Abstract
Elicitor-induced natural defense machinery of plants could be proposed as an alternative, non-conventional, and ecologically-friendly approach for plant protection. In this work, the abiotic elicitor, calcium chloride (CaCl2), was used for inducing resistance in tea plants against blister blight disease caused by Exobasidium vexans. Foliar application of elicitor resulted in around 80 % disease inhibition over the control set, during the peak time of blister blight incidence at the experimental garden of Darjeeling Tea Research and Development Centre. A significant increase in the activities of defense enzymes like phenylalanine ammonia lyase (PAL), peroxidase, polyphenol oxidase, and β-1,3-glucanase along with higher accumulation of total phenolics was observed. Treated plants also had elevated transcript levels of thaumatin, catalase, PAL, cinnamate 4-hydroxylase, and flavonoid 3′-hydroxylase genes compared to control plants. Further, the treatment-induced nitric oxide (NO) production was confirmed by real-time visualization of the NO burst using a fluorescent probe and spectrophotometric analysis. The result suggested that CaCl2 induced an array of plant defense responses making this compound a potential phytosanitary product with a challenging issue and a rather attractive option for sustainable organic tea cultivation practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pathogen attacks are responsible for losses in agricultural yield, and the use of toxic chemicals remains the general means of control. Extensive use of chemical phytosanitary products has become a major environmental concern over the last few decades. In this scenario, significant research efforts have been expended for the identification and development of newer and safer compounds, which are capable of triggering plant immune responses (Goupil and others 2012). These compounds (elicitors) are molecules that are capable of mimicking the perception of a pathogen by a plant, thereby triggering induction of a sophisticated defense response in plants. Plants are equipped with an array of defense mechanisms to protect themselves against attack by herbivorous insects and microbial pathogen. These inducers include avirulent pathogens (Hammerschmidt 1999), plant growth promoting rhizobacteria (Vivekananthan and others 2004; Acharya and others 2011a), and biotic and abiotic elicitors (Michael and others 2001; Acharya and others 2011b). Elicitation of plants with elicitor molecules results in the activation of a series of defense responses, including cell wall reinforcement by deposition of lignin and induction of an array of defense enzymes (Desender and others 2007). The corresponding plant defense responses following treatment include an oxidative burst leading to cell death, changes in cell wall composition, synthesis of antimicrobial compounds such as phytoalexins, activation of defense genes, and priming of host cells (Kuć 2006). So, these non-chemical disease control strategies are gaining importance in conventional agricultural practices diminishing negative side effects on both the environment and human health (Walling 2001; Ajay and Baby 2010; Harm and others 2011).
Induced resistance in plants can be achieved with applications of various elicitors like salicylic acid (SA) and its related compounds, such as benzo (1,2,3) thiadiazole-7- carbothioic acid S-methyl ester, 2,6-dichloro-isonicotinic acid, and dl-3-amino-n-butyric acid (Hong and others 1999; Walters and others 2005); jasmonic acid (JA) and its related compounds like methyl jasmonate (Moreno and others 2010; Yang and others 2011); benzothiadiazole (BTH) (Anttonen and others 2003; Lin and others 2011); chitosan and its derivatives (Dos Santos and others 2012; Yan and others 2012); arachidonic acid, cupric chloride (CuCl2), copper sulfate (CuSO4), isonicotinic acid, and oxalic acid (Coquoz and others 1995; Aziz and others 2006; Tian and others 2006; Acharya and others 2011b). The induction of resistance was achieved by the over expression of different defense gene products including PO, PPO, β-1,3-glucanase, and PAL (Maxson-Stein and others 2002; Pal and others 2011), along with several other enzymes and by higher accumulation of total phenolic content (Anand and others 2009; Acharya and others 2011a, b).
Tian and others (2006) used calcium chloride (CaCl2) as an elicitor for the post-harvest treatment of pear fruits and they found the induction of an increased level of defense enzyme activity. Calcium plays a fundamental role in plant growth and development. Many extracellular signals and environmental cues including hormones, light, biotic and abiotic stress factors, and elicit change in cellular calcium levels, termed as calcium signatures (Reddy 2001; Rudd and Franklin-Tong 2001; White and Broadley 2003). Plant cells also utilize a Ca2+ signal as a pivotal early signaling event in response to pathogen perception. In plant cells, the calcium ion is a ubiquitous intracellular second messenger involved in numerous signaling pathways. Cytosolic Ca2+ elevation is an important event in pathogen signaling that triggers plant innate immune responses (Dangl and others 1996).
Tea, the oldest known beverage, yields many health benefits to humans is made from the tender leaves of the tea plant (Camellia sinensis). This important horticulture crop has a key role in the economy in terms of export earnings of the country. Like other crops, tea plants also suffer from various biotic and abiotic stresses. Among the different pathogens that effect tea production, the fungal disease, blister blight, caused by Exobasidium vexans is by far the most serious disease of tea in Asia (Arulpragasam 1992; Sowndhararajan and others 2012). For the past five decades or so, several toxic chemicals have been used continuously as a general means for controlling the blister blight disease (Saravanakumara and others 2007). Around 24–28 rounds of sprays are required to keep this disease under control (Ajay and Baby 2010). The main aims of this study were (i) to evaluate the ability of calcium chloride to induce resistance at the field level against blister blight disease in tea; (ii) to determine its relation with defense enzyme activity and polyphenol content; and also (iii) to provide possible defense mechanism involved.
Materials and Methods
Plant Material
All the experiments were conducted with healthy C. sinensis (L) Kuntze plants (cultiver AV-2) raised in the experimental tea garden of Darjeeling Tea Research and Development Centre (DTR&DC), Tea Board of India, Kurseong.
