Abstract
Adaptation of quinoa (Chenopodium quinoa Willd.) to new regions demands acclimation to day-length, in addition to a host of other abiotic factors. To further elucidate the effects of photoperiod on development of quinoa, two differently adapted cultivars, Achachino (short day) from Bolivia and Titicaca (day-length neutral), were subjected to continuous long (17.5 h) and short (10 h) photoperiod conditions as well as a shift between the two to trigger possible adaptive mechanisms initiated by changes in leaf soluble sugar and ABA concentration. Our findings show both cultivars responding to an increase in photoperiod with significant increases in soluble sugar concentrations and a simultaneous increase in ABA. However, Titicaca exhibited a much stronger ABA response to increase in photoperiod, whereas the increase for Achachino falls within the range of natural diurnal variation. Achachino also showed increasing sensitivity to long photoperiods throughout all reproductive growth stages, resulting in continued flowering, stem elongation and disruption of seed formation, whereas Titicaca was capable of maintaining full seed set under all the photoperiod conditions. Discernible photoperiod-dependent chlorosis of the lower leaves of Titicaca was observed under long photoperiods compared to short photoperiods, implying multi-faceted adaptive responses to changes in photoperiod which may also involve nitrogen and carbon dynamics. Both ABA and sugar signals are possibly involved in regulating the photoperiod-adaptive capability of each cultivar, leading to pronounced differences in growth and reproductive development patterns between the contrasting cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1998, quinoa (Chenopodium quinoa Willd.) was selected by the Food and Agricultural Organisation of the United Nations as a vital tool to enable future food security and 2013 has been nominated as the international year of quinoa (Food and Agriculture Organisation of the United Nations 2011). Although quinoa has been cultivated in South America for thousands of years, its rising popularity in Western nations is considerably more recent and has been propelled by increasing culinary interest (Jacobsen 2003). The prospect for expanding quinoa cultivation in harsh environments is supported by its inherent ability to resist stressful growing conditions, and the hardiness of quinoa is determined by physiological mechanisms, as well as morphological characteristics and life cycle strategies (Jacobsen and others 2003). Quinoa is a halophyte, and tolerates high soil saline levels (Adolf and others 2012). Consequently, it has the ability to thrive where arable land and agricultural productivity is otherwise restricted by prolonged droughts and increasing soil salinity as well as in less vulnerable regions. However, the large-scale expansion of quinoa as an alternative crop is significantly handicapped by its photoperiodic sensitivity (Christiansen and others 2010). To make the process of adapting quinoa to more regions within and outside South America more systematic, it is essential that we understand adaptive ranges and physiology behind responses to ranges of photoperiods in contrasting cultivars (Bertero and others 2004; James and Lawn 2011), which is a concept that previously has also been applied to the investigation of the differential adaptation potential of two contrasting common bean varieties (Wentworth and others 2006). Quinoa is generally classified as a facultative short day plant (Fuller 1949) and is affected greatly by photoperiods longer than 12 h, particularly during reproductive growth stages (Christiansen and others 2010). The photoperiodic responses of quinoa are complex in nature (Bertero and others 1999). Bud induction and flowering are not the major problems for adaptation of quinoa to areas with long day conditions during the growing season (Fuller 1949). However, during bud development and after the onset of flowering, quinoa becomes increasingly sensitive to long day conditions. The sensitivity is expressed as cessation, or delay, of reproductive development and late or fully halted maturity (Christiansen and others 2010; Galwey 1993). Studies involving photoperiod effects on plants have in the past focused on more widely cultivated crops such as cereals and legumes, model species and ornamentals (Distelfeld and others 2009; Lawn and James 2011). Although photoperiod effects in quinoa have been previously recorded (Bertero and others 1999; Christiansen and others 2010; Fuller 1949), to our knowledge none or very little work has been carried out with regards to underlying physiological mechanisms involved in photoperiodic control of development in quinoa and how it translates to cultivar performance in agronomic terms. Thus, the knowledge base in this area has essentially remained obscure, acting as a possible constraint on geographic cultivation expansion of quinoa.
