Abstract
The effect of two chemical elicitors (acibenzolar-S-methyl benzo-[1,2,3]- thiadiazole-7-carboxylic acid S-methyl ester [Boost 500SC]) and salicylic acid in inducing resistance in tea plants against blister blight disease caused by Exobasidium vexans Massee, was studied. Treatments with elicitors resulted in reduced severity of blister blight disease in nursery plants on challenge with the pathogen. There was a significant increase in the activities of defense enzymes like phenylalanine ammonia lyase, peroxidase and β-1,3-glucanase on elicitor treatments in tea leaves challenged with the pathogen than on unchallenged leaves. Acibenzolar-S-methyl (ASM) at 0.14% registered the lowest disease severity (25.2%), whereas treatments with salicylic acid were inferior. Under field conditions, the application of ASM at 0.14% resulted in disease protection of 25%. When ASM was applied in alternate rounds with a standard fungicide, the disease protection improved to 46.8%. The importance of incorporating ASM as a component in integrated disease management and also its importance in organic tea cultivation is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blister blight caused by the obligate parasitic fungus Exobasidium vexans Massee is the most destructive foliar disease of tea. The disease is known to occur in almost all tea-growing areas of Asia, but it is most serious in India, Sri Lanka, Indonesia and Japan (Baby 2002). The disease is prevalent during monsoon months (June–November) and reaches epidemic proportions. In southern India it is reported to cause a crop loss of more than 50% besides adversely affecting the quality of made tea (Baby et al. 1998). A large number of chemical and plant-based fungicides were evaluated against the disease and it was found that only very few chemical fungicides are effective (Baby 2002). These fungicides are sprayed at 7-day intervals throughout the disease season and an average of 24–28 rounds of fungicide sprays is required to keep the disease under control. Large-scale application of pesticides pollutes the environment and their residues can cause various health hazards to human beings. Of late, the regulatory authorities are stringent in fixing limits to pesticide residues in food products including tea (Barooah 2008). In this context, non-chemical disease control strategies are gaining importance.
Plants possess built-in strategies to combat invasions by pathogens. These defense mechanisms may be constitutive or induced (Kuć 1995). There are certain defense mechanisms that are activated upon pathogen infection. This type of disease resistance is known as systemic acquired resistance (SAR). SAR develops either locally or systemically and is associated with the production of pathogenesis related (PR)-proteins (Ward et al. 1991). Besides pathogens, there are certain chemicals which upon application to plants mimic the host–pathogen interaction leading to SAR; these chemicals are known as SAR elicitors (Gullino et al. 2000). Chemical elicitors like salicylic acid (Mayers et al. 2005), jasmonic acid (Cohen et al. 1993), DL-β-aminobutyric acid (BABA) (Hong et al. 1999), oxalic acid (Mucharromah and Kuć 1991) and acibenzolar-S-methyl benzo-(1,2,3)-thiadiazole-7-carboxylic acid S-methyl ester (ASM) (Gullino et al. 2000) have been successfully employed in controlling diseases of various crop plants, The induction of resistance by chemical elicitors can form an important component in the integrated plant disease management program. In the present work attempts were made to study two different elicitors, viz., salicylic acid and ASM (Boost 500SC®), in controlling blister blight of tea.
Materials and methods
Elicitors and test dosages
Elicitors tested were acibenzolar-S-methyl (Boost 500SC®) obtained from Syngenta India Limited and salicylic acid (AR grade) from Merck. For spore germination studies and greenhouse experiments, ASM was tested at concentrations of 0.1% and 0.14%, whereas concentrations for salicylic acid were 100 and 250 ppm. For field evaluation ASM was tested at 0.1%, 0.14% and 0.18%, and salicylic acid was tested at 100, 250 and 500 ppm.
Spore germination study
Basidiospores of E. vexans were collected from naturally infected blister lesions as suggested by Baby et al. (2004). The spore mass was made into a suspension containing 106 spores ml−1 with respective concentrations of elicitors. The fungicide hexaconazole (0.28%) was included as check. Spore suspension prepared with distilled water was kept as a control. The spore suspension was placed in a cavity slide and kept in petri dishes containing distilled water to provide 100% humidity. Spore germination was observed under the microscope at 24 h and percent spore germination was calculated. A spore was considered as germinated if the length of the germ tube reached half the length of the spore. Fifty observations were made in each treatment and the mean values were recorded. The experiment was repeated twice.