Treatment
Healthy 25-year-old bushes of tea, cultiver AV-2, were treated with calcium chloride (1 % foliar application) at an interval of 15 days from May to October. Similar bushes treated with sterile distilled water were served as controls. The experiment was conducted for two seasons. There were three replications with each replicate consisting of 50 bushes. The experiment was laid out at the same elevation.
Field Trial and Percent Disease Index (PDI)
The incidence of disease in tender leaves was recorded at 30-day intervals for a period of 5 months (May–September). Three samples of fifty shoots of the same age (two leaves and a bud) and of uniform size were collected from individual plots, and they were assessed for the presence of blister spots. A shoot was considered as infected if an active blister lesion of any developmental stage was present. Disease was scored by following the method of Saravanakumara and others (2007) on a six-point scale (where 0 = no disease, 1 = 1 % leaf area affected, 3 = 2–10 % leaf area affected, 5 = 11–25 % leaf area affected, 7 = 26–50 % leaf area affected, 9 ≥ 50 % leaf area affected), and the PDI was calculated using the following formula:
Assay of the Defense-Related Enzymes
Enzyme Extraction
Tender leaves from treated and control bushes were plucked for assay during the peak time of blister blight severity at Darjeeling (late July to end of August) 24 h after the last treatment cycle. The leaf tissues collected from control and treated sets were homogenized with liquid nitrogen. Five hundred mg of powdered sample was extracted with 2 ml of different buffers containing 0.1 % polyvinylpyrrolidone and 20 µl of 1 mM phenylmethane sulphonyl fluoride to assay different enzymes: 0.1 M sodium acetate buffer (pH 5.0) for β-1,3 glucanase; 0.1 M sodium phosphate buffer (pH 7.0) for POD and PPO; and 0.1 M borate buffer (pH 8.7)for PAL. All the extraction procedures were conducted at 4 °C. The homogenate was centrifuged at 10,500×g for 20 min at 4 °C. The supernatants were used as the crude enzyme source for the enzymatic assay. Then, it was transferred to a 2-ml centrifuge tube and stored at −80 °C for further use.
Peroxidase (PO) Assay
Peroxidase activity was determined spectrophotometrically, as described (Hammerschmidt and others 1982), with some modifications. The reaction mixture consisted of 1.5 ml of pyrogallol, 0.05 ml of enzyme extract, and 0.5 ml of 1 % hydrogen peroxide in a total volume of 2.5 ml. The change in absorbance at 420 nm was recorded at each 30 s interval for 3 min. The enzyme activity was expressed as changes of absorbance of reaction mixture (∆ OD change) min−1 g−1 protein.
Polyphenol Oxidase (PPO) Assay
PPO activity was estimated as previously described (Mayer and others 1965). Two hundred μl of 0.01 M catechol was added to the reaction mixture containing 200 μl of enzyme extract and 1.5 ml of 0.1 M sodium phosphate buffer (pH 6.5). Enzyme activity was expressed as change in absorbance at 495 nm (∆ OD change) min−1 g−1 protein.
Phenylalanine Ammonia Lyase (PAL) Assay
PAL was assayed following the method of Dickerson and others (1984) determining the conversion of l-phenylalanine to transcinnamic acid spectrophotometrically at 290 nm. Enzyme extract (0.4 ml) was incubated with 0.5 ml of 0.1 M borate buffer (pH 8) and 12 mM l-phenylalanine in the same buffer for 30 min at 30 °C. Enzyme activity was expressed as synthesis of transcinnamic acid (n mol) min−1 g−1 protein.
β-1,3 Glucanase assay
β-1,3 glucanase activity was estimated according to the method of Pan and others (1991) with slight modifications. Crude enzyme extract of 50 µl was added to 50 µl of 1 % laminarin and incubated at room temperature for 30 min. The reaction was stopped by adding 300 µl of dinitrosalicylic acid and heated for 10 min in a boiling water bath. The resulting colored solution was diluted with distilled water, and the absorbance was recorded at 520 nm. The enzyme activity was expressed as µmol glucose equivalent produced min−1 g−1 protein.
Total Protein Estimation
The standard Bradford assay (1976) was employed, using bovine serum albumin as a standard, to test the protein concentration of each extract.
Analysis of Defense-Related Gene Expression by Semi-quantitative RT-PCR
Expression of the genes was analyzed by semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extracted with TRIzol Reagent (Invitrogen, USA) from the control and treated sets of tea leaves. The cDNA was synthesized from the total RNA using RevertAid M-MuLV Reverse Transcriptase (Fermentas, USA) according to the manufacturer protocol. Twenty µl reaction volume contained 1 µg of RNA, 0.5 µg of Oligo(dT), 20 units of RiboLock RNase Inhibitor (Fermentas, USA), 4 µl of 5× reaction buffer (250 mM Tris–HCl (pH 8.3), 250 mM KCl, 20 mM MgCl2, 50 mM DTT), 2 µl of 10 mM each deoxynucleoside triphosphates (dNTP Mix; Fermentas, USA), and 200 units of RevertAid M-MuLV Reverse Transcriptase. The reaction was carried out at 45 °C for 60 min followed by 70 °C for 10 min. To analyze the expression of a specific gene, 1 µl of the cDNA was taken in a 50 µl PCR mixture containing 1× DreamTaq PCR buffer, 0.2 mM of each dNTPs, 1 µM of each gene specific primer, and 1.25 units of DreamTaq DNA polymerase (Fermentas, USA). Thaumatin, catalase (CAT), PAL, cinnamate 4-hydroxylase (C4H), and flavonoid 3′-hydroxylase (F3H) genes were amplified individually (Table 1). Actin gene primers were used as internal controls for expression studies (Table 1). Linearity between the amount of input RNA and the final RT-PCR products was verified and confirmed. After standardizing the optimal amplification at the exponential phase, PCR cycles were carried out under the following conditions: 94 °C for 4 min, then 30 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 60 s with a final extension step of 7 min at 72 °C in a thermal cycler (Applied BioSystem, USA). The PCR products were electrophoresed in agarose gel, stained with ethidium bromide, visualized in UV transilluminator, and then photographed.