Photoperiodic control of plant development is a multi-faceted process involving light sensing mechanisms, phytohormone responses and subsequent modification of source-sink relationships (Dorais and others 1996; Seung and others 2011) where photoperiod may function as the sole abiotic input responsible for these responses (Gonziález and others 2009). The day-length sensitive reaction in short day adapted quinoa may partly be mediated by the presence or absence of light sensitive hormone-signalling (Christiansen and others 2010). Although absicisic acid (ABA), generally, is thought of as associated with dormancy regulation and drought responses, it may hold more of a multi-purpose signalling role. Few studies have investigated possible links between changes in ABA concentrations and day-length responses in a range of plant species. Nan and others (2002) reported that leaf ABA levels were significantly higher in wheat plants grown under continuous light, as compared to those only receiving 18–21 h of light and in spinach, ABA concentrations increased two to three fold during the first day after plants were transferred from short to long days (Zeevaart 1971). More recent studies and reviews are increasingly suggesting a stronger involvement of ABA in photoperiodic plant responses and adaptive mechanisms than what has so far been considered (Chu and others 2005; Seung and others 2011). Slight variations in environmental conditions can lead to substantial variation in carbohydrate metabolism (Ramel and others 2009), and the availability of carbohydrates, in the form of sucrose and hexose, has a regulatory function in a broad range of developmental and physiological processes and may also act as direct signalling molecules, thereby activating specific or hormone crosstalk pathways (Finkelstein and Gibson 2001; Gibson 2003). It has been suggested that soluble sugars and ABA can have synergetic and antagonistic, as well as additive effects on different physiological processes. In this manner they interact to regulate floral transition and organogenesis under the influence of light signals (Finkelstein and Gibson 2001). Consequently, different day-length and light intensity environments may induce changes in the concentration and composition of the soluble sugars in plant tissues, leading to modifications of plant growth and development. Clarifying the mechanisms behind photoperiod-regulated physiological and developmental responses is without doubt a challenge, as they are part of a very complex signalling network with large variation between both species and cultivars. In quinoa, the possible links between ABA and soluble sugars in relation to photoperiod adaptation have not previously been investigated. Therefore, the objectives of this study were to compare the effects of photoperiod on plant development of two contrastingly adapted quinoa cultivars (short day and day-length neutral), as well as exploring the possible involvement of ABA and soluble sugar responses in photoperiod adaptation in the two cultivars.
Materials and Methods
Plant Material and Growing Conditions
Potted quinoa plants of two cultivars, Titicaca and Achachino, were grown from seeds in controlled environment walk-in style growth chambers (Conviron, Winnipeg, Canada) at the University of Copenhagen, Faculty of Science, Taastrup, Denmark. Seeds were propagated from plants grown under optimum cultivar specific photoperiod conditions. Titicaca (previously Q52) is a day-length neutral cultivar developed at the Faculty of Life Sciences, Taastrup, Denmark (55°40′N, 12°18′E, 28 m above sea level). Achachino is a traditional short day cultivar of the Real type from southern Bolivia (20°28′S, 66°50′W, 3,653 m above sea level). The growth chambers were set to 22 /15 °C day/night air temperature. The photosynthetic flux density (PPFD) for all the chambers was approximately 600 μmol m−2 s−1, at upper plant height, provided by metal halide lamps (Osram, HQI 400 W). Four seeds were sown directly into each of the 4 L pots used in this study. Peat-based potting soil (Pindstrup, Substrate No. 1; Ryomgaard, Denmark) was used as a growth medium. For the duration of the study, all pots were kept moist by drip irrigation and supplied with a standard nutrient solution (Pioner NPK Makro 14-3-23 + Mg combined with Pioner Mikro + Fe; Lyngby, Denmark; EC: 1.3 mS cm−1; pH 5.5). Plants were thinned to two per pot after two leaves had fully emerged and one pot represents one repetition. This approach allowed for the collection of fresh leaf material for ABA and soluble sugar analysis without causing significant damage to each replication. There were eight replications of each cultivar in each treatment. Only one plant from each pot was harvested for biomass data collection (n = 8). To avoid positioning effects, within each chamber the pots were moved every couple of days. Four different photoperiod treatments were managed separately in four growth chambers, as integrals of uninterrupted full light. The treatments consisted of continuous