Plant material and greenhouse experiment
Six-month-old tea seedlings, variety ‘BSS-1’, highly susceptible to blister blight disease, were used for the greenhouse experiment. Each treatment consisted of 50 plants which were arranged in two sets of 25 plants and kept at 25°C. Elicitors were sprayed on the 3rd leaves of the plants using a hand sprayer. Three days after application, the 1st leaves of the plants were inoculated with spore suspension of E. vexans (106 spores ml−1) in one set of plants and the other set was left uninoculated and served as control for pathogen inoculation. In inoculated plants, disease severity on the 10th day after inoculation was assessed on a 0–4 scale (Premkumar 2005), where 0 = leaves with no infection, 1 = leaves with one or two lesions, 2 = leaves with three to five lesions, 3 = leaves with more than five lesions, 4 = stalk infection. Disease severity was calculated according to the following formula:
where ‘n’ is the number of leaves at each infection level, ‘v’ is the grade for each group of leaves , ‘N’ is the number of leaves assessed and ‘V’ is the highest grade of the scale (in this case 4). The experiment was repeated twice
Induction of defense mechanisms
Extraction of enzymes
Leaves (1st leaves) were collected from the plants on the 3rd, 5th and 7th days after inoculation and were immediately extracted with appropriate buffer solution at 4°C. The homogenate was centrifuged for 20 min at 10,000 rpm at 4°C. Buffers used were sodium phosphate buffer (0.1 M, pH 6.0) for peroxidase, sodium acetate buffer (0.5 M, pH 5.0) for ß-1,3-glucanase, and borate buffer (0.1 M, pH 8.7) for phenylalanine ammonia lyase. All the enzymes were assayed spectrophotometrically (Specord S100, Analytik Jena, Jena, Germany). The experiment was repeated twice.
Peroxidase (PO) activity
The PO activity was assayed as described by Mahadevan and Sridhar (1996). The reaction mixture consisted of 0.5 ml of enzyme extract, 0.5 ml of 0.1 M phosphate buffer (pH 6.0), 0.5 ml of 50 mM guaiacol and 0.5 ml of 60 mM of H2O2. The linear absorbance at 480 nm was monitored for 3 min at 30°C. The reaction mixture without enzyme extract served as blank. The enzyme activity was expressed as change in absorbance, min−1 g−1 f.wt.
Phenylalanine ammonia lyase (PAL) activity
The PAL activity was assayed as described by Sadasivam and Manickam (1996). The reaction mixture consisted of 0.2 ml enzyme extract, 0.5 ml borate buffer (pH 8.0; 1.1 M) and 1 ml of L-phenyl alanine (0.1 M), incubated at 32°C for 60 min. The reaction was stopped by adding 0.5 ml of 1 M trichloroacetic acid. The absorbance was measured at 290 nm. A standard curve was prepared using trans cinnamic acid and the enzyme activity in the sample was expressed as µmol t-cinnamic acid produced min−1 mg−1 protein.
β-1,3-glucanase activity
β-1,3-glucanase activity was assayed by the laminarin-dinitrosalicylic acid method (Pan et al. 1991). The reaction mixture was prepared by mixing 62.5 µl of 4% laminarin (Sigma, St. Louis, MO, USA) and 62.5 µl of plant extract. The mixture was incubated at 40°C for 10 min and the reaction was stopped by adding 375 µl of dinitrosalicylic acid reagent with subsequent heating for 5 min in a boiling water bath. The resulting colored solution was diluted to 4.5 ml with distilled water and vortexed. Products released were estimated for reducing groups in a spectrophotometer at 500 nm. The enzyme activity was expressed as µg glucose min−1 g−1 f.wt.
Field experiments
Tea fields planted with highly susceptible ‘Assam’ seedlings (42 years old) were selected for the field experiments. The experiment was in a randomized block design with nine treatments replicated three times with 50 bushes each. The spraying was carried out with a motorized air blast sprayer once in 7 days throughout the disease season (June–November). Disease incidence was assessed during every plucking round. One hundred shoots of the same age (three leaves and a bud) and of uniform size were collected randomly from the harvest and each shoot was examined against light for blister infection (Premkumar and Baby 2005). A shoot was considered as infected if an active blister lesion of any developmental stage was present. The number of infected shoots out of 100 shoots represents percent disease incidence. The experiment was conducted for two consecutive seasons and the mean data are presented.
Statistical analysis
In all the experiments, the data obtained were subjected to analysis of variance (ANOVA) and the significance of difference between the treatments was determined using Duncan’s Multiple Range Test (P = 0.05).
Results
Effect of elicitors on spore germination and induction of resistance
Percent inhibition of spore germination of E. vexans was less than 8% with both elicitors (Table 1). On the other hand, it was 93% with the fungicide hexaconazole.
