Abstract
The drought tolerance of Salicornia brachiata seedlings was assessed by monitoring growth, nutrient uptake, electrolyte leakage, lipid peroxidation, and biochemical responses under drought conditions simulated with 0, 10, 20, and 30 % polyethylene glycol (PEG 6000). After 7 days of drought induction, plants were harvested for measurement of various parameters. The biomass decreased and the plant height remained unchanged with PEG treatment. The total plant water content (TWC%) decreased by 11 % at the highest concentration of PEG (30 %). The electrolyte leakage and lipid peroxidation of shoots increased by 17 and 5 %, respectively, in 30 % PEG-treated plants. K+ and Ca2+ contents of shoots increased in a dose-dependent manner. However, in roots K+ content decreased and Ca2+ content remained unaffected by PEG treatment. Mg2+ content increased at high concentrations of PEG (20–30 %) in shoots and decreased at the highest concentration of PEG (30 %) in roots. Total free amino acids, proline, and polyphenol contents increased progressively with increase in severity of the drought stress. Total sugar content and reducing sugar content increased in 10 and 20 % PEG-treated plants and decreased in 30 % PEG-treated plants. Our results suggest that proline and other free amino acids, sugars, and polyphenols are the main compatible solutes in S. brachiata for maintenance of osmotic balance, protection of cellular macromolecules, detoxification of the cells, and scavenging of free radicals under drought stress. A greater accumulation of compatible solutes also facilitates the maintenance of nutrient uptake and adequate tissue water status and protection of membranes under drought conditions in S. brachiata. The results from the present study suggest that S. brachiata can be used for restoration of arid and semiarid lands of coastal ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drought or water deficit stress is the major environmental factor that severely affects plant productivity in various regions of the world. It has been reported that less than 10 % of the world’s arable lands may be free of major environmental stresses, with drought and salinity stresses being the most widespread (Ashraf and Foolad 2007). Drought stress induces cellular hyperosmolarity and ion disequilibrium in plants. To cope with drought stress, several physiological and biochemical alterations take place in plants. These changes are aimed at countering water loss within the plant cell by reducing stomatal opening and maintenance of photosynthesis. When plants experience drought or salt stress, overproduction of low-molecular-weight compatible solutes takes place in plant cells (Hasegawa and others 2000; Serraj and Sinclair 2002; Ashraf and Foolad 2007). These solutes include proline, sugars, polyols, trehalose, and quaternary ammonium compounds (QACs) such as glycine betaine, alanine betaine, proline betaine, choline O-sulfate, hydroxyproline betaine, and pipecolate betaine (Rhodes and Hanson 1993; Hare and others 1998; Ashraf and Foolad 2007). The compatible solutes are low-molecular-weight, highly soluble compounds that are usually nontoxic at high cellular concentrations (Hasegawa and others 2000; Parida and Das 2005; Ashraf and Foolad 2007). The accumulation of these solutes is proportional to the change in external osmolarity and within species-specific limits (Hasegawa and others 2000). The usual functions of compatible solutes are protection of cellular structures and osmotic balance supporting continued water influx or reduced efflux (Hasegawa and others 2000; Parida and Das 2005). Some of these compatible solutes also protect cellular components from dehydration injury and they are commonly referred to as osmoprotectants (Ashraf and Foolad 2007).
In addition to the synthesis of these osmolytic compounds, specific proteins and translatable mRNA are induced and upregulated by drought and salinity stresses (Wang and others 2009; Balsemão-Pires and others 2011). In addition to the low-molecular-weight organic osmolytes, some inorganic osmolytes also have vital functions in growth, metabolism, and water homeostasis of plants under water-deficit conditions (Loutfy and others 2012). It has been reported that K+ has several functions such as regulation of stomatal opening, osmotic balance, charge balance, protein biosynthesis, maintenance of turgor pressure, and reduction of transpiration under drought stress (Marschner 1995; Loutfy and others 2012). Calcium also affects growth and metabolism of plants under stress conditions by controlling several physiological processes of plants such as absorption and translocation of water and solute, stomatal control, respiration, transpiration, cell division, and cell wall synthesis (McLaughlin and Wimmer 1999; Loutfy and others 2012). Besides K+ and Ca2+, other important elements transported by roots are utilized directly as inorganic osmolytes for reducing water loss in plants under drought stress or to indirectly control the biosynthesis of potential organic osmolytes (Loutfy and others 2012).
Salicornia brachiata Roxb. is a stem-succulent halophytic plant that belongs to the family Chenopodiaceae and grows in salt marshes. In saline environments, halophytes frequently experience salt and drought stresses. The salt tolerance of Salicornia has been studied previously (Wang and others 2009; Parida and Jha 2010). In our previous study, S. brachiata grew optimally at a salinity level of 200 mM NaCl under hydroponic culture, and at higher salinity levels up to 600 mM, plant growth was significantly decreased but without any visible symptoms of wilting (Parida and Jha 2010). Salicornia brachiata counteracts the ionic stress resulting from high concentrations of potentially toxic salt ions within plant cells by elevated levels of some antioxidative enzymes (Parida and Jha 2010). Salicornia grows in an environment that is physiologically dry as a result of limited water absorption caused by salt-induced osmotic stress. Therefore, understanding the drought tolerance capacity of S. brachiata is imperative. The drought tolerance mechanism of this plant species has not yet been studied. To address the drought tolerance strategies in S. brachiata, seedlings were grown under hydroponic culture and the effects of PEG-mediated drought stress on growth, shoot and root mineral contents, electrolyte leakage, lipid peroxidation, photosynthetic pigments, and accumulation of organic-compatible solutes were evaluated. To our knowledge, this is the first report on the physiological and biochemical responses to PEG-mediated drought stress in the extreme halophyte S. brachiata.