short day (S), short day shifted to long day (SL), continuous long day (L) and long day shifted to short day (LS). The changes in photoperiod for LS and SL were administered during early reproductive development stages, corresponding to stage 2–6 as classified by Jacobsen and Stølen (1993), with all pots (eight of each cultivar) within the specific treatments being very close to the same exact stage at the time of the photoperiod change. The additional hours of light were divided up and timed to extend the morning and afternoon of the short period. The long day treatment of 17.5 h reflects the maximum day length under Danish field conditions during the growing season (Christiansen and others 2010). The short day treatment of 10 h is sufficiently below the critical 12 h threshold for optimum development and reproductive success of short day adapted plants. By switching the plants between photoperiods, we sought to trigger measurable ABA and soluble sugar responses. The L and LS-treated plants for both cultivars reached the early reproductive stages and plant size designated for the change in photoperiod a week earlier, that is, at 25 days after sowing (DAS), than the plants subjected to S and SL treatments, that is, at 32 DAS. The difference in days of the timing of the photoperiod shift compared to LS was added to the SL treatment after the change, thereby allowing for both LS and SL treated plants to spend the same amount of time under altered photoperiods. There were no visible morphological differences between cultivars within the treatment groups at the time of the photoperiod change for the LS or SL treatments. The daily PPFD integral under long day was 37.8 mol m−2 day−1 and under short day was 21.6 mol m−2 day−1. One plant per pot, from each cultivar and all treatments, was selected randomly and harvested for further processing and analysis of leaf areas and biomass at the end of the study. The study period lasted 41 DAS for L and LS treatments and 48 DAS for the S and SL treated plants. Leaves were harvested, and projected leaf area was measured using a Li-3100 Leaf Area Meter (Li-Cor Inc., Lincoln, Nebraska, USA). All plant parts were dried to constant weight at 70 °C and dry weight was determined. Specific leaf area (SLA) was calculated as leaf area (cm2) per unit of leaf dry weight (g). Growth stages throughout the study were recorded according to the system previously established by Jacobsen and Stølen (1993), where stage 0 is purely vegetative, stages 1–7 cover small steps in reproductive development, 8 is onset of flowering, stages 9–13 are the entire flowering process and 14–21 are seed set through maturation. The on-set of flowering (development stage 8) was noted at the appearance of the first open flower in the inflorescence of each plant. Stem height was measured from soil level to the base of the inflorescence.
For the S and SL treatments, the plants that were not harvested were allowed to develop to maturity. The same procedure was not carried out for the remaining plants from the long day treatments (L and LS) due to ceased panicle development of Achachino under those conditions and concerns over constraints of chamber height and distance to lamps. Continued flowering and reshooting occurred from the inflorescence of Achachino under L at the termination point of the experiment.
ABA Extraction and Analysis
Plant material used for ABA and sugar analysis was from the youngest, fully expanded leaves only. Leaves were taken from four (n = 4) randomly chosen plants out of the available 8, for each cultivar and each treatment. Samples were collected the day before the changes in photoperiod were implemented (Day0) and the two following days (Day1, Day2). The leaves were frozen in liquid nitrogen immediately after excision and stored at −80 °C until final processing. All collections of fresh plant materials and measurements were carried out from 10:30 to 12:30 h. ABA was extracted from 50 mg powdered freeze-dried leaf samples, dissolved in 1 ml Mili-Q, purified water (ELGA Purelab Ultra, Veolia Water Solutions and Technologies, France) and shaken overnight at 4 °C. The ABA concentration was quantified by enzyme linked immunosorbent assay using a monoclonal antibody for ABA (AFRC MAC 252) as specified in Asch and others (2001).
Soluble Sugar Analysis
The procedure followed for the extraction of water-soluble sugars is described in detail by Liu and others (2004) using powdered freeze-dried leaf material (50–100 mg). Hexose (glucose and fructose) and sucrose concentrations were analysed by HPLC (Hewlett-Packard 1100, Waldbronn, Germany) equipped with an RI detector (Hewlett-Packard 1047A, Waldbronn, Germany).
Data Analysis and Statistics
Means (n = 4 for soluble sugar and ABA analysis, and 8 for direct physical measurements) of the measured variables were subjected to analysis of variance (ANOVA) and LSD applied as post-ANOVA test with SAS 9.2 software (SAS Institute Inc., Cary, NC, USA). Standard errors of the means (±SE) were calculated and compared at P < 0.05.