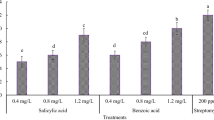
Plants treated with elicitors had a lesser disease severity compared with untreated plants on challenge inoculation. Among the elicitors, ASM at 0.14% was significantly superior to the others and it provided 40.8% protection (Table 2). This was followed by ASM at 0.1% and salicylic acid at 250 ppm. Elicitor treatments resulted in increased activity of β-1,3-glucanase, phenylalanine ammonia lyase and peroxidase on all the days of observation when compared with untreated plants (Figs. 1, 2, 3). The activity increased further in plants challenge-inoculated with the pathogen. The increase in activity reached a maximum on the 5th day and declined on the 7th day in all the treatments (Figs. 1, 2, 3). The activity of all these enzymes was highest in plants treated with ASM at 0.14%, followed by ASM at 0.1% and salicylic acid at 250 ppm.
Activity of phenlyalanine ammonia lyase in tea leaves on elicitor treatments at different intervals. Vertical bars indicate standard errors of the means. Within the same section of the figure, values with a common letter do not differ significantly according to Duncan’s multiple range test at P = 0.05
Effect of elicitors in controlling blister blight under field conditions
Application of ASM at 0.14% resulted in 25% protection from the disease. This was followed by ASM at 0.1% and salicylic acid at 250 ppm. The increase in dosage of salicylic acid to 500 ppm resulted in higher disease incidence compared with salicylic acid sprayed at lower dosages. Furthermore, there was no significant difference in protection achieved with ASM sprayed at 0.18% and 0.14%. On the other hand, when fungicide treatment was given alternately with ASM (0.18%) sprays, the protection achieved was improved further (to 46.8%). The highest protection was achieved with standard fungicide treatment (64.8%) (Table 3).
Discussion
Systemic acquired resistance is the enhanced state of disease resistance in plants developed due to infection by a necrotizing pathogen or by application of abiotic elicitors (Delany et al. 1994). For the development of resistance by elicitors, plants need an incubation period before being challenged with a pathogen. In the present study, plants were challenge-inoculated with the pathogen 3 days after the application of elicitors. A period of 3 days for development of resistance in pepper to Phytophthora when treated with ASM was reported by Baysal et al. (2005) and a period of 1 to 7 days by Doubrava et al. (1988). In the present study, spatial separation was maintained between elicitor-treated leaves and challenge-inoculated leaves and the disease severity was significantly low in elicitor-treated plants. The major feature of SAR is the protection of plant organs from pathogenic infection spatially distant from the elicitor-treated site (Sticher et al. 1997; Vallad and Goodman 2004).
The treatment with elicitors triggered the activity of all the defense enzymes in young tea plants. The defense enzyme PAL is the key enzyme of the phenylpropanoid pathway, catalyzing the transformation of L-phenylalanine into trans-cinnamic acid, which is the prime intermediary in the biosynthesis of flavanoid phytoalexins (Bowles 1990) and phenolics (Dixon and Lamb 1990), which are antimicrobial compounds. The defense enzyme β-1,3-glucanase is a pathogenesis-related protein (PR-2) which hydrolyzes β-1,3-glucan, one of the major components of fungal cell walls (Kauffmann et al. 1987). Peroxidase (PR-9) catalyzes the last step of the biosynthesis of lignin and other oxidative phenols. The reinforcement of the plant cell wall by phenolics and lignin increases plant resistance to cell-wall-degrading enzymes and toxins produced by pathogens, and acts as a mechanical barrier to the penetration of cell wall by the pathogen (Nicholson and Hammerschmidt 1992). The SAR is associated with the production of pathogenesis-related proteins (Kessman et al. 1994) and/or the activation of the phenyl propanoid pathway (Stadnik and Buchenauer 2000). In the present study, there was an increase in activity of defense enzymes with a consequent reduction in disease severity. It is therefore clear from the results that the variation in disease severity between treatments depends on the ability of the elicitor to activate defense enzymes. ASM at 0.14% was the best treatment and salicylic acid was inferior. There are many examples of ASM inducing SAR in many plant–pathogen interactions like, pepper—Phytophthora capsici (Baysal et al. 2005), tomato—Xanthomonas euvesicatoria (Roberts et al. 2008), cyclamen—Fusarium oxysporum f.sp. cyclaminis (Elmer 2006) and tobacco—Peronospora tabaci (Perez et al. 2003). Both of the elicitors tested had shown negligible fungitoxicity as evidenced by a low level of inhibition on spore germination (<8%) of E. vexans in vitro. The elicitors of SAR have less or no antimicrobial properties and are capable of preventing pathogenic infection by increasing crop resistance by activating the SAR signal transduction pathway (Anfoka 2000).