Materials and Methods
Plant Materials, Growth Conditions, and Stress Treatment
Seeds of S. brachiata Roxb. collected from salt marshes in Bhavnagar, Gujarat, India (latitude 21°35′N and longitude 72°16′E) were sown in plastic pots filled with soil:sand:peat (2:1:1). Seedlings were raised in the experimental greenhouse under nonsaline conditions as described in our previous study (Parida and Jha 2010). One-month-old healthy seedlings of uniform size were selected for hydroponic culture in Hoagland’s nutrient medium containing 200 mM NaCl supplemented with PEG 6000 (0, 10, 20, and 30 % w/v). The hydroponic cultures were maintained in a plant growth chamber (Model-1000 TLH, JEIO Tech Co. Ltd., Seoul, South Korea) under a photoperiod of 14 h at light intensities of 500 μmol m−2 s−1 and at 25 ± 2 °C room temperature and 60 % relative humidity. The cultures were continuously aerated with an air bubbler. The nutrient solution was replaced with freshly prepared solution at 3 day intervals. The shoot and root samples were harvested after 7 days of treatment for measurement of various parameters.
Measurement of Growth Parameters
Plant height and fresh and dry weights of shoots and roots of eight seedlings from each treatment were measured after 7 days of treatment. For measurement of fresh and dry weights, shoots and roots from control and PEG-treated plants were excised and the fresh weight was recorded immediately. Afterward, these plant parts were wrapped in preweighed aluminum sheets, kept in an incubator at 70 °C for 48 h, and cooled in a desiccator before the dry weight was recorded. Total plant water content (TWC) was calculated as follows: TWC% = [(FW − DW)/FW] × 100, where FW is the fresh weight of shoot and root and DW is dry weight of shoot and root.
Electrolyte Leakage
Shoot samples from control and PEG-treated plants were excised into 1 cm-long pieces (1 g of fresh weight in total for each treatment), washed in sterile water, and introduced into falcon tubes containing 10 ml of deionized water. After gently shaking in a rotary shaker (120×g) at room temperature for 4 h, the electrical conductivity of the bathing solution (EC1) was measured with a conductivity meter (Seven Easy, Mettler Toledo, Switzerland). Then, the solution containing shoot samples was boiled at 99 °C for 20 min until the destruction of membrane integrity, leading to leakage of the entire electrolyte from cells. After cooling to room temperature, the electrical conductivity of the bathing solution was measured (EC2). Electrolyte leakage was calculated as follows: Electrolyte leakage (%) = (EC1/EC2) × 100.
Lipid Peroxidation
The extent of lipid peroxidation was estimated by determining the concentration of malondialdehyde (MDA) produced by the thiobarbituric acid (TBA) reaction following the method of Draper and Hardley (1990). Shoot material (0.5 g) was homogenized in 2 ml of 0.1 % (w/v) TCA solution. The homogenate was centrifuged at 15,000×g for 10 min, and 1 ml of the supernatant was added to 4 ml of 0.5 % (w/v) TBA in 20 % (w/v) TCA. The mixture was incubated at 90 °C for 30 min; the reaction was stopped by placing the reaction tubes in an ice water bath. Samples were centrifuged at 10,000×g for 5 min and the absorbance of the supernatant was read at 532 nm. The value for nonspecific absorption at 600 nm was subtracted. The concentration of MDA was calculated from the extinction coefficient of 155 mM−1 cm−1.
Estimation of Ion Contents
The shoot and root samples were dried in an oven at 70 °C for 48 h for analysis of ions. After drying, preweighed shoot and root samples were homogenized and placed in a 100 ml volumetric flask. The flasks containing samples were placed on a hot plate after adding a 10 ml mixture of HNO3 and HClO4 (9:4) in a digestion chamber and digested until the production of red NO2 fumes ceased. The contents were further evaporated until the volume was reduced to 3–5 ml. Completion of digestion was confirmed when the liquid became colorless. After cooling the volumetric flask, 20 ml of deionized water was added, the volume was made up to 100 ml, and the solution was filtered through Whatman No. 1 filter paper. Aliquots of this solution were used for the determination of Na+, K+, Ca2+, Mg2+, Mn2+, Zn2+, Cu2+, and Fe2+ content of shoots and roots by inductively coupled plasma atomic absorption spectrometry (Optima 2000DV, PerkinElmer, Waltham, MA, USA).
Estimation of Photosynthetic Pigments
Fresh shoots (0.2 g) were thoroughly homogenized in chilled 100 % N,N-dimethylformamide (DMF) with a mortar and pestle in the dark at 4 °C; then the homogenate was centrifuged at 10,000×g for 10 min. The supernatants were collected, and absorptions at 664.5, 647, and 461 nm were recorded using a UV–Visible spectrophotometer (SpectramaxPlus 384, Molecular Devices, Sunnyvale, CA, USA). Chlorophyll a (Chl a), chlorophyll b (Chl b), and total chlorophyll were estimated using the equations of Inskeep and Bloom (1985). Carotenoids were estimated using the equation of Chamovitz and others (1993).
