcazar and others (2010) found that overexpression of ADC2 enhanced Arabidopsis drought tolerance through increase in Put level; however, overexpression of SAMDC increased Spm level.
Furthermore, changes in the content of free Put, Spd, and Spm as well as in the activity of PA biosynthetic enzymes were observed when water-stressed plants were treated with EBL and/or Spm. In treated hybrid Giza 10 plants, a significant increase in free Put content and in ADC activity was observed under drought conditions. However, the response to these foliar treatments in stressed hybrid Giza 129 plants involved a significant increase in the levels of free Spd and Spm, which correlated with stimulation of the activities of ODC and SAMDC. It seems that exogenous applications improved plant adaptation to water stress by their role on the activity of PA biosynthetic enzymes. It is interesting to underline that treated plants have evolved various defense mechanisms to cope with the potential damage of drought. These could include decreasing ROS content by increasing the levels of endogenous PAs.
Protective effects of EBL and/or Spm were further manifested in the form of reduced oxidative damage to proteins. Leaf protein carbonylation, a marker of oxidative stress, was increased when plants were subjected to drought conditions (Fig. 4). However, the exogenous applications were able to rescue maize from drought-induced carbonyl accumulation (Fig. 4), and thus prevented protein oxidation. This finding could reflect changes in antioxidant capacity and/or increased degradation of carbonylated proteins in stressed treated plants. Because enhanced carbonyl content signifies damage caused by ROS, the resulting decrease in carbonyl level after foliar applications can be regarded as evidence for EBL and/or Spm-induced efficient scavenging of ROS. From previous results, it appears that the inhibition in carbonyl content (Fig. 4) together with a concomitant increase in PAs pool (Fig. 1) in stressed treated plants proved the efficiency of PAs to prepare the cell to meet and combat stress by serving as scavengers of free radicals and as membrane surface stabilizers through interaction with the negatively charged groups of the membrane (Groppa and Benavides 2008; Alcazar and others 2010). Hence, in treated plants, maintenance of elevated levels of free PAs as well as low content of carbonyl could be associated with drought tolerance and contribute to prevent oxidative injury in these plants.
Further, ethylene production, observed as a consequence of drought treatments, was significantly prevented in maize leaves sprayed with Spm or with EBL plus Spm (Fig. 5). This result confirms those already reported by Huang and others (2014). It is interesting to underline that a negative correlation between total PA content and ethylene levels was detected in these plants, which suggests that the increase in PA concentration inhibited ethylene synthesis. In this concern, Garnica and others (2009) showed that PAs inhibited the accumulation of transcripts of 1-aminocyclopropane-1-carboxylic acid (precursor of ethylene) synthase. On the other hand, data in the same figure also demonstrated that the single foliar application of EBL stimulated the production of ethylene in maize leaves. In line with these observations, Arteca and Arteca’s (2008) data pointed to a positive BR effect on the ethylene biosynthesis.
Results in Fig. 6 pointed out that the RuBPC activity was decreased by increasing water stress levels. Drought stress also affected PA levels and ethylene production (Figs. 1 and 5). It seems likely that drought inhibits RuBPC activity by altering the PA pool and increasing ethylene release. In this respect, Huang and others (2014) reported that PA metabolism may be involved in the change in RuBPC activity, which was confirmed by the fact that RuBPC activity varies with the levels of endogenous PAs. Deviating from the response generated by water stress, the application of EBL and/or Spm had a favorable impact on RuBPC activity, even if given as a follow-up treatment with drought stress (Fig. 6). This result is concordant with Zhang and others (2008) and Huang and others (2014). Foliar applications may increase RuBPC activity by changing the PA levels and decreasing the damage by ethylene release (Figs. 1 and 5). It appears that the change in the endogenous PA level induced by the exogenous applications leads to a change in the RuBPC activity, which supports the function of PAs. Indeed, PAs are involved in regulating the physical and chemical properties of membranes and the functions and modulation of enzyme activity (Alcazar and others 2010). Spd and Spm, but not Put accumulation, may confer stress tolerance by interacting with membranes either by inhibiting the transbilayer movement of phospholipids or by stabilizing the molecular complexes of thylakoid membranes (Bouchereau and others 1999).