Under field conditions, none of the elicitors provided protection comparable to standard fungicide treatment. Among the elicitors, the highest protection achieved was 25% with ASM at 0.14%, which is not satisfactory. An increase in dosage of ASM to 0.18% did not provide any significant improvement in protection. When ASM at 0.18% was used alternately with a standard fungicide schedule, the percent protection improved to 46.8%, which is satisfactory. This clearly indicated that application of ASM alone is not feasible for controlling tea blister blight disease under field conditions. The protection achieved with integrated treatment, though not comparable to standard treatment, is important in that the total number of applications of fungicides was reduced to half. This indicates that ASM works better under low disease pressure. In tea, the crop is harvested continuously at intervals of 10–15 days. Therefore, it is necessary to spray fungicides at closer intervals to protect the newly developed leaves from the disease and because of this, many rounds of fungicides are sprayed during a disease season (Premkumar and Baby 2005). By integrating ASM in the fungicide schedule there is the possibility of reducing the number of fungicide sprays. Another possibility exists in the usage of ASM in clonal tea fields where disease pressure is relatively low (Baby 2006), compared with the seedling tea fields used in the present study. There is yet another use for ASM in blister blight control in tea under organic cultivation. In organic cultivation no fungicides are permitted for control of blister blight disease except for copper fungicides, the usage of which is restricted to 3 kg a.i. ha−1 per season (Radhakrishnan 2004). ASM, being an activator, fits well into organic cultivation and its application alternating with copper sprays allows us to distribute the permissible quantity of copper fungicides more effectively.
In conclusion, ASM can stimulate resistance in tea to blister blight disease. Since the level of control with application of ASM alone was not satisfactory under field conditions, it can be integrated in fungicide schedules for achieving better control of the disease.
References
Anfoka, G. H. (2000). Benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester induces systemic resistance in tomato (Lycopersicon esculentum Mill. Cv. Volledung) to cucumber mosaic virus. Crop Protection, 19, 401–405.
Baby, U. I. (2002). An overview of blister blight disease of tea and its control. Journal of Plantation Crops, 30(2), 1–12.
Baby, U. I. (2006). Varietal resistance of certain south Indian tea clones to blister blight. Newsletter of UPASI Tea Research Foundation, 16(2), 2.
Baby, U. I., Balasubramanian, S., Ajay, D., & Premkumar, R. (2004). Effect of ergosterol biosynthesis inhibitors on blister blight disease, the tea plant and quality of made tea. Crop Protection, 23, 795–800.
Baby, U. I., Ravichandran, R., Ganesan, V., Parthiban, B., & Sukumar, S. (1998). Effect of blister blight on the biochemical and quality constituents of green leaf and CTC tea. Tropical Agriculture, 75, 452–456.
Barooah, A. K. (2008). Residue limits of agrochemicals and heavy metals in tea. In P. Ghosh (Ed.), Field management in tea (Darjeeling 2008) (pp. 234–246). West Bengal, India: Tea Research Association, Nagarkata Sub-Station.
Baysal, O., Turgut, C., & Mao, G. (2005). Acibenzolar-S-methyl induced resistance to Phytophthora capsici in pepper leaves. Biologia Plantarum, 49, 599–604.
Bowles, D. J. (1990). Defense-related proteins in higher plants. Annual Review of Biochemistry, 59, 873–907.
Cohen, Y., Gisi, U., & Niderman, T. (1993). Local and systemic protection against Phytophthora infestans induced in potato and tomato plants by jasmonic acid methyl ester. Phytopathology, 83, 1054–1062.
Delany, T. P., Friedrich, L., Kessmann, H., Uknes, S., Veernooj, B., & Ward, E. (1994). The molecular biology of systemic acquired resistance. In M. Daniels (Ed.), Advances in molecular genetics of plant microbe interaction (Vol. 3, pp. 339–347). Dordrecht, the Netherlands: Kluwer Academic.
Dixon, R. A., & Lamb, C. V. (1990). Molecular communication in interactions between plants and microbial pathogens. Annual Review of Plant Physiology and Plant Molecular Biology, 41, 339–367.
Doubrava, N. S., Dean, R. A., & Kuć, J. (1988). Induction of systemic induced resistance to anthracnose caused by Colletotrichum lagenarium in cucumber by oxalate and extracts from spinach and rhubarb leaves. Physiological and Molecular Plant Pathology, 33, 69–79.
Elmer, W. H. (2006). Effects of acibenzolar-S-methyl on the suppression of Fusarium wilt of cyclamen. Crop Protection, 25, 671–676.