Estimation of Total Phenol Content
Leaf samples (1 g) from the control and CaCl2-treated sets were homogenized in 10 ml of 80 % methanol and agitated for 15 min at 70 °C (Zieslin and Ben Zaken 1993). One ml of the methanolic extract was added to 5 ml of distilled water and 0.25 ml of 1 N Folin–Ciocalteau reagent, and the solution was kept at 25 °C. Phenolic content was measured spectrophotometrically at 725 nm using gallic acid as a standard. The amount of phenolics was expressed as μg gallic acid g−1 fresh weight.
Quantification of Phenolic Compounds by HPLC
Samples prepared from tea leaves for phenolic estimation were analyzed with an HPLC system (Agilant, USA) equipped with an Agilent DAAD detector and an Agilent Eclipse plus C18 column (100 mm × 4.6 mm, 3.5 μm). The mobile phases were (A) acetonitrile and (B) 0.1 % phosphoric acid. The linear gradient conditions were as follows: 0–5 min, 10 % A in B; 5–15 min, 10–20 % A in B; 15–25 min, 20-90 % A in B with a flow rate of 0.8 ml min−1; and 20 μl of injection volume. UV–Vis absorption spectra were recorded on-line from 190 to 600 nm during the HPLC analysis (Yao and others 2004). Samples were injected three times into the sample loop, and the mean of the peak areas of individual compounds was taken for quantification. Solutions of each standard, at various concentration levels, were injected into the HPLC system. The peak areas and thus the calibration curves and response factors were recorded under the same conditions as for the samples. Gallic acid (GA), caffeine, epicatechin (EC), epigallocatechin (EGC), epigallocatechin gallate (EGCG), epicatechin gallate (ECG), gallocatechin gallate (GCG), and catechin gallate (CG) (M.P. Biomedicals. USA) were used as standards. The DAAD detection was conducted at 278 nm for the quantification (Seeram and others 2006). Concentrations were calculated by comparing peak areas of reference compounds with those in the samples run under the same elution conditions.
Nitric Oxide Estimation
Production of NO was estimated by hemoglobin assay (Delledonne and others 2001) during the peak time of blister blight severity period 24 h after the treatment cycle. Leaf tissues of control and treated sets were incubated in a reaction mixture containing 10 mM l-arginine, 10 mM hemoglobin, in a total volume of 5 ml of 0.1 M phosphate buffer (pH 7.4). Production of NO was measured spectrophotometrically at 401 nm, and NO levels were calculated using an extinction coefficient of 38,600 M−1 cm−1(Salter and Knowles 1998). After 2 h of incubation, NO content in the reaction mixture was measured as nmol of NO produced mg−1 fresh weight h−1 and compared with the water control.
Real-time NO production was visualized using membrane permieant fluorochrome 4-5-diaminofluorescein diacetate (DAF-2DA) dye (Bartha and others 2005). The lower epidermis of the control and treated sets of leaves was peeled off and placed in a brown bottle containing 1 ml of loading buffer (10 mM KCl, 10 mM Tris HCl, pH 7.2) with DAF-2DA at a final concentration of 10 μM for 20 min in dark. Fluorescence was observed with a Leica DMLS microscope at an excitation wavelength of 480 nm and emission wavelength of 500–600 nm.
Statistical Analysis
Data from two consecutive seasons were analyzed by student’s t test using Microsoft® Office Excel (Microsoft®, USA), and in all the cases, results are mean ± SD (standard deviation) of at least three individual experimental data, each in triplicate. Values of P < 0.05 were considered statistically significant. The relation between changes in enzyme activity, NO level, and corresponding PDI of the treated set has been evaluated by correlation analysis using Microsoft® Office Excel (Microsoft®, USA).
Results
Under field conditions, calcium chloride-treated C. sinensis plants (cultivar AV-2) recorded a significantly lower incidence of disease than the untreated plants. Blister blight incidences were observed at an interval of 30 days from May to September (150 days) after the first spray, and PDI was calculated accordingly. During the peak time of blister blight incidence at Darjeeling tea garden in the month of July, disease incidence of blister blight was significantly reduced about 80 % when compared to untreated controls (Fig. 1).
Effects of foliar application of elicitors on blister blight disease index. Field photograph of tea plants (cultivar AV-2): a control set; b CaCl2-treated set; and c graphical presentation of disease index. Arrows indicate presence of symptoms on tender leaves. Results are mean ± SD of three separate experiments, each in triplicate
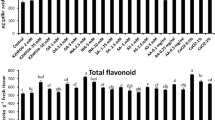
Simultaneously, defense-related enzyme activity and polyphenol accumulation levels were also compared between the CaCl2 treated and the untreated control plants. Elicitor treatment showed higher induction of PO, PPO, PAL, and β-1,3 glucanase level along with higher polyphenol accumulation. The induction of the PO enzyme and β-1,3 glucanase enzyme was significantly higher, and about 61 % increase in enzyme accumulation was observed in tea plants treated with CaCl2 over the control set (Table 2). Likewise, higher production of PPO activity was noted in tea plants, followed by induction of PAL enzyme activity (Table 2). Correlation analysis (Table 3) between the defense enzymes and corresponding PDI is an indication for an inverse relationship. Accumulation of phenols in tea plants sprayed with CaCl2 was 7.5 % higher compared to untreated controls (Table 2).