Estimation of Total Free Amino Acids
Total free amino acids were extracted and determined following the methods described earlier by Parida and others (2007). The shoot (0.5 g) was homogenized in 80 % ethanol with a pestle and mortar. The homogenate was centrifuged at 5,000×g for 10 min and the supernatant was taken. The extraction was repeated four to five times and the supernatants were combined. An appropriate volume (5–10 ml) of this ethanolic extract was evaporated to dryness on a boiling water bath and the residue was dissolved in 5 ml of 0.2 M citrate buffer (pH 5.0). The above sample (2 ml) was placed in a test tube and 1 ml of ninhydrin reagent (4 % ninhydrin in methyl cellosolve and 0.2 M acetate buffer in the ratio of 1:1) was added to it. The samples were boiled for 20 min and cooled; the volume was made up to 10 ml with distilled water. Absorbance was recorded at 570 nm. Total free amino acids were calculated from a standard curve prepared against glycine (0–100 μg).
Estimation of Proline
After 7 days of PEG treatment, shoot samples were harvested for extraction and estimation of proline. The proline was extracted using 3 % sulfosalicylic acid and estimated following the method of Ringel and others (2003) using ninhydrin reagent. The absorbance was measured at 520 nm.
Extraction and Estimation of Total Soluble Sugar, Reducing Sugar, and Starch
Total soluble sugar, reducing sugar, and starch content were extracted and estimated according to the procedures described by Parida and others (2007). For extraction of total soluble sugar and reducing sugar as well as starch, 1 g of shoot tissue was homogenized in 80 % ethanol, refluxed for 15 min in a water bath at 70 °C, and centrifuged at 5,000×g for 10 min. The pellet was re-extracted twice with 80 % ethanol and the supernatants were pooled. The supernatant was used for estimation of total soluble sugar and reducing sugar. The pellet left after extraction of the soluble sugar was solubilized in 52 % perchloric acid for determination of starch. Total soluble sugar and starch were estimated by the anthrone-sulfuric acid method using 0.2 % anthrone in concentrated H2SO4 as reagent. Spectrophotometric readings were taken at 630 nm. A standard curve was plotted with 0–100 mg of glucose. The starch concentration was determined by multiplying the obtained value by 0.9 for conversion of the glucose value to starch.
Reducing sugar was estimated following the alkaline copper method as described by Parida and others (2007) using arsenomolybdate reagent. Absorbance was recorded at 510 nm and reducing sugar content was determined from a standard curve prepared against pure glucose (0–50 μg).
Estimation of Total Polyphenol
Total polyphenol was determined according to the procedures of Chandler and Dodds (1983). Fresh shoots (0.5 g) were homogenized in 5 ml of 80 % ethanol using a chilled pestle and mortar with subsequent centrifugation at 10,000×g for 20 min. The supernatant was preserved and residue re-extracted with 2.5 ml of 80 % ethanol and centrifuged, and the supernatants were pooled and evaporated to dryness. The residue was dissolved in 5 ml of distilled water. In a test tube, 3 ml aliquots were taken to which 0.5 ml of Folin–Ciocalteau’s reagent (1 N) was added and kept for 3 min. Then, 2 ml of 20 % freshly prepared Na2CO3 solution was added to each tube and mixed thoroughly. The solution was boiled in a water bath for exactly 1 min, cooled, and then the absorbance was measured at 650 nm against a reagent as a blank. A standard curve was prepared using 10–100 μg of catechol (Sigma). From the standard curve, the concentrations of phenols in the unknown samples were calculated.
All the spectrophotometric analyses were performed using a UV–Visible spectrophotometer (SpectramaxPlus 384) and Soft-Max Pro v5.2 software (Molecular Devices) at end point mode.
Statistical Analysis
All the experiments were conducted with a minimum of three replicates and results were expressed as mean ± standard deviation (SD). All data were subjected to one-way analysis of variance (ANOVA) and Duncan’s multiple-range test (P ≤ 0.05) using the Sigma Plot v12.0 statistical software (Systat Software Inc., Chicago, IL, USA).
Results
Effects of PEG-Mediated Drought Stress on Growth
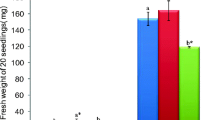
The effects of PEG-mediated drought stress on growth were assessed by plant height, fresh and dry weights of shoots and roots, shoot and root water content, and total plant water content (TWC%). The shoots of S. brachiata became slightly flaccid within 7 days of drought induction in a dose-dependent manner by PEG treatment (Fig. 1). There were no significant differences in plant height observed with respect to treatment (Table 1; Fig. 1). However, fresh and dry weights of shoots and roots decreased gradually with increase in PEG concentration. After 7 days of drought, shoot fresh weight, root fresh weight, shoot dry weight, and root dry weight decreased by 13, 72, 65, and 45 %, respectively, in 30 % PEG-treated plants compared to control (Table 1). However, the water content of shoots and roots and total plant water content were adequately maintained in S. brachiata seedlings exposed to PEG-induced drought stress. After 7 days of treatment, shoot water content, root water content, and total plant water content (TWC) decreased slightly by 10, 16, and 11 % in 30 % PEG-treated plants compared to control (Table 1).
Electrolyte Leakage and Lipid Peroxidation
Electrolyte leakage in shoots of S. brachiata increased by 21, 9, and 17 %, respectively, in 10, 20, and 30 % PEG-treated plants as compared to control (Fig. 2a). The level of lipid peroxidation was measured in terms of MDA content in shoots. In S. brachiata, the MDA content of shoots remained unchanged at low concentrations of PEG (0–10 %) and increased marginally by 5 % at high concentrations of PEG treatment (20–30 %) with respect to control (Fig. 2b).
