Abstract
Mature xerophytes access groundwater and minimize the risk of water and nutrient deficits in arid environments. However, how their young seedlings respond to the availability of water and nutrients before they reach groundwater is largely unknown. We investigated the effects of different drought regimes (controlled, medium-drought (MD), and severe-drought (SD)] and nitrogen (N; with or without) addition on biomass and physio-biochemical responses in Alhagi sparsifolia seedlings. Both drought stresses significantly increased superoxide dismutase (O2•−), hydrogen peroxide (H2O2), malondialdehyde, and oxidized-glutathione in leaves and roots, thereby impairing growth and metabolism. Furthermore, there is a significant accumulation of fructose and glucose, but lower sucrose and starch, possibly due to higher sucrose synthase, α-amylase, β-amylase and hexokinase but lower sucrose phosphate synthase and fructokinase. Drought-stressed seedlings also displayed higher abscisic and, jasmonic acids, strigolactones, glucose-6-phosphate dehydrogenase (G-6-PDH), phosphoenolpyruvate carboxylase, O2•−-H2O2-scavenging enzymes, but lower gibberellin, cytokinin, and indole-acetic acid. However, N-addition quantifies the productivity of drought-stressed seedlings by improving the leaf relative water content (LRWC), biomass, chlorophyll-a, sucrose-synthesizing enzymes (SPP and SPS), and hormones. It also increased the G-6-PDH in stressed seedlings to satisfy the need for NADPH and reduced the sucrose and starch degrading enzymes, leading to higher starch and sucrose levels. Upregulation of O2•−-H2O2 -scavenging enzymes under N-supply reduced lipid peroxidation and improved the ascorbate–glutathione redox states. N addition might be an effective strategy to improve drought resistance in A. sparsifolia seedlings to manage and conserve its vegetation in hyper-arid conditions in the face of future climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water and nutrients are essential for plant growth, survival, and distribution. In desert ecosystems, these two components are essential for the establishment and maintenance of phreatophytes (Arndt et al. 2004). For adaptation to arid environments, plants need rapid root elongation to obtain groundwater resources and minimize the risk of water and nutrient deficiencies (Canham et al. 2015). Seedlings, however, are more vulnerable to water deficits (McDowell et al. 2008), since their roots are small and cannot access the groundwater. This limits the spontaneous emergence of phreatophyte seedlings and inhibits the regeneration of natural vegetation (Tariq et al. 2022). Therefore, it is crucial to understand how phreatophyte seedlings respond to the availability of water and nutrients before reaching groundwater resources.
Drought conditions lead to a reduction in photosynthesis and carbon fixation, causing disturbances in carbohydrate metabolism and the distribution of dry mass (Chaves et al. 2002). Consequently, water-stressed plants deplete their stored reserves, including starch, due to increased respiration (Sami et al. 2016). This imbalance between the accumulation and utilization of photo-assimilates (Abid et al. 2016), affects the activity of carbohydrate-metabolizing enzymes, with stress triggering the upregulation of starch-degrading enzymes like alpha-amylase (AMY) and beta-amylase (BAM) to enhance soluble sugar production for osmotic balance (Du et al. 2020).
Furthermore, the enzyme soluble acidic (SAI) catalyzes sucrose hydrolysis into fructose and glucose (Li et al. 2002), with its low activity contributing to enhanced sucrose accumulation for osmoregulation in stressed leaves (Kaur et al. 2007). Studies, such as those conducted by Liu et al. (2004) on soybean and Xu et al. (2015a) on rice, demonstrate that drought stress can reduce SAI and sucrose synthase (SuSy) activity, respectively, resulting in increased sucrose accumulation to optimize osmotic regulation. Consequently, the allocation of organic carbon shifts towards moderating osmoregulation, leading to an increase in root-to-shoot ratios due to reduced carbon availability for shoot growth. Several studies have shown that plants under dehydration accumulate higher levels of fructose, glucose, sucrose, and raffinose compared to normal conditions (Fàbregas and Fernie 2019), aiding in the regulation of osmotic potential and mitigation of oxidative stress damages.