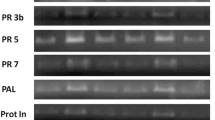
To elucidate the effect of CaCl2 treatment on defense-related genes, expression in the transcript level was determined by semi-quantitative RT-PCR analysis. Figure 2 reveals that differential alteration of defense-related genes like PAL, thaumatin, and CAT genes occurred on treatment. Expression of the two phenylpropanoid biosynthetic pathway genes C4H and F3H increased markedly indicating a higher accumulation of flavan-3-ol compounds (Table 4).
HPLC analysis showed interesting results in the total phenolic extracts from control and elicitor-treated plants. Phenolic compounds present in the samples were identified by comparing both retention times and UV–Vis spectra with those of pure standards (Fig. 3). GA, EC, EGC, and EGCG were produced in significantly higher amounts in the elicitor-treated plants, whereas caffeine and CG content were higher in the control plant sets. No significant change in ECG content was observed between the control and treated plants. GCG content was only detected in the elicitor-treated set of plants (Table 4).
Furthermore, an attempt has been made to evaluate the status of NO during the same period in the leaves of treated and untreated tea plants (Fig. 4). CaCl2 treatment stimulated NO production about 2.35 fold over the controls (Fig. 4). To gain a precise view of NO production during the interaction between the elicitor and tea plants, a NO-specific fluorophore DAF-2DA on leaf peals, which converts fluorescent triazol derivative upon reaction with NO, showed increased fluoresce under a fluorescent microscope (Leica DMLS) in the elicitor-treated set (Fig. 4).
Calcium chloride treatment and nitric oxide status in C. sinensis leaves. Real-time determination of NO in leaf epidermal cells by DAF-2DA staining. NO generation was detected by green fluorescence. a Control; b CaCl2-treated set; c spectrophotometric analysis of NO production in control and treated plants of C. sinensis. Results are mean ± SD of three separate experiments, each in triplicate
Discussion
Protecting plants with the application of elicitor are so far a better alternative to the wide application of pesticides in crop protection (Sticher and others 1997). To defend themselves against attack from various pathogens present in their surrounding environment, plants are equipped both with pre-formed, constitutive chemical, and mechanical barriers as well as with inducible defense systems (Montesano and others 2003), which need appropriate stimuli or signals to activate them. Elicitors are usually capable of triggering various modes of the plant defense system resulting in the activation of a series of defense responses including cell wall reinforcement by deposition of lignin, an oxidative burst leading to a hypersensitive response, synthesis of antimicrobial compounds, and activation of defense genes. In this study, the role of defense-related gene products such as PO, PPO, PAL, β-1,3 glucanase, CAT, thaumatin, C4H, F3H, and phenolics during the CaCl2-mediated elicitation of resistance in tea plants against blister blight disease was evaluated.
This study demonstrated that CaCl2 treatment might have caused the significant reduction in disease incidence in the treated set compared to the water-treated controls. The analysis of defense-related enzymes revealed a higher accumulation of PO, PPO, PAL, and β-1,3-glucanases in the CaCl2-treated sets. This observation supports the findings of Tian and others (2006) and Acharya and others (2011b), whereas pear fruits and Raphanus leaves showed higher induction of PO, PPO, PAL, and β-1,3-glucanase enzymes upon respective treatment with several elicitors. Ajay and Baby (2010) showed that foliar application of salicylic acid (SA) and acibenzolar-S-methyl benzo-(1,2,3)-thiadiazole-7-carboxylic acid S-methyl ester (ASM) in tea plants induced higher PO, PAL, and β-1,3-glucanases activity over the untreated control and reduced disease incidence significantly. Similarly, higher accumulation of all the four defense enzymes along with reduced disease severity was observed when carrot plants were treated with different elicitors to fight against the pathogen Alternaria radicinia (Jayaraj and others 2009).
Furthermore, transcript analysis showed over expression of thaumatin, catalase, PAL, C4H, and F3H genes, which strengthens our earlier observations. Elicitor treatment enhancement of transcript levels of various plants was observed by several researchers. Over expression of thaumatin genes from rice has been demonstrated to reduce infection of rice by Rhizoctonia solani (Grover and Gowthaman 2003), of wheat by Fusarium graminearum (Chen and others 1999), of tobacco by Alternaria alternata (Velazhahan and Muthukrishnan 2003), and of carrot by Alternaria dauci, Alternaria petroselini, A. radicina, Botrytis cinerea, R. solani, and Sclerotinia sclerotiorum (Punja 2005). Increased CAT activity might have reduced the harmful effect of H2O2 accumulation. Our observations also coincide with the findings of Tasgin and others (2006) and Pal and others (2011), whereas CAT activity in wheat plants and rice plants was found to be increased due to treatment with salicylic acid and Cymbopogan citrus leaf extract respectively. PAL activity could be induced by elicitor treatment (Ajay and Baby 2010). Suspension culture of Oryza sativa cv BL-1 treated with the elicitor N-acetylchitooligosaccharide showed over expression of several defense-related genes including PAL (Yamaguchi and others 2005). Catalysis of PAL is the first step of the phenylpropanoid biosynthesis pathway, and the expression of C4H and F3H genes indicates a higher accumulation of catechin compounds. Catechin compounds are reported to act as growth and defense inducing agents (Prithiviraj and others 2007). F3H catalyzes one of the key steps of the flavonoid biosynthesis pathway yielding a large family of flavonoid compounds which are mainly involved in various biological activities (Khlestkina and others 2011). A direct relationship between the F3H expression level and disease resistance in different plants like chickpea (Cho and others 2005), avocado fruits (Ardi and others 1998), and wheat (Giovanini and others 2006) against different pathogens has already been reported earlier.