Results
Plant Development and Growth
Both the long day initiated treatments (L and LS) resulted in faster and more complete plant development for Titicaca compared to Achachino (Fig. 1a, P < 0.05). Titicaca reached flowering for both L and LS around 35–36 DAS and continued panicle development towards complete seed fill. Achachino, however, followed a different developmental progression. Under L and LS treatments, onset of flowering was delayed until around 40 DAS. Further reproductive development for Achachino under L and LS abated after flowering was initiated and none of the treatment reached seed fill stages (stage 14) prior to plant harvest (Fig. 1a). At the end of the study period, both cultivars of the L treated plants had reached the limit for plant height of the growth chambers. Stem growth progressed similarly for both cultivars under L and LS until around 29 DAS (Fig. 1b). After 29 DAS both Achachino and Titicaca for the LS treatment ended with the same stem height and both were shorter than the L treated stem heights. Achachino under L had more stem growth than Titicaca for the same treatment (Fig 1b, P < 0.001). None of the L and LS plants reached a plateau in growth within the study period. Under S and SL conditions, both cultivars developed at a slower rate and did not reach comparable plant size with L and LS for photoperiod change until 32 DAS, a week later compared to 25 DAS for the L and LS treatments. All plants of Titicaca were flowering at 34 DAS under SL and 35–36 DAS for S, as was Achachino for both S and SL (Fig. 1c). Under S and SL both cultivars developed beyond flowering during the study period, with Titicaca reaching the most developed stage under SL (Fig. 1c, P < 0.005). Stem elongation for both cultivars under S and SL treatments was indistinguishable until around 41 DAS, when Titicaca under SL proceeded towards zero gain in stem elongation for both S and SL, but on different DAS so S-treated Titicaca grew taller (Fig 1d). Stem growth for Achachino did not reach a plateau for either the S or SL treatment by the end of the study and both reached the same stem height (Fig. 1d). For both cultivars and treatments, Achachino reached a taller stem height compared to Titicaca (Fig. 1d, P < 0.001). We not only observed continuous flowering and stem elongation, but also severely reduced seed size and lack of seed hardening in Achachino compared to Titicaca under SL. In Achachino, L and LS both exhibited reshooting between the branches of the panicle structure towards the end of the study. Clear developmental differences between the cultivars and treatments were visible at this point (Fig. 1b, d).
Plant development and stem height for both cultivars and all treatments (mean ± SE, n = 8). a Plant development and b stem height for L and LS treatments; c plant development and d stem height for the S and SL treatments. The horizontal dashed line in a, c indicates development stage 8, which signifies the onset of flowering. The arrows in b, d indicate the time at which photoperiod was changed in the LS and SL treatments, where the dashed horizontal line indicates identical stem height at that time
Even under continuous short photoperiod (S), which is its preference, Achachino appeared to have slower inflorescence development and seed fill compared to Titicaca (Fig. 2). When photoperiod was extended from short to long (SL), Achachino became more branched with a less compact inflorescence compared to growth under S. Titicaca maintained similar plant structure under both S and SL. In Titicaca, differences were most obvious in the reproductive structure, which appeared to increase in size under extended photoperiod (SL). An additional observation was a discernible chlorosis of the lower leaves, which occurred in Titicaca for SL, with initial yellowing being visible two days after the photoperiod change (Fig. 2). It is important to note that this intense leaf chlorosis of the lower leaves for the SL treated plants did not lead to leaf abscission or death. In comparison, Titicaca S showed signs of chlorosis of the older leaves as well, although neither until towards the end of the study, nor with the same intensity or rapidity as Titicaca SL. Achachino SL likewise exhibited increased chlorosis of the lower leaves compared to Achachino S, but not at the same rate or to the extent as that of Titicaca. Under continuous long photoperiod treatments (L), both cultivars showed increasing chlorosis of the lower leaves compared to the LS treatment, but the yellowing was less intense and slower in appearing compared to SL.
Plants from the short (S) and short to long photoperiod (SL) treatments, 49 days after sowing. From left to right Titicaca and Achachino from the SL treatment, followed by Titicaca and Achachino subjected to S treatment. Rapid yellowing of the lower leaves for the SL treatment was visible shortly after the change in photoperiod (Color figure online)
Aboveground Biomass Accumulation and Specific Leaf Area
There were no significant differences in leaf area between the two cultivars, or between any of the day-length treatments. Leaf biomass showed a tendency towards greater total leaf mass for both the L and SL treatments compared to the S and LS treatments, but not with statistical significance (Table 1, P = 0.056). However, specific leaf area for both cultivars showed a strong inclination towards a lower value (higher density), at time of harvest, for both the L and SL treatments compared to S and LS treatments (Table 1, P < 0.001).