During periods of water shortage, plants tend to produce excessive reactive oxygen species (ROS), resulting in cellular damage to organelles and molecules (Zhang et al. 2020; Wahab et al. 2022). To counterbalance these detrimental effects, plants have evolved osmotic adjustment and antioxidant defense systems (Tariq et al. 2019a; Wahab et al. 2022). Enzymes such as peroxidase (POD), ascorbate peroxidase (APX), catalase (CAT), and superoxide dismutase (SOD) primarily eliminate ROS, enhancing stress resilience (Wahab et al. 2022). Furthermore, osmolytes like proline, glycine betaine, soluble proteins, and soluble sugars aid in maintaining cell turgidity, improving osmotic potential, enzyme activity, macromolecule protection, membrane stability, antioxidant mechanisms, and ROS detoxification, thereby promoting stress tolerance (Lukić et al. 2020; Ullah et al. 2022; Wahab et al. 2022). The diversion of assimilatory products to anti-stress mechanisms underlines why water deficit negatively impacts growth and biomass production.
Phytohormones play pivotal roles in normal and stressful conditions, orchestrating various physiological, biochemical, and molecular responses in plants, including drought tolerance (Iqbal et al. 2022; Ullah et al. 2022). Accordingly, drought increases abscisic acid (ABA) (Fleta-Soriano et al. 2015) and strigolactones (SLs) (Tariq et al. 2023) contribute significantly to drought tolerance. For instance, ABA regulates stress-responsive genes, osmolyte accumulation, dehydrin and LEA protein synthesis, and other stress-related proteins (Sreenivasulu et al. 2012). Drought-induced elevation in ABA levels contributes to the modulation of root growth and development, which is indispensable for ensuring sufficient water supply (Sharp et al. 2004). Moreover, Tariq et al. (2023) reported that strigolactones (SLs) modulate shoot and root architecture in nutrient-limited soil and facilitate P uptake by roots (Ruyter-Spira et al. 2011).
Furthermore, jasmonic acid (JA) and salicylic acid (SA) contribute to drought tolerance by regulating stomatal closure, ROS elimination, root growth, and enhancing cellular water conductivity (Iqbal et al. 2022). Salicylic acid (SA) accumulation has been reported to improve drought resistance (Miura and Tada 2014), by upregulating the antioxidant potential and protecting the photosynthetic apparatus from oxidative stress (Iqbal et al. 2022). Recent findings suggest that ethylene (ETH) stabilizes stress signals, enhancing plant adaptation to drought (Nazir et al. 2024). Moreover, these hormones, along with gibberellic acid (GA), indole acetic acid (IAA), and cytokinins (CTK), synergistically coordinate defense mechanisms (Iqbal et al. 2022; Rai et al. 2023) and promote growth and development, aiding plants in effectively coping with environmental stresses (Jiang and Asami 2018; Ullah et al. 2022).
Furthermore, the intricate interplay among hormones regulates the production of osmolytes, stomatal regulation, root growth, anti-oxidant potential, and other stress-responsive genes (Fleta-Soriano and Munné-Bosch 2016; Liu et al. 2022; Iqbal et al. 2022). Moreover, phytohormones also modulate photoassimilates, synthesis, distribution, and carbon balance in plants (Aksenova et al. 2012), with CK and ABA, for instance, regulating the source-sink relationship (Pospisilova et al. 2005). For example, CTK regulates growth and carbohydrate consumption and enhances starch biosynthesis by increasing the sink capacity (Ramawat and Merillon 2013). Thus, understanding the complex interactions among hormones and their regulation of antioxidant mechanisms, osmolyte production, and carbon balance is crucial for elucidating defense mechanisms under stress conditions.
A. sparsifolia Shap. (Camelthorn, family Fabaceae) is a perennial xerophytic shrub in central and eastern Asia. In China, this species is distributed throughout the Xinjiang Autonomous Region and Gansu, Inner Mongolia, and Qinghai provinces. In Xinjiang, it is primarily found in the Taklimakan Desert, which is hyperarid, saline, nutrient deficient, and water scarce. It has several ecological and social benefits including preventing desertification, dunes, and soil salinization and improving livelihoods (Tariq et al. 2022). Yet its abundance and habitat are threatened by rapid population growth, overgrazing, overharvesting, agricultural expansion, urbanization, and industrialization (Tariq et al. 2022, 2024). Its revegetation and restoration are thus urgently required. However, the availability of water is crucial for the growth and establishment of A. sparsifolia (Gui et al. 2013). Therefore, planting and establishing seedlings for revegetation and restoration in hyper-arid conditions are extremely challenging (Tariq et al. 2022). As a result, improving its seedling drought stress resilience is critical to its successful establishment.