Phenolics are involved in phytolaxin accumulation, biosynthesis of lignin, and the formation of structural barriers, and play a major role in resistance against pathogens. Greater accumulation of phenolics due to elicitor-treated plants reduces pathogen attack and makes the plants more resistant to pathogen attack. The role of phenolic substances in disease resistance (Nicholson and Hammerschmidt 1992) and their accumulation by the phenyl propanoid pathway due to various elicitor treatments have already been documented earlier (Sánchez-Estrada and others 2009; Dong and others 2010; El Modafar and others 2012). Previously, Jayaraj and others (2009) and Acharya and others (2011b) showed that carrot and R. sativus leaves, respectively, upon treatment with several elicitors, accumulated higher amount of phenolics over controls. In the present study, tea plants when sprayed with CaCl2 induced higher accumulation of polyphenols. HPLC analysis of phenolic compounds revealed higher accumulation of GA, caffeine, several catechhins (GCG and CG), and epicatechins (EC, EGC, EGCG). It has also been previously reported that flavonoids are the most abundant chemical group in tea leaves and among them, flavan-3-ol compounds contribute almost 20–25 % of the dry weight of the leaves (Punyasiri and others 2004). These compounds are important for plants to adapt to various environmental conditions and also involved in the resistance to pathogens by acting as feeding deterrents (Gould and Lister 2006). The resistance of apple cultivars (Treutter and Feucht 1990) to Venturia inaequalis and avocado (Prusky and others 1996; Prusky 1996) to anthracnose has been attributed to high levels of EC, suggesting that induced epicatechin may be directly or indirectly involved in the resistance mechanism of tea against blister blight. The catechins and epicatechins are converted to proanthocyanidins via lucoanthocyanidin reductase (Tanner and others 2003) and the anthocyanidin (Xie and others 2003) reductase pathways, respectively. These proanthocyanidins are widely distributed plant defense compounds (Treutter and Feucht 1999) and have a general toxicity toward fungi, yeast, and bacteria (Scalbert 1991). In the present investigation, the enhanced accumulation of catechins and epicatechins might have induced the accumulation of proanthocyanidins, which in turn could have resulted in the observed defense enhancement. Thus, the high level of flavonoid accumulation in tea plants might be an indication of enhanced resistance against blister blight. From this observation, it can also be inferred that CaCl2 treatment might reduce the need for pesticide treatments.
Ca2+ is a well-established important intracellular messenger in plant defense signaling, which is relayed by the calcium sensor that quickly converts the signal to second messengers like NO and cyclic nucleotides. Furthermore, several recent reports have painted a picture of the signaling role of NO, which is associated with numerous physiological roles (Baudouin 2011) including defense responses in plants (Acharya and others 2005; Acharya and Acharya 2007; Hong and others 2008; Acharya and others 2011a, b; Gupta and others 2013). Lum and others (2002) showed that Ca2+ is required for H2O2-induced NO production in guard cells of mung bean. Several scientists reported that in plants Ca2+ or Ca2+- bound CaM might directly interact with the plant NOS-like enzyme (Delledonne and others 1998; Modolo and others 2005; del Rίo and others 2004). It has also been reported that H2O2 acts together with NO during programmed cell death (Zago and others 2006). Thus, NO appears as an early signaling component, possibly orchestrating a number of downstream signaling pathways (Perchepied and others 2010). In this study, CaCl2-treated tea plants showed greater NO production than the control set. Table 3 indicates that the production of NO might have a relationship with defense enzyme accumulation and PDI. This result signifies that higher accumulation of NO might have played a role in the up-regulation of defense enzyme activity, which in turn could have resulted in the reduced disease incidence. Recently, Gupta and others (2013) have also indicated the relationship between the elevated level of defense enzymes and phenols with the higher production of NO in the toxin-treated callus tissue. They also showed that co-treatment of A. alternata toxin with an NOS inhibitor (L-NAME) or NO scavenger (cPTIO) reduced the increased NO production to basal levels in R. serpentina callus showing a strong unidirectional correlation with plant defense regulators. A similar observation was recorded by Zhao and others (2007), whereas co-treatment of tobacco cells with cPTIO and oligochitosan did not increase PAL activity.
Taken together, our results demonstrate that the elicitor CaCl2 has a greater induction effect on the production of defense gene products and molecules in tea plants. This elicitor capacity of CaCl2 appeared to be associated with the higher induction of NO and defense molecules, which might have induced protection against the blister blight causing pathogen E. vexans. The increased level of NO in the treated plants suggests that NO might be the signal molecule for the stimulatory effect on induced resistance in higher accumulation of defense-related gene products and total phenolics.
Conclusion
In conclusion, the defense chemicals induced upon treatment with CaCl2 have reduced disease incidence, and thereby simultaneously increased yield would have been recorded. Our results also suggest that NO acts as a key signaling molecule in elicitor-mediated transduction cascades. NO might also be associated with the higher accumulation of defense enzymes and total phenolics. Thus, the use of elicitors can be an invaluable tool for the stimulation of defense mechanisms in plants and may open a new horizon in eco-friendly disease control and sustainable organic agriculture practices.