The biomass allocation data for the plants that were grown under the S and SL treatments, in which both cultivars developed seeds, show differences between treatments within cultivars (Table 2). Under S, Achachino transitioned to reproductive development and seed fill at the same time as Titicaca and also had shorter stem height than under SL (Fig. 1), where it continued to elongate the main stem and the stems within the panicle, in conjunction with continuous flowering rather than a complete progression to seed fill and maturation; consequently, the amount of vegetative growth was nearly tripled for Achachino subjected to the SL treatment compared to S (P < 0.01). The vegetative growth of Titicaca did not differ between the two treatments. Harvest data for Titicaca showed the SL treatment resulted in a significant increase in panicle dry matter accumulation compared to S (P < 0.01). Further analysis indicated similar trends with regards to yield and seed weight, but not with statistical significance. The same comparison for Achachino showed no difference in whole panicle dry weight, but did reveal severely reduced seed development for the SL treatment compared to S-treated plants (P < 0.05). Overall, the data indicated that regardless of the photoperiod conditions, Achachino yielded less compared to Titicaca (Table 2).
ABA Responses
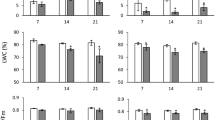
The ABA concentrations were compared within all 4 treatments for each day and here the only significant response was found for Day1 for the day-length neutral Titicaca, with the SL treatment showing a high peak in ABA concentration compared to S, L and LS (P < 0.001). When comparing the ABA concentration development for each treatment over time, between the three sampling days (Day0–Day2), no significant changes in leaf ABA concentration were detected for the L, LS or S treatments. However, for the SL treated plants, significant changes in leaf ABA concentration were detected within 24 h of increasing the photoperiod (Day0–Day1), for the SL treated plants for both Titicaca and Achachino (P < 0.001 and P < 0.05, respectively). The ABA response was significantly more pronounced for Titicaca compared to Achachino, and on Day2 ABA concentrations for Titicaca dropped to levels resembling those of Day0 (Fig. 3c). Considering that the individual day analysis showed no significance between the four treatments for Achachino during all three days, including Day1, therefore, the significant difference detected from Day0 to Day1 for the SL treated plants must be attributed to natural diurnal variation of concentrations rather than a treatment-dependent response (Fig. 3c).
Soluble Sugar Responses
The same comparison approach carried out for ABA was applied to the soluble sugar analysis.
Sucrose and hexose concentrations for the L, LS and S treatments did not exhibit significant fluctuations between sampling days for Titicaca or Achachino. Only the plants subjected to SL conditions showed a response with a significant increase of hexose and sucrose concentrations over time (P < 0.05) in both cultivars from Day0 to Day1. The significantly increased hexose and sucrose levels, caused by the added productivity as a result of the increases in light integral and photoperiod, were sustained through to Day2 for Titicaca, but not with similar significance for Achachino when making the comparison between levels of the four treatments for Day2 (Fig. 3a, b).
Discussion
Based on the results of the present study and the previous studies by Christiansen and others (2010) and Bertero and others (1999), it is clear that quinoa is a facultative short day plant rather than qualitative, indicating that regardless of cultivar adaptation, quinoa is capable of flowering under a wide range of day-lengths. As seen in the present and previous studies (Christiansen and others 2010), this does, however, not guarantee seed yield, which underscores the importance of including plant development beyond flowering in future quinoa adaptation studies. Our observations of long photoperiods noticeably affecting quinoa development after the onset of flowering are in accordance with what has been documented in other quinoa focused studies (Bertero and others 1999) and other short day species (Roberts and Summerfield 1987), particularly with regards to variation in critical photoperiods between differently adapted cultivars of tropical legumes (James and Lawn 2011; Lawn and James 2011). For Titicaca, neither seed set nor development towards maturity was affected by the photoperiod treatments, implying that it is truly day-length neutral. The vegetative growth response of Achachino after exposure to long photoperiods is similar to that of tomatoes, as reported by Dorais and others (1996), and tropical legumes (James and Lawn 2011). The indications of differences in resource allocations seen between the plants of the two cultivars that were grown to maturity from the S and SL treatments, are also in