Exposure of maize plants to drought conditions induced a significant decrease in NR activity, along with lower nitrate uptake (Fig. 7). This result is concordant with Fresneau and others (2007) and Casadebaig and others (2008). Moreover, water stress decreased grain protein content (Fig. 7), which could be due to (a) enhanced activity of proteases (Fig. 7; Hameed and others 2011) (b) increased degradation of oxidized proteins (Xiong and others 2007), and/or (c) altered stability of protein (Zhu 2001). In contrast to negative effects of water stress, the application of EBL and/or Spm alone or as a follow-up treatment to the water-stressed plants elevated NR activity as well as nitrate content (Fig. 7). This improvement in the activity of NR could be an expression of the impact of BRs on the (a) translation and/or transcription of NR (Bajguz 2000) and/or (b) uptake of nitrate by acting at the level of the membrane (Mai and others 1989). It seems possible that the reason behind this enhancement is that BRs maintained the fluidity of the plasma membrane that was altered by water injury and improved the uptake of nitrate, an inducer of NR activity.
Involvement of BRs and PAs in drought response is evident also from the data in Fig. 7, which illustrated that the exogenous treatments significantly relieved the drought-induced reduction in grain protein content, which is possibly the result of the well-documented effect of BRs on transcription and/or translation thereby changing the pattern of total proteins (Bajguz 2000). Previous studies confirmed the stimulating role of BRs and PAs (applied alone) in the enhancement of protein content (Behnamnia and others 2009; Anjum and others 2011; Radhakrishnan and Lee 2013). Moreover, decreased protein content in stressed plants was accompanied by enhancing levels of protein carbonylation (Fig. 4). Foliar applications completely reversed this trend by reducing the formation of protein carbonyls and increasing the protein content in water-stressed plants implying that cellular protein is being protected from ROS-mediated oxidative damage. In addition, the increased PA pool (Fig. 1) probably plays an important role in the stabilization of membrane integrity and protein structure. PAs have a function to protect against stress via their stabilizing protein structure and preventing the proteins from degradation by conjugation to proteins (Groppa and Benavides 2008). Spm and Spd may play an important role in the post-transcriptional modifications of proteins and could stabilize the conformation and function of proteins (Huang and others 2014).
One of the most commonly induced adaptive responses of plants to drought is the accumulation of organic solutes. In this investigation, enhanced total free amino acid concentrations in stressed plants treated with EBL and/or Spm were observed (Fig. 8). Increased total free amino acid concentrations may regulate osmotic adjustment, protect cellular macromolecules, store nitrogen, and maintain cellular pH (Szabados and Savoure 2010; Talaat and Shawky 2014). Additionally, data in Fig. 9 clearly demonstrated that drought stress significantly reduced the relative water content. However, foliar treatments alleviated the drought-induced RWC reduction. This result corroborates the findings of Farooq and others (2009), Yiu and others (2009), Yuan and others (2010), Anjum and others (2011), and Radhakrishnan and Lee (2013). The ability of exogenous applications to maintain high RWC in stressed plants might be attributed to their contribution to osmotic adjustment by increasing the internal total free amino acid concentration (Fig. 8).
As complementary mechanisms to cope with ROS accumulation in response to drought conditions, plant cells use a wide array of non-enzymatic antioxidants such as flavonoids and phenolic compounds (Mittler 2002). Results in Fig. 10 showed that stressed plants accumulated phenols and flavonoids; however, their concentrations were higher when stressed plants were treated with EBL and/or Spm, suggesting a limited contribution of these antioxidant compounds in the selection of maize water stress resistance. In this concern, Krishna (2003) pointed out that the increase in stress tolerance in BR-treated potato tubers is associated with the accumulation of phenolic and terpenoid compounds.
Taken as a whole, findings in this approach reveal that exogenous applications to water-stressed plants significantly modified the PA concentration by altering the activities of both PA biosynthetic and degradative enzymes (Figs. 1, 2, 3), prevented protein oxidation by suppressing the accumulation of carbonyls (Fig. 4), decreased damage by ethylene release (Fig. 5), elevated protein content by enhancing nitrate uptake and NR activity as well as by inhibiting protease activity (Fig. 7), enhanced osmotic adjustment by osmoprotector solutes accumulation (Fig. 8), reversed the detrimental effects of water deficit on RWC (Fig. 9), and combated oxidative stress by increasing non-enzymatic antioxidant (flavonoids and phenolic compounds) concentrations (Fig. 10). Obviously, EBL and/or Spm applications have been found as potent water stress alleviators. Moreover, this positive effect of the foliar treatments was more significant in hybrid Giza 129 than in hybrid Giza 10, confirming that the former genotype is more drought-tolerant than the latter. Indeed, the genotype hybrid Giza 129 seems to be relatively more tolerant than the other one at least partly due to its higher levels of nitrogenous compounds (Spm, Spd, and free amino acids) and protein content. Furthermore, results of this study concerning the interaction between EBL and Spm indicate that exogenous EBL cooperated efficiently with exogenous Spm altering the contents of analyzed biochemical parameters in plants subjected to drought conditions. The highest stimulation was observed for the mixture of EBL with Spm, whereas the lowest was in the plants treated with Spm alone. Obtained results confirm the role of this dual application in masking the effect of water stress and its possible role toward stress amelioration. As a result, it is hoped that advances in this area of exogenous application of plant growth regulators under stressful conditions could bring us one step closer to meeting the demands for increased food production to feed the growing world population.