References
Acharya R, Acharya K (2007) Evaluation of nitric oxide synthase status during disease progression in resistant and susceptible varieties of Sesamum indicum against Macrophomina phaseolina. Asian Australas J Plant Sci Biotechnol 1:40–44
Acharya R, Mukhia M, Sen S, Acharya K (2005) Nitric oxide: a common antipathogenic factor of plants. Indian J Exp Biol 43:100–103
Acharya K, Chakraborty N, Dutta AK, Sarkar S, Acharya R (2011a) Signaling role of nitric oxide in the induction of plant defense by exogenous application of abiotic inducers. Arch Phytopathol Plant Prot 44:1501–1511
Acharya K, Chandra S, Chakraborty N, Acharya R (2011b) Nitric oxide functions as a signal in induced systemic resistance. Arch Phytopathol Plant Prot 44:1335–1342
Ajay D, Baby UI (2010) Induction of systemic resistance to Exobasidium vexans in tea through SAR elicitors. Phytoparasitica 38:53–60
Anand T, Chandrasekaran A, Raguchander T, Prakasam V, Samiyappan R (2009) Chemical and biological treatments for enhancing resistance in chilli against Colletotrichum capsici and Leveillula taurica. Arch Phytopathol Plant Prot 42:533–551
Anttonen M, Hukkanen A, Tiilikkala K, Karjalainen R (2003) Benzothiadiazole induces defense responses in berry crops. Acta Hortic 567:177–182
Ardi R, Kobiler I, Jacoby B, Keen NT, Prusky D (1998) Involvement of epicatechin biosynthesis in the activation of the mechanism of resistance of avocado fruits to Colletotrichum gloeosporioides. Physiol Mol Plant Pathol 53:269–285
Arulpragasam PV (1992) Disease control in Asia. In: Wilson KC, Clifford MN (eds) Tea cultivation to consumption. Chapman & Hall, London, pp 353–374
Aziz A, Trotel-Aziz P, Dhuicq L, Jeandet P, Couderchet M, Vernet G (2006) Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 96:1188–1194
Bartha B, Kolbert Z, Erdei L (2005) Nitric oxide production induced by heavy metals in Brassica juncea L. Czern. and Pisum sativum L. Acta Biol Szeged 49:9–12
Baudouin E (2011) The language of nitric oxide signalling. Plant Biol 13:233–242
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen WP, Chen PD, Liu DJ, Kynast R, Friebe B, Velazhahan R, Muthukrishnan S, Gill BS (1999) Development of wheat scab symptoms is delayed in transgenic wheat plants that constitutively express a rice thaumatin-like protein gene. Theor Appl Genet 99:755–760
Cho S, Chen W, Muehlbauer FJ (2005) Constitutive experssion of the flavanone 3-hydroxylase gene related to pathotype-specific ascochyta blight resistance in Cicer arietinum L. Physiol Mol Plant Pathol 67:100–107
Coquoz JL, Buchala AJ, Meuwly Ph, Métraux JP (1995) Arachidonic acid induces local but not systemic synthesis of salicylic acid and confers systemic resistance in potato plants to Phytophthora infestans and Alternaria solani. Phytopathol 85:1219–1224
Dangl JL, Dietrich RA, Richberg MH (1996) Death don’t have no mercy: cell death programs in plant–microbe interactions. Plant Cell 8:1793–1807
del Rίo LA, Corpas FJ, Barroso JB (2004) Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry 65:783–792
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394:585–588
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98:13454–13459
Desender S, Andrivon D, Val F (2007) Activation of defence reactions in Solanaceae: where is the specificity? Cell Microbiol 9:21–30
Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL (1984) Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Plant Pathol 25:111–123
Dong J, Wan G, Liang Z (2010) Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol 148:99–104
Dos Santos NST, Athayde Aguiar AJA, de Oliveira CEV, Verissimo de Sales C, de Melo E, Silva S, Sousa da Silva R, Stamford TCM, de Souza EL (2012) Efficacy of the application of a coating composed of chitosan and Origanum vulgare L. essential oil to control Rhizopus stolonifer and Aspergillus niger in grapes (Vitis labrusca L.). Food Microbiol 32:345–353
El Modafar C, Elgadda M, El Boutachfaiti R, Abouraicha E, Zehhar N, Petit E, El Alaoui-Talibi Z, Courtois B, Courtois J (2012) Induction of natural defense accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci Hort 138:55–63
Giovanini MP, Puthoff DP, Nemacheck JA, Mittapalli O, Saltzmann KD, Ohm HW, Shukle RH, Williams CE (2006) Gene-for-gene defense of wheat against the Hessian fly lacks a classical oxidative burst. Mol Plant-Microbe Interact 19:1023–1033
Gould KS, Lister C (2006) Flavonoid functions in plants. In: Andersen ØM, Markham KR (eds) Flavonoids: chemistry, biochemistry and applications. CRC Press, Boca Raton, pp 397–411
Goupil P, Benouaret R, Charrier O, ter Halle A, Richard C, Eyheraguibel B, Thiery D, Ledoigt G (2012) Grape marc extract acts as elicitor of plant defence responses. Ecotoxicology 21:1541–1549
Grover A, Gowthaman R (2003) Strategies for development of fungus-resistant transgenic plants. Curr Sci 84:330–340