agreement with what was reported for tomato and sweet pepper plants (Dorais and others 1996), where extended photoperiods resulted in further shoot development and carbohydrate accumulation in leaves being favoured over fruit development. In contrast, the day-length neutral pepper plants responded to the added hours of light by significantly increasing fruit yield (Dorais and others 1996) which is what the present data suggest are also the case for the photoperiod-neutral Titicaca subjected to the SL treatment. In the day-length neutral Titicaca, sucrose concentration was fairly stable between treatments, only the SL treatment showed significant variation with an increase after the shift from short to long days, compared to the other treatments, which was to be expected from suddenly increasing the period of carbon gain. If this increase had been caused by the increases in average diurnal temperature due to the added hours of light, we would have detected a constant higher level of sugars in the L compared to S-treated plants. But this was not the case, we have to consider the soluble sugar response as a specific result of a shift to longer photoperiod. The significantly lower specific leaf area found in the L and SL treatments, for both Titicaca and Achachino, compared to the S and LS-treated plants, indicates that leaf growth is acclimated instantaneously to the light environment and that it is determined by the last imposed duration of the photoperiod treatment. Leaf components such as leaf thickness, nitrogen and carbon status have in other studies been shown to rapidly change as the existing leaves acclimate to changing light levels (Rodríguez-Calcerrada and others 2008; Wentworth and others 2006). In the present case, we suspect that the increased leaf density found in the SL and L treatments for both cultivars could be a result of starch accumulation, as reported for tomatoes (Dorais and others 1996). The significant increase in ABA for Titicaca, caused by the extended photoperiod, is consistent with earlier findings in spinach (Zeevaart 1971) and for wheat (Nan and others 2002). Increased ABA levels in spinach were by Zeevaart (1971) suggested to possibly inhibit other phytohormones, hereunder gibberellins (GA), which would otherwise play an antagonistic role in relation to ABA throughout numerous developmental plant processes (Weiss and Ori 2007). Regulation of transitioning from vegetative to reproductive development, amongst others, depends on the balance of GAs and ABA and hence also the ability of the plants to mobilize these hormones in response to changing environmental cues (Domagalska and others 2010; Li and others 2011; Liu and others 2005; Wingler and others 2006) and in turn the circadian clock appears to also actively regulate the signalling function of ABA by affecting everything from metabolism to perception and activity of the hormone (Seung and others 2011). The ABA concentration increase, we measured in Titicaca under the SL treatment, was of much greater magnitude than the increase in Achachino, and only Titicaca developed to seed maturity under the SL and L photoperiod treatments. Wentworth and others (2006) also noted differences between two common bean varieties in their ability to synthesise ABA and subsequent acclimation potential in response to abiotic stress. Although the ABA response for Achachino was shown to be statistically significant, further analysis suggests that the increase for ABA in Achachino can be attributed to diurnal variations rather than treatment effects. Both ABA and sugar accumulation can occur under various stress conditions (Nan and others 2002; Ramel and others 2009), but more recent theories and experimental data (Finkelstein and Gibson 2001; Gibson 2003; Rolland and others 2006; Wingler and others 2006) increasingly support the notion that variation in sugar production and plant hormone concentrations is not entirely unrelated and sugar levels may act as simultaneous or causal signalling molecules in integrating responses to environmental stimuli (Wingler and others 2006), thereby dictating the hormone (that is, ABA) dependent regulation of plant growth (Finkelstein and Gibson 2001; Gibson 2003, Gibson 2005; Pourtau and others 2004; Wingler and others 2006). Although a sudden switch between photoperiods will never occur under natural conditions, by triggering the response and causing a peak in ABA and sugar accumulation, masking of responses and mechanisms due to very small changes in concentrations caused by gradually increasing photoperiods and challenges with timing of measurements is avoided. The intense chlorosis in the lower leaves of Titicaca under long photoperiods, which did not lead to immediate leaf death or abscission, suggests a simultaneous re-mobilization of leaf-nitrogen as a result of the ABA and sugar concentration increases and would further support the notion that they both play an important part in regulating the source-sink relationships