N is vital for the growth, and overall function of plants. It plays various functions in the process of photosynthesis, respiration, storage, and transformation of photoassimilates, and many other activities (Vrede et al. 2004). A prolonged drought condition may severely hinder the mobility of N, resulting in a deficiency of N that ultimately limits tree growth (Mahieu et al. 2009). Trees are also impeded from metabolizing N by drought through disruption of enzyme activities associated with N metabolism. It’s a primary factor limiting the growth and productivity of trees and shrubs, particularly in arid regions where water scarcity further restricts their availability (Tariq et al. 2022). Clearly, this scenario illustrates a strong correlation between N availability and drought due to the severe restrictions imposed by drought on its accessibility and mineralization.
There is compelling evidence suggesting that the exogenous application of N plays a crucial role in enhancing the resilience of plants facing water deficit (Zhou et al. 2011; Tariq et al. 2019a; Zhang et al. 2020a, b) This improvement is attributed to enhancements in both enzymatic and non-enzymatic antioxidant systems, as well as regulation of photoassimilates and osmolytes (Tariq et al. 2019a; Li et al. 2020;). Additionally, N application facilitates the synthesis of proteins, and structural carbohydrates, leading to accelerated cell division and enhanced growth and biomass production (Huang et al. 2018). Moreover, the positive effects of N application on stress resistance and biomass production are associated with enhancements in hydraulic conductance (Xu et al. 2015b), N uptake and assimilation (Tariq et al. 2019a), cell wall elasticity, growth hormones, and increased meristematic tissues (Lawlor 2002).
In conditions of limited water availability, maintaining plant hydration is crucial for metabolic processes, and studies have indicated that leaf relative water content (LRWC) tends to increase following N addition, potentially due to the accumulation of osmolytes (Tariq et al. 2019a; Zhang et al. 2021a,b). The presence of N is indispensable for fundamental processes such as photosynthesis, respiration, and carbohydrate metabolism (Zhang et al. 2021a). There is a lack of comprehensive knowledge regarding how N addition affects sugar metabolism and its interplay with endogenous hormones and antioxidant mechanisms under conditions of water deficit. Furthermore, the majority of research has focused on non-woody plants, particularly crop species, with little attention given to understanding the effects of N application on drought resistance and metabolic alterations in shrubs like A. sparsifolia, which thrive in hyperarid, nutrient-deficient environments.
Previously, A. sparsifolia has been studied for its root’s transcriptomic analysis under drought (Wu et al. 2015), biomass and NP allocation patterns under drought, and N nutrition (Zhang et al. 2020). Plant adaptation is complex, so relying on limited indicators may be unreliable. It's crucial to assess multiple comprehensive indices to understand adaptation strategies. For instance, a plant's tolerance to environmental stress depends largely on its enzymatic and non-enzymatic antioxidant potential (Wahab et al. 2022), osmolytes production (Sami et al. 2016), and phytohormonal regulations (Fleta-Soriano et al. 2015; Fleta-Soriano and Munné-Bosch 2016; Neves et al. 2017; Iqbal et al. 2022). However, the physiological adaptation mechanisms of A. sparsifolia seedlings based on their sugar metabolism, phytohormones, and antioxidant potential have not yet been assessed under drought and N addition. Therefore, this study aimed to (a) determine the impact of drought on growth and biochemical changes in photosynthetic pigments, sugar metabolism, phytohormones, osmolytes, and antioxidant capacity in both leaves and roots of A. sparsifolia, and (b) decipher how N application affects these responses under water deficit.
2 Materials and Methods
2.1 Seedling Establishment
This study was conducted at the Cele National Station of Observation and Research for Desert-Grasslands Ecosystem at the southern fringe of the saline, nutrient-poor hyperarid Taklimakan Desert (37°00′N, 80°43′E). The area has a mean annual temperature of 11.9 °C, with extreme temperatures of 41.9 and −23.9 °C. The vegetation is sparse around oases, dominated by shrubs and sub-shrubs such as A. sparsifolia, Calligonum mongolicum, Karelinia caspica, and Tamarix ramosissima. The pot experiment was conducted in an outdoor nursery from May to September 2022. Healthy and viable seeds of A. sparsifolia were collected from the desert and planted in 42-L plastic pots (30 cm in diameter at the bottom, 35 cm in diameter at the top, 50 cm high, with a bottom hole) containing 38 kg of homogenized topsoil (0-30 cm) collected from around the study area. The soil was aeolian loamy sand with the following chemical properties (g kg−1): total N, 0.23; organic C, 2.99; total K, 23.11; total P, 0.60. Water was supplied to each pot (n = 1 seedling) on a three-day cycle to field capacity (18% w/w) during the first 30 days of the experiment.