Gupta NS, Banerjee M, Basu SK, Acharya K (2013) Involvement of nitric oxide signal in Alternaria alternata toxin induced defense response in Rauvolfia serpentina Benth. ex Kurz calli. Plant Omics J 6:157–164
Hammerschmidt R (1999) Induced disease resistance: how do induced plants stop pathogens? Physiol Mol Plant Pathol 55:77–84
Hammerschmidt R, Nuckels EM, Kuć J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber in Colletotrichum lagenarium. Physiol Plant Pathol 20:73–82
Harm A, Kassemeyer H–H, Seibicke T, Regner F (2011) Evaluation of chemical and natural resistance inducers against downy mildew (Plasmopara viticola) in grapevine. Am J Enol Vitic 62:184–192
Hong JK, Hwang BK, Kim CH (1999) Induction of local and systemic resistance to Colletotrichum coccodes in pepper plants by dl-β-amino-n-butyric acid. J Phytopathol 147:193–198
Hong JK, Yun BW, Kang JG, Raja MU, Kwo E, Sorheagn K, Chu C, Wang Y, Loake GJ (2008) Nitric oxide function and signaling in plant resistance. J Exp Bot 59:147–154
Jayaraj J, Rahman M, Wan A, Punja ZK (2009) Enhanced resistance to foliar fungal pathogens in carrot by application of elicitors. Ann Appl Biol 155:71–80
Khlestkina EK, Salina EA, Matthies IE, Leonova IN, Börner A, Röder MS (2011) Comparative molecular marker-based genetic mapping of flavanone 3-hydroxylase genes in wheat, rye and barley. Euphytica 179:333–341
Kuć J (2006) What’s old and what’s new in concepts of induced systemic resistance in plants and its application. In: Tuzun S, Bent E (eds) Multigenic and induced systemic resistance in plants, vol 20. Springer, New York, pp 9–20
Lin JH, Gong DQ, Zhu SJ, Zhang LJ, Zhang LB (2011) Expression of PPO and POD genes and contents of polyphenolic compounds in harvested mango fruits in relation to benzothiadiazole-induced defense against anthracnose. Sci Hortic 130:85–89
Lum HK, Butt YK, Lo SC (2002) Hydrogen peroxide induces a rapid production of nitric oxide in mung bean (Phaseolus aureus). Nitric Oxide 6:205–213
Maxson-Stein K, He SY, Hammerschmidt R, Jones AS (2002) Effect of treating apple trees with acibenzolar-S-methyl on fire blight and expression of pathogenesis-related protein genes. Plant Dis 8:785–790
Mayer AM, Harel E, Shaul RB (1965) Assay of catechol oxidase, a critical comparison of methods. Phytochemistry 5:783–789
Michael O, Walter K, Bob D, Theodor S (2001) Induced disease resistance in plants by chemicals. Eur J Plant Pathol 107:19–28
Modolo LV, Augusto O, Almeida IM, Magalhaes JR, Salgado I (2005) Nitrite as the major source of nitric oxide production by Arabidopsis thaliana in response to Pseudomonas syringae. FEBS Lett 579:3814–3820
Montesano M, Brader G, Palva ET (2003) Pathogen derived elicitors: searching for receptors in plants. Mol Plant Pathol 4:73–79
Moreno FD, Blanch GP, del Castillo MLR (2010) (+)-Methyl jasmonate-induced bioformation of myricetin, quercetin and kaempferol in red raspberries. J Agric Food Chem 58:11639–11644
Nicholson RL, Hammerschmidt R (1992) Phenolic-compounds and their role in disease resistance. Annu Rev Phytopathol 30:369–389
Pal TK, Bhattacharya S, Chakraborty K (2011) Induction of systemic resistance in rice by leaf extract of Cymbopogan citrus and Ocimum sanctum against seath blight disease. Arch Appl Sci Res 3:392–400
Pan S, Ye XS, Kuć J (1991) Association of β-1,3-glucanase activity and isoform pattern with systemic resistance to blue mold in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiol Mol Plant Pathol 39:25–39
Perchepied L, Balague C, Riou C, Claudel-Renard C, Riviere N, Grezes-Besset B, Roby D (2010) Nitric oxide participates in the complex interplay of defense-related signaling pathways controlling disease resistance to Sclerotinia sclerotiorum in Arabidopsis thaliana. Mol Plant-Microbe Interact 23:846–860
Prithiviraj B, Perry LG, Badri DV, Vivanco JM (2007) Chemical facilitation and induced pathogen resistance mediated by a root-secreted phytotoxin. New Phytol 173:852–860
Prusky D (1996) Pathogen quiescence in post harvest diseases. Annu Rev Phytopathol 34:413–434
Prusky D, Hamadan H, Ardi R, Keen NT (1996) Induction of biosynthesis of epicatechin in avocado suspension cells treated with an enriched CO2 atmosphere. Physiol Mol Plant Pathol 48:171–178
Punja ZK (2005) Transgenic carrots expressing a thaumatin-like protein display enhanced resistance to several fungal pathogens. Can J Plant Pathol 27:291–296
Punyasiri PAN, Tanner GJ, Abeysinghe ISB, Kumar V, Campbell PM, Pradeepa NHL (2004) Exobasidium vexans infection of Camellia sinensis increased 2,3-cis isomerisation and gallate esterification of proanthocyanidins. Phytochemistry 65:2987–2994
Reddy ASN (2001) Calcium: silver bullet in signalling. Plant Sci 160:381–404
Rudd JJ, Franklin-Tong VE (2001) Unravelling response-specificity in Ca2+ signalling pathways in plant cells. New Phytol 151:733–749
Salter M, Knowles GR (1998) Assay of NOS activity by the measurement of conversion of oxyhemoglobin to methemoglobin by NO. In: Titheradge MA (ed) Nitric oxide protocols. Humana Press, Totowa, pp 61–65