Mineral Ion Content in Shoot and Root
After 7 days of PEG treatment, K+ and Ca2+ contents of shoots of S. brachiata increased gradually with an increase in PEG concentration (Table 2). However, in roots, the K+ content decreased gradually with an increase in PEG concentration and the Ca2+ content remained unaffected by PEG treatment. The Mg2+ content of shoots remained unchanged at low concentrations of PEG (0–10 %) and increased at high concentrations of PEG (20–30 %). In contrast, the Mg2+ content of roots decreased significantly at highest concentration of PEG (30 %). The iron (Fe2+) content of shoots decreased progressively as a result of an increase in PEG concentration in growth medium and there was a concomitant increase in root Fe2+ content. Uptake of Zn2+ by shoots and roots showed a reverse trend compared to Fe2+, that is, an increased level of Zn2+ in shoots and a decreased level of Zn2+ in roots with increase in PEG concentration (Table 2). The Mn2+ content of shoots increased gradually at low concentrations of PEG treatment (0–20 %), whereas at 30 % PEG treatment, the Mn2+ content decreased to control levels. In roots, the Mn2+ content increased gradually at low concentrations of PEG treatment (0–20 %) and then decreased at 30 % PEG treatment. After 7 days of PEG treatment, there was no significant change in Cu2+ content of shoots, whereas the Cu2+ content of roots was elevated in PEG-treated plants (0–30 % PEG) compared to control (Table 2).
PEG-Mediated Drought Induced Changes in Photosynthetic Pigments
There was no significant change in Chl a, Chl b, or total chlorophyll content in 10 % PEG-treated plants compared to control (Fig. 3). However, Chl a, Chl b, and total chlorophyll content decreased approximately by 27 in 20 % PEG-treated plants (Fig. 3). In contrast, Chl a, Chl b, and total chlorophyll content increased by 13, 17, and 15 %, respectively, in 30 % PEG-treated plants (Fig. 3). Likewise, the total carotenoid content increased by 10.5 and 27 %, respectively, in plants treated with low (10 %) and high (30 %) concentrations of PEG and decreased by 18 % in plants treated with the moderate concentration of PEG (20 %, Fig. 3d). Compared to control, the Chl a/b ratio showed no significant differences at all concentrations of PEG treatment. After 7 days of PEG treatment, the Chl a/b ratios were 1.86 ± 0.04, 1.88 ± 0.05, 1.85 ± 0.02, and 1.79 ± 0.08 in control and 10, 20, and 30 % PEG-treated plants, respectively.
Changes in Total Free Amino Acids and Proline
In S. brachiata, both total free amino acids and proline levels increased significantly with PEG treatment. After 7 days of PEG treatment, total free amino acid levels increased progressively by 38, 106, and 80 %, respectively, in 10, 20, and 30 % PEG-treated plants compared to control (Fig. 4a). A dramatic increase in the proline content was observed in S. brachiata with PEG-induced drought stress. After 7 days of PEG treatment, the proline level increased from 1.34 μmol g−1 DW in control to 26.26, 27.15, and 36.78 μmol g−1 DW in 10, 20, and 30 % PEG-treated plants, respectively (Fig. 4b).
Effects of PEG-mediated drought stress on a total free amino acids, b proline, c total sugar, d reducing sugar, e starch, and f polyphenol content in shoots of S. brachiata. Values are the mean ± SD (n = 3). Different letters on the top of the error bars indicate significantly different means at P ≤ 0.05
Changes in Total Sugar, Reducing Sugar, and Starch Content
In S. brachiata, total sugar and reducing sugar contents increased significantly in 10 and 20 % PEG-treated plants and decreased at 30 % PEG treatment (Fig. 4c, d). After 7 days of PEG treatment, total sugar content increased by 17 and 18 %, respectively, in 10 and 20 % PEG-treated plants and decreased by 13 % in 30 % PEG-treated plants compared to control (Fig. 4c). Similarly, reducing sugar content increased by 21 and 31 %, respectively, in 10 and 20 % PEG-treated plants and decreased by 16 % at the highest concentration of PEG (30 %) treatment (Fig. 4d). In contrast to sugar, starch content showed a reverse trend by PEG treatment. After 7 days of PEG treatment, starch content increased by 7 % and 38 %, respectively, in 10 and 30 % PEG treated plants and decreased by 16 % in 20 % PEG treated plants compared to control (Fig. 4e).
Effects of PEG-Mediated Drought Stress on Total Polyphenol
In S. brachiata, total polyphenol content increased gradually with and increase in PEG concentration (Fig. 4f). After 7 days of PEG treatment, total polyphenol content increased by 46, 99, and 171 %, respectively, in 10, 20 and 30 % PEG-treated plants compared to control (Fig. 4f).