2.2 Drought-Stress and N Application
One-month-old seedlings with uniform height were exposed to water deficit and N addition: (controlled, medium-drought (MD), and severe-drought (SD)] and N supplementation (0- and 4.0 gN⋅m−2 yr.−1). There were 12 replications of each treatment. The pots were maintained at a soil-relative water content (the ratio of soil water to water content at water-holding capacity) of 70–75% (well-watered, CK), 45–50% (medium drought), and 25–30% (severe drought) according to the following equation (Xu et al. 2009)
Wsoil is the current soil weight (soil + pot + water), Wpot is the weight of the empty pot, DWsoil is the weight of dry soil, and WFC is the weight of soil at field capacity (soil + pot + water).
We rotated all pots every week to minimize positional effects. Moreover, a canopy made of transparent plastic film was used to cover the pots when it rained. The N application (pure urea 64%N) was applied once per month to the upper surface after watering. Five months fresh plants were harvested and separated into roots and leaves, which were wrapped in tinfoil, immediately immersed in liquid N, and stored at − 80 °C for physiological analysis.
2.3 Measurements of Relative Water Content (LRWC), Height, and Biomass
An electronic balance was used to measure the fresh weight (FW) of fully expanded leaves collected from each pot. The leaves were then soaked in distilled water for four hours at 4 °C in the dark. To determine the turgid weight (TW), the samples were weighed and transferred to an oven at 70 °C for 24 h before measuring their dry weight (DW). According to the following equation, the LRWC values were determined for each sample:
Each plant was then split into above-ground (shoot) and below-ground (root), which were dried for 24 h at 70 °C before measurement of DW (g) using an electric balance.
2.4 Estimation of Concentrations of Photosynthetic Pigments
The concentrations of chlorophyll a (Chl a), and chlorophyll b (Chl b), in the fresh leaves were assessed using 80% acetone extracts following the method of Lichtenthaler and Wellburn (1983). We measured absorbances for Chl a, and Chl b at 663, and 646 respectively, using a U-1800 spectrophotometer (Hitachi, Tokyo, Japan).
2.5 Determination of Metabolites and Enzymes of Sugar Metabolism
The concentration of glucose and fructose was determined according to Johnson et al. (1964). Moreover, sucrose was determined using van Handel’s (1968) method. Citric acid was conducted using a standard method (Moellering and Gruber 1966). Starch was determined calorimetrically at 620 nm following a previous method (Hansen and Møller 1975).
The enzymatic activities of sugar metabolism were determined in leaf and roots extracts using appropriate ELISA kits following the manufacturer’s instructions (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China): SuSy (YJ302947), SPP (YJ550955), SPS (YJ302949), AMY (YJ190046), BAM (YJ190047), G6PDH (YJ513908), 6-PGDH (YJ241016), G6P (YJ550940), FrK (YJ364900), HK (YJ530492), PEPC (YJ224906) and Inv (YJ306942) (Sun et al. 2023).
2.6 Estimation of ROS and Malonaldehyde Concentrations
O2•– production rate was estimated by homogenizing samples (0.2 g) in phosphate buffer (pH 7.8) and centrifuging them at 5000 × g for 10 min. The reaction mixture consisting of 1 mL of supernatant, 0.1 mL of 10 mM hydroxylammonium chloride, and 0.9 mL of 65 mM phosphate buffer was used. A 20-min incubation at 25 °C was followed by the addition of 7 mM α-naphthylamine and 17 mM sulphanilamide. After adding ethyl ether, we centrifuged for five minutes at 1500 × g and measured the absorbance at 530 nm.
Hydrogen peroxide (H2O2) was determined by measuring the absorbance of the titanium-peroxide complex at 410 nm. The reaction mixture (supernatant: 1 mL, titanium reagent: 0.1 mL; ammonia: 0.2 mL), was centrifuged at 3000 × g for 10 min and washed with acetone and then centrifuged again at 10,000 × g for five minutes. In the next step, 1-M H2SO4 (3 mL) was added and the absorbance was read at 410 nm.