Gullino, M. L., Leroux, P., & Smith, C. M. (2000). Uses and challenges of novel compounds for plant disease control. Crop Protection, 19, 1–11.
Hong, J. K., Hwang, B. K., & Kim, C. H. (1999). Induction of local and systemic resistance to Colletotrichum coccodes in pepper plants by DL-β-amino-n-butyric acid. Journal of Phytopathology, 147, 193–198.
Kauffmann, S., Legrand, M., Geoffroy, P., & Fritig, B. (1987). Biological function of pathogenesis-related proteins: four PR proteins of tobacco have 1,3-β-glucanase activity. EMBO Journal, 6, 3209–3212.
Kessman, H., Staub, T., Hofmann, C., Maetzke, T., Herzog, J., & Ward, E. (1994). Induction of systemic acquired resistance in plants by chemicals. Annual Review of Plant Pathology, 32, 439–459.
Kuć, J. (1995). Induced systemic resistance—an overview. In R. Hammerschmidt & J. Kuć (Eds.), Induced resistance to disease in plants (pp. 169–175). Dordrecht, the Netherlands: Kluwer Academic.
Mahadevan, A., & Sridhar, R. (1996). Methods in physiological plant pathology (4th ed.). Chennai, India: Sivakami.
Mayers, C. N., Lee, K. C., Moore, C. A., Wong, S. M., & Carr, J. P. (2005). Salicylic acid-induced resistance to Cucumber mosaic virus in squash and Arabidopsis thaliana: contrasting mechanisms of induction and antiviral action. Molecular Plant-Microbe Interactions, 18, 428–434.
Mucharromah, E., & Kuć, J. (1991). Oxalate and phosphates induce systemic resistance against diseases caused by fungi, bacteria and viruses in cucumber. Crop Protection, 10, 265–270.
Nicholson, R. L., & Hammerschmidt, R. (1992). Phenolic compounds and their role in disease resistance. Annual Review of Phytopathology, 30, 369–389.
Pan, S. Q., Ye, X. S., & Kuć, J. (1991). Association of β-1, 3 glucanase activity and isoform pattern with systemic resistance to blue mold in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiological and Molecular Plant Pathology, 39, 25–39.
Perez, L., Rodriguez, M. E., Rodriguez, F., & Roson, C. (2003). Efficacy of acibenzolar-S-methyl, an inducer of systemic acquired resistance against tobacco blue mould caused by Peronospora hyoscyami f.sp. tabacina. Crop Protection, 22, 405–413.
Premkumar, R. (2005). Report of plant pathology division. Annual Report No. 35UPASI Tea Research Institute, Valparai, India.
Premkumar, R., & Baby, U. I. (2005). Blister blight control—a review of current recommendations. Planters’ Chronicle, 101(5), 26–36.
Radhakrishnan, B. (2004). Package of practices for organic tea. Planters’ Chronicle, 99(1), 19–39.
Roberts, P. D., Momol, M. T., Ritchie, L., Olson, L. M., Jones, J. B., & Balogh, B. (2008). Evaluation of spray programs containing famoxadone plus cymoxanil, acibenzolar-S-methyl, and Bacillus subtilis compared to copper sprays for management of bacterial spot on tomato. Crop Protection, 27, 1519–1526.
Sadasivam, S., & Manickam, A. (1996). Biochemical methods (2nd ed., pp. 190–191). Chennai, India: New Age International (P) Limited.
Stadnik, M. J., & Buchenauer, H. (2000). Inhibition of phenylalanine ammonia-lyase suppresses the resistance induced by benzothiadiazole in wheat to Blumeria graminis f.sp. tritici. Physiological and Molecular Plant Pathology, 57, 25–34.
Sticher, L., Mauch-Mani, B., & Metraux, J. P. (1997). Systemic acquired resistance. Annual Review of Phytopathology, 35, 235–270.
Vallad, G. E., & Goodman, R. M. (2004). Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Science, 44, 1920–1934.
Ward, E. R., Ukness, S. J., Williams, S. C., Dincher, S. S., Wiederhold, L., Alexander, A., et al. (1991). Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell, 3, 1085–1094.
Acknowledgments
The authors express their thanks to Dr. N. Muraleedharan, Adviser, Dr. P.Mohan Kumar, Director, and Dr. R. Premkumar, Head, Plant Pathology Division, for use of the facilities and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ajay, D., Baby, U.I. Induction of systemic resistance to Exobasidium vexans in tea through SAR elicitors. Phytoparasitica 38, 53–60 (2010). https://doi.org/10.1007/s12600-009-0068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-009-0068-x