Discussion
In plants, drought stress is induced by several methods ranging from withdrawal of water to the use of chemicals such as polyethylene glycol (PEG) or mannitol (Kumar and others 2011). It has been well established that PEG-induced drought stress mimics withdrawal of water from plants (Perez-Alfocea and others 1993). It has been suggested that PEG can be successfully used to decrease the water potential of culture medium and also of plants as long as it does not enter the roots (Lawlor 1970). Because PEG is a neutral polymer and is highly soluble in water, it has been widely used to impose drought stress in plants (Zgallai and others 2005). It has also been reported that exposing the plant root system to PEG 6000 solution has no other toxic symptoms at the plant level (Emmerich and Hardegree 1990; Zgallai and others 2005; Kumar and others 2011). In the present investigation, S. brachiata seedlings were exposed to different levels of drought stress induced by applying PEG 6000 in the growth medium. As observed from the growth behavior of S. brachiata in Table 1, shoot as well as root growth was affected in a dose-dependent manner as a consequence of PEG-mediated drought stress within the short treatment duration of 7 days. Our results agree with earlier reports of growth inhibition by PEG-induced osmotic stress in Sesuvium portulacastrum (Slama and others 2007) and in tomato (Zgallai and others 2005). However, total plant water content (TWC) decreased by 11 % in S. brachiata as a result of PEG-induced drought stress, which suggests that in this plant shoot and root water status is little impaired by drought stress. Our results contrast with an earlier report of severe reduction in relative water content (RWC, ca. 50 %) in the halophyte S. portulacastrum (Slama and others 2007) by PEG-induced osmotic stress. In contrast with our results, a severe reduction in RWC (ca. 56 %) has also been reported in the drought-sensitive pigeon pea plant as a consequence of PEG-mediated drought stress (Kumar and others 2011). It has also been reported that water deficit caused by the application of PEG to the culture medium resulted in a more than twofold reduction in the water content of drought-sensitive spring wheat seedlings (Filek and others 2012). In concurrence with our results, Diaz-Lopez and others (2012) reported that plant water status is maintained adequately in the drought-tolerant plant Jatropha curcas under severe and mild drought stress. In our previous study, growth of S. brachiata seedlings was improved under mild salt stress (200 mM) and slightly affected by high salinity up to 600 mM (Parida and Jha 2010). Munns (2002) has suggested that plants showing drought tolerance would also exhibit salinity tolerance. Our results agree with Munns (2002) and suggest that S. brachiata is also a drought-tolerant plant. MDA, a product of lipid peroxidation in plants exposed to adverse environmental constraints including drought, is a useful indicator of free radical formation in tissue (Hernandez and Almansa 2002). As observed from our data on S. brachiata, the lipid peroxidation level and electrolyte leakage from shoot tissue increased slightly by 5 and 17 %, respectively, in 30 % PEG-treated plants compared to control. In contrast to our results, a higher increase in electrolyte leakage (about 28–42 %) and a twofold increase in MDA have been observed as a result of PEG-mediated drought stress in the drought-sensitive spring wheat genotypes (Filek and others 2012). These results indicate that the membrane integrity of S. brachiata is maintained and the plant is less affected by drought-induced oxidative damage. Our results contrast with those from earlier reports where increased levels of electrolyte leakage and lipid peroxidation during drought stress led to severe damage to membrane integrity in many plants (Guo and others 2006; Silva and others 2010).
The correlation between drought and mineral nutrition of plants is extremely multifarious and plant growth is adversely affected by drought-induced nutritional imbalances. K+ is usually considered a very important cationic osmolyte and Ca2+ stabilizes the membrane systems or affects the capability of biomembranes to selectively absorb some ions (Basu and others 2010). As seen from our data, there was a significant increase in the K+, Ca2+, and Mg2+ content of the shoots of S. brachiata, with a concomitant decrease in root K+ and Mg2+ content as a result of drought treatment. These results suggest that drought stress induces increased absorption and transportation of K+, Ca2+, and Mg2+ in S. brachiata. The drought-induced increase in K+ content suggests that this cation may have an important role as an inorganic osmolyte during drought stress in S. brachiata. The drought-induced increase in Ca2+ content indicates that there is a role for this cation in membrane protection during drought stress in S. brachiata. In agreement with our results, Diaz-Lopez and others (2012) observed an increase in K+ and Ca2+ content in the leaves of Jatropha and a decrease in these cations in the roots when under severe drought stress. Our results contrast with those of an earlier report in which there were decreased levels of K+, Ca2+, and Mg2+ in the leaves of the perennial halophyte Suaeda fruticosa under high salinity conditions (Khan and others 2000). It has been reported that Mg2+ concentrations modulate ionic currents across the chloroplast and the vacuolar membranes, thereby regulating ion balance in the cell and stomatal opening (Shaul 2002). Mg2+ has a significant role in photosynthesis. It is the central atom of the chlorophyll molecule, and fluctuations in its levels in the chloroplast regulate the activity of key photosynthetic enzymes, ultimately affecting photosynthesis in plants (Shaul 2002). The drought-induced increase in Mg2+ content of S. brachiata indicates that the photosynthetic apparatus of this plant may remain unaffected by drought stress. The drought-induced effects on micronutrients vary between plant species (Hu and Schmidhalter 2005; Chen and others 2011). In our experiment, drought induced a decrease in Fe2+ and an increase in Zn2+ levels in shoots, whereas the Mn2+ and Cu2+ content of both shoots and roots remained unaffected by drought stress in S. brachiata. In roots, Fe2+ levels increased and Zn2+ levels decreased as a consequence of drought stress. The concentrations of some micronutrients went up and others went down during drought stress in S. brachiata. This may be due to the effect of drought on the availability of micronutrients, competitive uptake, transport, or partitioning within the plant organs. The change in micronutrient levels in S. brachiata might be the adaptive responses of the plant to drought stress. The lack of interaction between Mn2+ and Cu2+ levels and drought stress indicates that these important cations are not disturbed by drought stress in S. brachiata.