Sánchez-Estrada A, Tiznado-Hernández ME, Ojeda-Contreras AJ, Valenzuela-Quintanar AI, Troncoso-Rojas R (2009) Induction of enzymes and phenolic compounds related to the natural defence response of netted melon fruit by a bio-elicitor. J Phytopathol 157:24–32
Saravanakumara D, Vijayakumar C, Kumar N, Samiyappan R (2007) PGPR-induced defense responses in the tea plant against blister blight disease. Crop Prot 26:556–565
Scalbert A (1991) Antimicrobial properties of tannins. Phytochemistry 30:3875–3883
Seeram NP, Henning SM, Niu Y, Lee R, Scheuller HS, Heber D (2006) Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J Agric Food Chem 54:1599–1603
Singh K, Rani A, Kumar S, Sood P, Mahajan M, Yadav SK, Singh B, Ahuja PS (2008) An early gene of the flavonoid pathway, flavanone 3-hydroxylase, exhibits a positive relationship with the concentration of catechins in tea (Camellia sinensis). Tree Physiol 28:1349–1356
Singh K, Kumar S, Rani A, Gulati A, Ahuja PS (2009) Phenylalanine ammonia-lyase (PAL) and cinnamate 4-hydroxylase (C4H) and catechins (flavan-3-ols) accumulation in tea. Funct Integr Genomics 9:125–134
Sowndhararajan K, Marimuthu S, Manianl S (2012) Biocontrol potential of phylloplane bacterium Ochrobactrum anthropi BMO-111 against blister blight disease of tea. J Appl Microbiol 114:209–218
Sticher L, Mauch-Mani B, Metraux JP (1997) Systemic acquired resistance. Ann Rev Phytopathol 35:235–270
Tanner GJ, Franki KT, Abrahams S, Watson JM, Larkin PJ, Ashton AR (2003) Purification, properties and cloning of leucoanthocyanidin NADPH reductase from Desmodium uncinatum. J Biol Chem 278:31647–31656
Taşgin E, Atici O, Nalbantoğlu B, Popova LP (2006) Effects of salicylic acid and cold treatments on protein levels and on the activities of antioxidant enzymes in the apoplast of winter wheat leaves. Phytochemistry 67:710–715
Tian SP, Qin GZ, Xu Y (2006) Induction of defense responses against Alternaria rot by different elicitors in harvested pear fruit. Appl Microbiol Biotechnol 70:729–734
Treutter D, Feucht W (1990) The pattern of flavan-3-ols in relation to scab resistance of apple cultivars. J Hortic Sci 65:511–517
Treutter D, Feucht W (1999) The role of flavan-3-ols and proanthocyanidin in plant defense. In: Dakshini KMM, Foy CL (eds) Principles and practices of plant ecology. CRC Press, Boca Raton, pp 307–338
Velazhahan R, Muthukrishnan S (2003/2004) Transgenic tobacco plants constitutively overexpressing a rice thaumatin-like protein (PR-5) show enhanced resistance to Alternaria alternata. Biol Plant 47:347–54
Vivekananthan R, Ravi M, Ramanathan A, Samiyappan R (2004) Lytic enzymes induced by Pseudomonas fluorescens and other biocontrol organisms mediate defence against the anthracnose pathogen in mango. World J Microbiol Biotechnol 20:235–244
Walling LL (2001) Induced resistance: from the basic to the applied. Trends Plant Sci 6:445–447
Walters D, Walsh D, Newton A, Lyon G (2005) Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathol 95:1368–1373
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot (London) 92:487–511
Xie DY, Sharma SB, Pavia NL, Ferreira D, Dixon R (2003) Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 299:396–399
Yamaguchi T, Ueki J, Minami E, Shibuya N (2005) Elicitor-induced activation of phospholipases plays an important role for the induction of defense responses in suspension-cultured rice cells. Plant Cell Physiol 46:579–587
Yan JQ, Cao JK, Jiang WB, Zhao YM (2012) Effects of preharvest oligochitosan sprays on portharvest fungal disease, storage quality, and defense responses in jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit. Sci Hortic 142:196–204
Yang SY, Chen YL, Feng LY, Yang E, Su XG, Jiang YM (2011) Effect of Methyl jasmonate on pericarp browning of postharvest lychees. J Food Process Preserv 35:417–422
Yao L, Jiang Y, Datta N, Singanusong R, Liu X, Duan J, Raymont K, Lisle A, Xu Y (2004) HPLC analyses of flavanols and phenolic acids in the fresh young shoots of tea (Camellia sinensis) grown in Australia. Food Chem 84:253–263
Zago E, Morsa S, Dat JF, Alard P, Ferrarini A, Inze D, Delledonne M, Van Breusegem F (2006) Nitric oxide- and hydrogen peroxide-responsive gene regulation during cell death induction in tobacco. Plant Physiol 141:404–411
Zhao MG, Zhao X, Wu YX, Zhang LX (2007) Enhanced sensitivity to oxidative stress in an Arabidopsis nitric oxide synthase mutant. J Plant Physiol 164:737–745
Zieslin N, Ben Zaken R (1993) Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem 31:333–339
Acknowledgments
Financial support for the research work and fellowship by the Tea Board of India, Ministry of Commerce is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandra, S., Chakraborty, N., Chakraborty, A. et al. Abiotic Elicitor-Mediated Improvement of Innate Immunity in Camellia sinensis . J Plant Growth Regul 33, 849–859 (2014). https://doi.org/10.1007/s00344-014-9436-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-014-9436-y