Conclusion
Elucidating the endogenous mechanisms that confer stress resistance is essential to providing insights into the potential of plants to adapt to environmental change. In sum, the present study illustrates that the dual application of EBL and Spm regulated stress response as a result of a complex sequence of biochemical reactions such as modifying the PA pool by altering PA biosynthetic and degradative enzyme activity, elevating protein content, production of free amino acids, phenolic, and flavonoids compounds as chemical defense compounds, as well as suppression of ethylene and carbonyl production. These observations provide strong evidence that the objective of avoiding drought damage can be achieved more easily and efficiently by using the combined application of EBL and Spm, which are non-toxic products and environmentally friendly substances. To our knowledge, this is the first report showing the effect of the dual application of EBL and Spm on PAs pool, protein level, and water stress amelioration.
References
Alcazar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio A (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou CM (2011) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci 197:177–185
Arteca RN, Arteca JM (2008) Effects of brassinosteroids, auxin, and cytokinin on ethylene production in Arabidopsis thaliana plants. J Exp Bot 59:3019–3026
Asthir B, Duffus CM, Smith RC, Spoor W (2002) Diamine oxidase is involved in H2O2 production in the chalazal cells during barley grain filling. J Exp Bot 53:677–682
Bajguz A (2000) Effect of brassinosteroids on nucleic acid and protein content in cultured cell of Chlorella vulgaris. Plant Physiol Biochem 38:209–215
Bartoli CG, Gomez F, Martinez DE, Guiamet JJ (2004) Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot 55:1663–1669
Behnamnia M, Kalantari KM, Ziaie J (2009) The effects of brassinosteroid on the induction of biochemical changes in Lycopersicon esculentum under drought stress. Turk J Bot 33:417–428
Bouchereau A, Aziz A, Larher F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 7:248–254
Casadebaig P, Debaeke P, Lecoeur J (2008) Thresholds for leaf expansion and transpiration response to soil water deficit in a rang of sunflower genotypes. Eur J Agron 28:646–654
Coombs J, Hall DO, Long SP, Scurlock JMO (1987) Techniques in bioproductivity and photosynthesis. Pergamon, Oxford
Cottenie A, Verloo M, Kiekens L, Velghe G, Camerlynck R (1982) Chemical analysis of plants and soils. Laboratory of analytical and agrochemistry. State University, Ghent, pp 14–24
Drapeau G (1974) Protease from Staphylococcus aureus. In: Lorand L (ed) Method of enzymology, vol 45b. Academic Press, New York
Duan J, Li J, Guo S, Kang Y (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165:1620–1635
Farooq M, Wahid A, Lee DJ (2009) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945
Fresneau C, Ghashghaie J, Cornic G (2007) Drought effect on nitrate reductase and sucrose-phosphate synthase activities in wheat (Triticum durum L.): role of leaf internal CO2. J Exp Bot 58:2983–2992
Garnica M, Houdusse F, Yvin JC, Garcia-Mina JM (2009) Nitrate supply induced changes in polyamine content and ethylene production in wheat plants grown with ammonium. J Plant Physiol 166:363–374
Groppa MD, Benavides MP (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
Gupta K, Dey A, Gupta B (2013) Plant polyamines in abiotic stress responses. Acta Physiol Plant 35:2015–2036
Hameed A, Bibi N, Akhter J, Iqbal N (2011) Differential changes in antioxidants, proteases, and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol Biochem 49:178–185
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41
Huang X, Zhou G, Yang W, Wang A, Hu Z, Lin C, Chen X (2014) Drought-inhibited ribulose-1,5-bisphosphate carboxylase activity is mediated through increased release of ethylene and changes in the ratio of polyamines in pakchoi. J Plant Physiol 171:1392–1400
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochem Biol Res Commun 43:1274–1279
Kasukabe Y, He L, Nada K, Misawa S, Iharu I, Tachibana S (2004) Overexpression of spermidine synthase enhances tolerance to multiple environmental stress and upregulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722
Kim D, Jeong SW, Lee CY (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 81:321–326
Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22:289–297
Kubis’ J (2008) Exogenous spermidine differently alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J Plant Physiol 165:397–406