Chlorophylls and carotenoids are important components of energy metabolism for almost all green plant systems. Plant metabolism is noticeably affected by significant alterations in chlorophyll and carotenoid levels (Li and others 2012). In S. brachiata Chl a, Chl b, and total chlorophyll increased in plants treated with the highest concentration of PEG (30 %), whereas the Chl a/b ratio remained unaffected by PEG-induced drought stress. It has been reported that Chl a, Chl b, and total chlorophyll content decrease and the Chl a/b ratio increases as a consequence of PEG-induced drought stress in pigeon pea (Kumar and others 2011) and maize (Mohammadkhani and Heidari 2007). The contrasting results in chlorophyll profiles between glycophytes like pigeon pea and maize and the halophyte Salicornia suggest that the halophytes and glycophytes have different adaptation mechanisms during drought stress. The increase in chlorophyll content in S. brachiata at the highest concentration of PEG treatment might possibly be due to the decrease in chlorophyllase activity and/or increased biosynthesis of chlorophyll (Iyengar and Reddy 1996). The unchanged level of the Chl a/b ratio in S. brachiata by drought induction suggests that the light-harvesting complexes of thylakoid membranes remain unaffected by short-term drought stress (Parida and others 2007). Stabilization and protection of the lipid phase of the thylakoid membrane involve carotenoids and they also quench the excited triplet state of chlorophyll and singlet oxygen (Foyer and Noctor 2009; Li and others 2012; Ramel and others 2012). The significant increase in carotenoid content in S. brachiata at the highest concentration of PEG treatment suggests the role of carotenoids as a scavenger of reactive oxygen species (ROS), like singlet oxygen, produced under drought conditions. There is substantial evidence for increased (Mohammadkhani and Heidari 2007) or decreased (Parida and others 2007; Loutfy and others 2012) carotenoid content under drought stress depending upon the plant species.
Various compatible solutes are accumulated in plants exposed to various types of stresses. Proline is the most abundant osmolyte not only in plants but also in bacteria (Yoshiba and others 1997; Parida and others 2007; Kumar and others 2011). A greater accumulation of proline in response to drought stress is well documented in many plants (Abdel-Nasser and Abdel-Aal 2002; Parida and others 2007; Slama and others 2007; Mostajeran and Rahimi-Eichi 2009; Kumar and others 2011). In S. brachiata, proline content increased rapidly under PEG-induced drought stress. This might be due to increased activity of enzymes involved in proline biosynthesis and/or decreased activity of proline-degrading enzymes (Parida and others 2008). The elevated level of proline in S. brachiata observed here in response to PEG-mediated drought stress also occurred when this plant was grown under high-salinity stress (Parida and Jha 2010). It appears that the increased accumulation of proline under drought and high salinity is an osmoregulatory process in S. brachiata. In addition to osmoregulation, the accumulation of proline during drought may have some other functions such as protection of enzymes and membrane stabilization (Van Rensburg and others 1993, Bandurska 2000; Parida and others 2007).
As seen from our data, there was also a significant increase in total free amino acid content in S. brachiata as a result of drought stress. This increase could be due to increased amino acid biosynthesis and/or decreased protein synthesis to a certain extent, as reported in Brassica napus (Good and Zaplachinski 1994) and wheat (Mattioni and others 1997), or it could be due to increased activity of protease (Parida and others 2007). Our results agree with those of several reports on a drought-induced increase in total free amino acid content in many plants (Bowne and others 2012; Djebbar and others 2012). Although there was a significant increase in free amino acids (ca. 1.8-fold) observed in S. brachiata under drought stress, there was also a rapid increase in proline content (ca. 28-fold) under the same stress condition. Thus, proline is considered the major compatible solute in S. brachiata under drought stress conditions. A greater accumulation of proline and other free amino acids permits the plants to counteract the effects of drought through osmotic adjustment and provides storage forms of nitrogen and carbon for future use when the plants recover from drought (Parida and others 2007).
The carbohydrate concentrations show variable responses to drought stress in different plant species. Generally, a decline in starch content in leaves with a concomitant increase in soluble sugars is common in many plant species exposed to drought stress (Abdel-Nasser and Abdel-Aal 2002; Parida and others 2007; Nemeskéri and others 2010; Diaz-Lopez and others 2012). The increased accumulation of sugars under drought stress suggests that sugars act as osmotic compounds and stabilize cell membranes (Parida and others 2007; Diaz-Lopez and others 2012). In contrast to the above reports, in S. brachiata starch content increased under severe drought stress (30 % PEG) accompanied by a decrease in total and reducing sugars. This increase in starch observed in S. brachiata under drought conditions could be due to the synthesis of starch during the CO2 assimilation process.
The polyphenols are regarded as powerful nonenzymatic ROS scavengers in plants (Matysik and others 2002; Leopoldini and others 2006; Yildiz-Aktas and others 2009). There is increasing evidence of the role of polyphenols in energy dissipation and ROS scavenging (Grace and Logan 2000; Edreva 2005; Yildiz-Aktas and others 2009). It has also been reported that polyphenols are used as substrates by the H2O2-scavenging enzyme peroxidase (Grace and Logan 2000; Edreva 2005). Our results demonstrated a drought-induced increase of the level of polyphenols in S. brachiata. In agreement with our results, there are several reports of increased levels of polyphenols in plants suffering from drought stress (Yaginuma and others 2002; Kirakosyan and others 2004; Parida and others 2007; Yildiz-Aktas and others 2009). An increased level of polyphenols in leaves is a common response to abiotic stresses, but the levels vary depending on plant species, tissue, plant stage, and severity and duration of stress (Yildiz-Aktas and others 2009).