and seed maturation (Liu and others 2005; Pourtau and others 2004; Wingler and others 2006). In the present experiment, we created the different day-length treatments by changing the daily duration of actinic light. As a consequence, both the light integral and temperature sum were affected. The abrupt changes in photoperiod provoked plant response mechanisms and within 24 h after changing from long to short days we detected a short, but strong transitional peak in ABA concentrations in Titicaca, which may indicate that also ABA is relevant in day-length signalling and plant adaptation to the light environment, particularly for the photoperiod-neutral quinoa cultivar. The content of soluble sugars also increased from short to long days, in both Achachino and Titicaca. There the increase in light integral could be considered to have an effect, but if that was true the sugar content of the L-treated plants should persistently be higher than that of the S-treated plants. Because this was not the case, we have to assume that the responses we measured were mainly due to the increase in photoperiod and not daily radiation amount. To clearly distinguish between the effects of day-length and light integral effects, a different experimental design has to be used where a change in day-length extension is created with incandescent bulbs, triggering the phytochrome system, but staying below actinic levels to separate day-length effects from those that depend on the light integral (Islam and others 2004). Overall, based on the plant development correlated with the photoperiod-induced concentration changes, our results suggest that both ABA and soluble sugar responses may be involved in quinoa cultivar specific day-length adaptation. In the future it could also be interesting to include physiologically active components such as proline, auxins and other phytohormones in the pursuit of further analysis of underlying photoperiod-response mechanisms. Ultimately, crop adaptation and yield potential are determined by the plasticity of a host of physiological components and mechanisms to respond not only to more urgent environmental variation in cases of drought and soil salinity, but also to something as basic as light quantity and quality. This study supports the notion reiterated by others (for example, Chu and others 2005; Gibson 2005; Rolland and others 2006; Seung and others 2011; Weiss and Ori 2007) that plant responses to the light environment, are a highly complex area of study, involving numerous components in a multi-dimensional network with branched and parallel signalling pathways leading to a myriad of physiological and morphological responses. Further studies aiming to gain more in-depth knowledge of physiological differentiation of contrasting cultivars (Bertero and others 2004; James and Lawn 2011, Lawn and James 2011; Wentworth and others 2006), as well as growth patterns and carbon and nitrogen dynamics in quinoa, will be important for the success and efficiency of future selection criteria for quinoa adaptation outside its native region (Bertero and others 2004; Haussman and others 2012; Wright and others 1996).
References
Adolf VI, Shabala S, Andersen MN, Razzaghi F, Jacobsen S-E (2012) Varietal differences of quinoa’s tolerance to saline conditions. Plant Soil. doi:10.1007/s11104-012-1133-7
Asch F, Andersen MN, Jensen CR, Mogensen VO (2001) Ovary abscisic acid concentration does not induce kernel abortion in field-grown maize subjected to drought. Eur J Agron 15:119–129
Bertero HD, King RW, Hall AJ (1999) Photoperiod-sensitive development phases in quinoa (Chenopodium quinoa Willd.). Field Crop Res 60:231–243
Bertero HD, de la Vega AJ, Correa G, Jacobsen S-E, Mujica A (2004) Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of multi-environmental trials. Field Crop Res 89:299–318
Christiansen JL, Jacobsen S-E, Jørgensen ST (2010) Photoperiodic effect on flowering and seed development in quinoa (Chenopodium quinoa Willd.). Acta Agr Scand 60:539–544
Chu L-Y, Shao H-B, Li M-Y (2005) Molecular mechanisms of phytochrome signal transduction in higher plants. Colloid Surf B 45:154–161
Distelfeld A, Li C, Dubcovsky J (2009) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12:178–184
Domagalska MA, Sarnowska E, Nagy F, Davis SJ (2010) Genetic analyses of interactions among gibberellin, abscisic acid, and brassinosteroids in the control of flowering time in Arabidopsis thaliana. PLoS One 5(11):e14012. doi:10.1371/journal.pone.0014012
Dorais M, Yelle S, Gosselin A (1996) Influence of extended photoperiod on photosynthates partitioning and export in tomato and pepper plants. New Zeal J Crop Hort 24:29–37
FAO (Food and Agriculture Organisation of the United Nations) (2011) The state of food insecurity in the world. The Food and Agriculture Organization of the United Nations, Rome
Finkelstein RR, Gibson S (2001) ABA and sugar interactions regulating development: cross-talk or voices in a crowd? Curr Opin Plant Biol 5:26–32