Liu JH, Moriguchi T (2007) Changes in free polyamine titers and expression of polyamine biosynthetic genes during growth of peach in vitro callus. Plant Cell Rep 26:125–131
Liu JH, Kitashiba H, Wang J, Ban Y, Moriguchi T (2007) Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotech 24:117–126
Lowry OH, Rosenbrough NJ, Aarr AL, Randaal RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Mai YY, Lin JM, Zeng XL, Pan RJ (1989) Effect of homobrassinolide on the activity of nitrate reductase in rice seedlings. Plant Physiol Commun 2:50–52
Makino A, Mae T, Ohira K (1988) Differences between wheat and rice in the enzymic properties of ribulose-1,5-bisphosphate carboxylase/oxygenase and the relationship to photosynthetic gas exchange. Planta 174:30–38
Minocha SC, Papa NS, Khan AJ, Samuelsen AI (1991) Polyamines and somatic embryogenesis in carrot. III. Effects of methylglyoxal bis (guanylhydrazone). Plant Cell Physiol 32:302–395
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mol R, Filek M, Machackova I, Matthys-Rochon E (2004) Ethylene synthesis and auxin augmentation in pistil tissues are important for egg cell differentiation after pollination in maize. Plant Cell Physiol 45:1396–1405
Moore S, Stein WH (1954) A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem 211:907–913
Radhakrishnan R, Lee I (2013) Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic Acid, and jasmonic acid signals in soybean. J Plant Growth Regul 32:22–30
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl-assay. Methods Enzymol 233:357–363
Ribaut JM, Betran J, Monneveux P, Setter T (2012) Drought tolerance in maize. In: Bennetzen JL, Hake SC (eds) Handbook of maize: its biology. Springer, New York, pp 311–344
Rivero RM, Ruiz JM, García PC, López-Lefebre LR, Sánchez E, Romero L (2001) Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci 160:315–321
Sawada S, Usuda H, Hasegawa Y, Tsukui T (1990) Regulation of rubisco activity in response to changes of the source/sink balance in single-rooted soybean leaves. Plant Cell Physiol 31:697–704
Singh JP (1988) A rapid method for determination of nitrate in soil and plant extracts. Plant Soil 110:137–139
Snedecor GW, Cochran WG (1980) Statistical Methods, 7th edn. Iowa State University Press, Ames
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Talaat NB, Shawky BT (2012) 24-Epibrassinolide ameliorates the saline stress and improves the productivity of wheat (Triticum aestivum L.). Environ Exp Bot 82:80–88
Talaat NB, Shawky BT (2013a) 24-Epibrassinolide alleviates salt-induced inhibition of productivity by increasing nutrients and compatible solutes accumulation and enhancing antioxidant system in wheat (Triticum aestivum L.). Acta Physiol Plant 35:729–740
Talaat NB, Shawky BT (2013b) Modulation of nutrient acquisition and polyamine pool in salt-stressed wheat (Triticum aestivum L.) plants inoculated with arbuscular mycorrhizal fungi. Acta Physiol Plant 35:2601–2610
Talaat NB, Shawky BT (2014) Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot 98:20–31
Talaat NB, Shawky BT, Ibrahim AS (2015) Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ Exp Bot 113:47–58
Xiong Y, Contento AL, Nguyen PQ, Bassham DC (2007) Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol 143:291–299
Yiu J, Liu C, Fang DY, Lai Y (2009) Waterlogging tolerance of Welsh onion (Allium fistulosum L.) enhanced by exogenous spermidine and spermine. Plant Physiol Biochem 47:710–716
Yuan G, Jia C, Li Z, Sun B, Zhang L, Liu N, Wang Q (2010) Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci Hortic 126:103–108
Zhang C, Huang Z (2013) Effects of endogenous abscisic acid, jasmonic acid, polyamines, and polyamine oxidase activity in tomato seedlings under drought stress. Sci Hortic 159:172–177
Zhang M, Zhai Z, Tian X, Duan L, Li Z (2008) Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). Plant Growth Regul 56:257–264
Zhou Q, Yu B (2010) Changes in content of free, conjugated and bound polyamines and osmotic adjustment in adaptation of vetiver grass to water deficit. Plant Physiol Biochem 48:417–425
Zhu JK (2001) Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol 4:401–406
Acknowledgments
This research was supported by the Academy of Scientific Research and Technology in Egypt and the Bulgaria-Egypt Joint Research Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talaat, N.B., Shawky, B.T. Dual Application of 24-Epibrassinolide and Spermine Confers Drought Stress Tolerance in Maize (Zea mays L.) by Modulating Polyamine and Protein Metabolism. J Plant Growth Regul 35, 518–533 (2016). https://doi.org/10.1007/s00344-015-9557-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-015-9557-y