Conclusions
In summary, the data presented in this work demonstrated that S. brachiata tolerates drought by maintaining water status even under severe drought conditions. The accumulation of some compatible solutes such as proline and other free amino acids was induced in S. brachiata under PEG-mediated drought stress. Osmotic regulation, protection of cellular macromolecules, nitrogen storage, pH maintenance, detoxification of the cells, and scavenging of free radicals are anticipated functions of free amino acid accumulation in S. brachiata. Polyphenols act as nonenzymatic ROS scavengers in S. brachiata in PEG-mediated drought. Increased levels of carotenoids in S. brachiata under severe drought conditions suggest that the function of carotenoids is scavenging of ROS produced by drought-induced oxidative stress. The induced accumulation of compatible solutes such as proline and nonenzymatic ROS scavengers such as polyphenols and carotenoids under drought conditions also has a role in protection of membrane integrity as evidenced from lower changes in electrolyte leakage and membrane lipid peroxidation in drought-stressed seedlings of S. brachiata. The slight reduction in growth parameters observed in S. brachiata suggests a higher energy cost to synthesize organic solutes for osmotic balance under stressful conditions, which seems to result in a growth penalty. The results from the present study suggest that S. brachiata can be used for restoration of arid and semiarid land, especially in coastal zones.
References
Abdel-Nasser LE, Abdel-Aal AE (2002) Effect of elevated CO2 and drought on proline metabolism and growth of safflower (Carthamus mareoticus L.) seedlings without improving water status. Pak J Biol Sci 5:523–528
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Balsemão-Pires E, Jaillais Y, Olson BJSC, Andrade LR, Umen JG, Chory J, Sachetto-Martins G (2011) The Arabidopsis translocator protein (AtTSPO) is regulated at multiple levels in response to salt stress and perturbations in tetrapyrrole metabolism. BMC Plant Biol 11:108
Bandurska H (2000) Does proline accumulated in leaves of water deficit stressed barley plants confine cell membrane injury? I. Free proline accumulation and membrane injury index in drought and osmotically stressed plants. Acta Physiol Plant 22:409–415
Basu S, Roychoudhury A, Saha PP, Sengupta DN (2010) Comparative analysis of some biochemical responses of three indica rice varieties during polyethylene glycol-mediated water stress exhibits distinct varietal differences. Acta Physiol Plant 32:551–563
Bowne JB, Erwin TA, Juttner J, Schnurbusch T, Langridge P, Bacic A, Roessner U (2012) Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol Plant 5(2):418–429
Chamovitz D, Sandmann G, Hirschberg J (1993) Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate limiting step in carotenoid biosynthesis. J Biol Chem 268:17348–17353
Chandler SF, Dodds JH (1983) The effect of phosphate, nitrogen and sucrose in the production of phenolics and solasidine in callus cultures of Solanum laciniatum. Plant Cell Rep 2:105–108
Chen W, Yao X, Cai K, Chen J (2011) Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol Trace Elem Res 142:67–76
Diaz-Lopez L, Gimeno V, Simon I, Martinez V, Rodriguez-Ortega WM, Gracia-Sanchez F (2012) Jatropha curcas seedlings show a water conservation strategy under drought conditions based on decreasing leaf growth and stomatal conductance. Agric Water Manag 105:48–56
Djebbar R, Rzigui T, Pétriacq P, Mauve C, Priault P, Fresneau C, Paepe MD, Florez-Sarasa I, Benhassaine-Kesri G, Streb P, Gakière B, Cornic G, Paepe RD (2012) Respiratory complex I deficiency induces drought tolerance by impacting leaf stomatal and hydraulic conductances. Planta 235:603–614
Draper HH, Hardley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Edreva A (2005) The importance of non-photosynthetic pigments and cinnamic acid derivatives in photoprotection. Agric Ecosyst Environ 106:135–146
Emmerich WE, Hardegree SP (1990) Polyethylene glycol solution contact effects on seed germination. Agron J 82:1103–1107
Filek M, Walas S, Mrowiec H, Rudolphy-Skórska E, Sieprawska A, Biesaga-Kościelniak J (2012) Membrane permeability and micro- and macroelement accumulation in spring wheat cultivars during the short-term effect of salinity- and PEG-induced water stress. Acta Physiol Plant 34:985–995
Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical Implications. In: Buchanan B, Dietz KJ, Pfannschmidt T (eds) Antioxidants and redox signaling, vol 11. Mary Ann Liebert, Inc., New Rochelle, pp 861–905
Good GA, Zaplachinski ST (1994) The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus. Physiol Plant 90:9–14
Grace SG, Logan BA (2000) Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos Trans R Soc Lond B 355:1499–1510
Guo Z, Ou W, Lu S, Zhong Q (2006) Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol Biochem 44:828–836
Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation under stress. Plant Cell Environ 21:535–553
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Hernandez JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115:251–257
Hu YC, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549
Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll a, b in N,N-dimethylformamide, 80% acetone. Plant Physiol 77:483–485
Iyengar ERR, Reddy MP (1996) Photosynthesis in high salt tolerant plants. In: Pesserkali M (ed) Handbook of photosynthesis. Marshal Dekar, Baton Rouge, pp 56–65
Khan MA, Ungar IA, Showalter AM (2000) The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte Suadea fruticosa (L.) Forssk. J Arid Environ 45:73–84