Fuller HJ (1949) Photoperiodic responses of Chenopodium quinoa Willd. and Amaranthus caudatus L. Am J Bot 36:175–180
Galwey NW (1993) The potential of quinoa as a multipurpose crop for agricultural diversification: a review. Ind Crop Prod 1:101–106
Gibson SI (2003) Sugar and phytohormone response pathways: navigating a signalling network. J Exp Biol 55:253–264
Gibson SI (2005) Control of plant development and gene expression sugar signalling. Curr Opin Plant Biol 8:93–102
González JA, Rosa M, Parrado MF, Hilal M, Prado FE (2009) Morphological and physiological responses of two varieties of a highland species (Chenopodium quinoa Willd.) growing under near-ambient and strongly reduced solar UV-B in a lowland location. J Photoc Photobiol B 96:144–151
Haussmann BIG, Rattunde HF, Weltzien-Rattunde E, Traoré PSC, vom Brocke K, Parzies HK (2012) Breeding strategies for adaptation of pearl millet and sorghum to climate variability and change in West Africa. J Agron Crop Sci 198:327–339
Islam N, Patil GG, Gislerød HR (2004) Effect of photoperiod and light integral on flowering and growth of Eustoma grandiflorum (Raf.). Shinn Scientia Hortic 103:441–451
Jacobsen S-E (2003) The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food Rev Int 19(1&2):167–177
Jacobsen S-E, Stølen O (1993) Quinoa-morphology and phenology and prospects for its production as a new crop in Europe. Eur J Agron 2:19–29
Jacobsen S-E, Mujica A, Jensen CR (2003) The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Rev Intern 19:99–109
James AT, Lawn RJ (2011) Application of physiological understanding in soybean improvement. II. Broadening phenological adaptation across regions and sowing dates. Crop Pasture Sci 62:12–24
Lawn RJ, James AT (2011) Application of physiological understanding in soybean improvement. I. Understanding phonological constraints to adaptation and yield potential. Crop Pasture Sci 62:1–11
Li W, Song Z, Emery RJ, Chinnappa CC (2011) Effects of day-length, light quality and ethylene on phytochrome B expression during stem elongation in Stellaria longpipes. Plant Growth Regul 63:291–300
Liu F, Jensen CR, Andersen MN (2004) Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: its implication in altering pod set. Field Crop Res 86:1–13
Liu F, Jensen CR, Andersen MN (2005) A review of drought adaptation in crop plants: changes in vegetative and reproductive physiology induced by ABA-based chemical signals. Aust J Agric Res 56:1245–1252
Nan R, Carman JG, Salisbury FB (2002) Water stress, CO2 and photoperiod influence hormone levels in wheat. J Plant Physiol 159:307–312
Pourtau N, Marès M, Purdy S, Quentin N, Ruël A, Wingler A (2004) Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta 219:765–772
Ramel F, Sulmon C, Gouesbet G, Couée I (2009) Natural variation reveals relationships between pre-stress carbohydrate nutritional status and subsequent responses to xenobiotic and oxidative stress in Arabidopsis thaliana. Ann Bot 104:1323–1337
Roberts EH, Summerfield RJ (1987) Measurement and prediction of flowering in annual crops. In: Atherton JG (ed) Manipulation of flowering. Butterworths, London, pp 17–50
Rodríguez-Calcerrada J, Reich PB, Rosenqvist E, Pardos JA, Cano FJ, Aranda I (2008) Leaf physiological versus morphological acclimation to high-light exposure at different stages of foliar development in oak. Tree Physiol 28:761–771
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Seung D, Risopatron JPM, Jones BJ, Marc J (2011) Circadian clock-dependent gating in ABA signalling networks. Protoplasma. doi:10.1007/s00709-011-0304
Weiss D, Ori N (2007) Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol 144:1240–1246
Wentworth M, Murchie EH, Gray JE, Villegas D, Pastenes C, Pinto M, Horton P (2006) Differential adaptation of two varieties of common bean to abiotic stress. J Exp Bot 57:699–709
Wingler A, Purdy S, MacLean JA, Pourtau N (2006) The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot 57:391–399
Wright GC, Nageswara RC, Basu MS (1996) A physiological approach to the understanding of genotype by environment interactions—a case study on improvement of drought adaptation in groundnut. In: Cooper M, Hammer GL (eds) Plant adaptation and crop improvement. CAB International/ICRISAT & IRRI, Wallingford, pp 365–381
Zeevaart JAD (1971) (+)−Abscisic acid content of spinach in relation to photoperiod and water stress. Plant Physiol 48:86–90
Acknowledgments
We would like to gratefully acknowledge the invaluable technical support from Jens Bertelsen and Lene Korsholm Jørgensen, as well as the financial support received from the Faculty of Science, University of Copenhagen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bendevis, M.A., Sun, Y., Shabala, S. et al. Differentiation of Photoperiod-Induced ABA and Soluble Sugar Responses of Two Quinoa (Chenopodium quinoa Willd.) Cultivars. J Plant Growth Regul 33, 562–570 (2014). https://doi.org/10.1007/s00344-013-9406-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-013-9406-9