Kirakosyan A, Kaufman P, Warber S, Zick S, Aaronson K, Bolling S, Chang SC (2004) Applied environmental stresses to enhance the levels of polyphenolics in leaves of hawthorn plants. Physiol Plant 121:182–186
Kumar RR, Karajol K, Naik GR (2011) Effect of polyethylene glycol induced water stress on physiological and biochemical responses in pigeonpea (Cajanus cajan L. Millsp.). Recent Res Sci Technol 3:148–152
Lawlor DW (1970) Absorption of polyethylene glycols by plants and their effects on plant growth. New Phytol 69:501–513
Leopoldini M, Russo N, Chiodo S, Toscano M (2006) Iron chelation by the powerful antioxidant flavonoid quercetin. J Agric Food Chem 54:6343–6351
Li X, Zhang L, Li Y, Ma L, Bu N, Ma C (2012) Changes in photosynthesis, antioxidant enzymes and lipid peroxidation in soybean seedlings exposed to UV-B radiation and/or Cd. Plant Soil 352:377–387
Loutfy N, El-Tayeb MA, Hassanen AM, Moustafa MFM, Sakuma Y, Inouhe M (2012) Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). J Plant Res 125:173–184
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Mattioni C, Lacerenze NG, Troccoli A, DeLeonardis AM, DiFonzo N (1997) Water and salt stress-induced alterations in proline metabolism of Triticum durum seedlings. Physiol Plant 101:787–792
Matysik J, Bhalu B, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
McLaughlin SB, Wimmer R (1999) Calcium physiology and terrestrial ecosystem processes. New Phytol 142:373–417
Mohammadkhani N, Heidari R (2007) Effects of water stress on respiration, photosynthetic pigments and water content in two maize cultivars. Pak J Biol Sci 22:4022–4028
Mostajeran A, Rahimi-Eichi V (2009) Effects of drought on growth and yield of rice (Oryza sativa L) cultivars and accumulation of proline and soluble sugars in sheath and blades of their different age leaves. Am Eurasian J Agric Environ Sci 5:264–272
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Nemeskéri E, Sárdi E, Remenyik J, Köszegi B, Nagy P (2010) Study of the defensive mechanism against drought in French bean (Phaseolus vulgaris L.) varieties. Acta Physiol Plant 32:1125–1134
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Parida AK, Jha B (2010) Antioxidative defense potential to salinity in the euhalophyte Salicornia brachiata. J Plant Growth Regul 29:137–147
Parida AK, Dasgaonkar VS, Phalak MS, Umalkar GV, Aurangabadkar LP (2007) Alterations in photosynthetic pigments, protein, and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotechnol Rep 1:37–48
Parida AK, Dasgaonkar VS, Phalak MS, Aurangabadkar LP (2008) Differential responses of the enzymes involved in proline biosynthesis and degradation in cotton genotypes during drought stress and recovery. Acta Physiol Plant 30:619–627
Perez-Alfocea F, Estan MT, Caro M, Guerrier G (1993) Osmotic adjustment in Lycopersicon esculentum and L. penneli under NaCl and polyethylene glycol 6000 iso-osmotic stress. Physiol Plant 87:493–498
Ramel F, Birtic S, Cuine S, Triantaphylides C, Ravanat JL, Havaux M (2012) Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol 158:1267–1278
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary ammonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44:357–384
Ringel C, Siebert S, Wienhaus O (2003) Photometric estimation of proline in quartz microplates: remarks on specificity. Anal Biochem 313:167–169
Serraj R, Sinclair TR (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ 25:333–341
Shaul O (2002) Magnesium transport and function in plants: the tip of the iceberg. Biometals 15:309–323
Silva EN, Ferreira-Silva SL, Fontenele A, Ribeiro RV, Viégas RA, Silveira JAG (2010) Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J Plant Physiol 167:1157–1164
Slama I, Ghanaya T, Hessini K, Messedi D, Savoure A, Abdelly C (2007) Comparative study of the effects mannitol and PEG osmotic stress on growth and solute accumulation in Sesuvium portulacstrum. Environ Exp Bot 61:10–17
Van Rensburg L, Kruger GHJ, Kruger H (1993) Proline accumulation as drought-tolerance selection criterion: its relationship to membrane integrity and chloroplast ultrastructure in Nicotiana tabacum L. J Plant Physiol 141:188–194
Wang X, Fan P, Song H, Chen X, Li X, Li Y (2009) Comparative proteomic analysis of differentially expressed proteins in shoots of Salicornia europaea under different salinity. J Proteome Res 8:3331–3345
Yaginuma S, Shiraishi T, Ohya H, Igarashi K (2002) Polyphenol increases in safflower and cucumber seedlings exposed to strong visible light with limited water. Biosci Biotechnol Biochem 66:65–72
Yildiz-Aktas L, Dagnon S, Gurel A, Gesheva E, Edreva A (2009) Drought tolerance in cotton: involvement of non-enzymatic ROS-scavenging compounds. J Agron Crop Sci 195:247–253
Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozki K, Shinozaki K (1997) Regulation of levels of proline as an osmolytes in plants under water stress. Plant Cell Physiol 38(10):1095–1102
Zgallai H, Steppe K, Lemeur R (2005) Photosynthetic, physiological and biochemical responses of tomato plants to polyethylene glycol-induced water deficit. J Integr Plant Biol 47:1470–1478
Acknowledgments
The financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi, India (Net Work Project-20), is gratefully acknowledged. The help of Sonal Mangukiya of Analytical Science Discipline, CSIR-CSMCRI, in running the samples for ion analysis by ICP-AAS is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parida, A.K., Jha, B. Physiological and Biochemical Responses Reveal the Drought Tolerance Efficacy of the Halophyte Salicornia brachiata . J Plant Growth Regul 32, 342–352 (2013). https://doi.org/10.1007/s00344-012-9303-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-012-9303-7