MDA was determined, following a standard method. Samples were homogenized with 5 ml of 1% trichloroacetic acid (TCA) and centrifuged at 5000 × g for 10 min. The supernatant was added with 205 TCA (4 mL, containing 0.5% thiobarbituric acid) and boiled for 30 min in a water bath at 95 °C. The reaction mixture was then cooled in an ice bath, and MDA was determined at reflectances of 450, 532, and 600 nm. MDA content was calculated using the following equation.
2.7 Determination of Reduced and Oxidized Ascorbate and Glutathione and Antioxidant Enzyme Activities
The concentrations of ascorbate–glutathione metabolites and enzymatic activities were determined by rinsing 1.0 g of fresh samples of leaves and roots, freezing in liquid N, grinding to a powder, and mixing with 10 mL of 0.1 mol L−1 phosphate-buffered saline (PBS) (pH 7.0). The extracts were then centrifuged at 10 000 at 4 °C for 20 min to obtain supernatants for identifying markers of oxidative stress using appropriate ELISA kits following the manufacturer’s instructions (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China): SOD (YJ203409), CAT (YJ203416), POD (YJ203419), PPO (YJ203490), GPX (YJ203440), APX (YJ320415), GR (YJ203471), MDHAR (YJ103677), DHA (YJ880342), DHAR (YJ803460), AsA (mlsh0046), DHAR (YJ880342), GSSG (YJ302944), and GSH (YJ299073) (Liu et al. 2020; Li et al. 2022).
2.8 Determination of Concentrations of Endogenous Phytohormones
ELISAs were used to evaluate the concentrations of phytohormones. Briefly, frozen foliar and root samples were ground into powders with liquid N and combined with 5 mL of 0.1 mol L−1 PBS (pH 7.0). The concentrations of IAA (YJ803465), ZR (YJ803411), ABA (YJ803413), SLs (YJ803429), GA (YJ803421) BR (YJ803463), CT (YJ803406GA), and SA (YJ803461) were determined using appropriate ELISA kits following the manufacturer’s instructions (Shanghai Enzyme-linked Biotechnology Co., Ltd. Shanghai, China), respectively (Wu et al. 2020).
3 Statistical Analysis
An analysis of variance (ANOVA) in SPSS (Chicago, IL, United States) was used to test treatment differences in biomass, photosynthetic pigments, sugar metabolism, and antioxidant mechanisms. Means were compared using Duncan’s multiple range tests (p > 0.05). The figure graphics were created using GraphPad Prism 8. OriginPro (Version 2024, OriginLab Corporation, Northampton, MA, USA) was used to perform Pearson correlation analyses of growth characteristics, photosynthetic pigments, sugar metabolism, phytohormones, and antioxidants. Principal component analysis (PCA) of standardized data collected using OriginPro (Version 2024, OriginLab Corporation, Northampton, Ma, USA) was used to determine the overall complexity of drought and N response and to identify traits showing similar trends. With the help of this analysis, we were able to investigate the physiological responses to stress and integrate all responses to the drought stress and N treatment in leaves and roots.
4 Results
4.1 Changes in Growth and Chlorophyll Concentration
Under MD, the seedling height, shoot dry weight root dry weight, and leaf relative water content (LRWC) were reduced by 27, 36, 19, and 15%, respectively. Under SD, the reductions were 47, 51, 32, and 24% respectively. In contrast, the root/shoot ratio (RSR) increased up to 1.34 under MD and 1.40-fold under SD stress.
However, N application considerably reduced the drastic effects of drought stress and improved LRWC, plant height, shoot dry weight, and root dry weight by 1.20, 1.29, 1.35, and 1.12-fold under MD and by 1.12, 1.08, 1.15, and 1.13-fold under SD stress. However, the N application reduced the RSR under MD and had no significant impact under SD stress (Table 1).
The concentration of Chl a and Chl b fell by 31, 22% under MD, and 28 and 47% under SD stress. The ratio of Chl a/Chl b reduced by 11% under MD stress but increased up to 1.4-fold under SD stress. However, the N application enhanced Chl a up to 13 and 1.20 folds under MD and SD stress but had little effect on Chl-b and the ratio of Chl-/Chl-b (Table 1).
4.2 Sugar Metabolism
Under MD, the leaf concentrations of glucose increased 1.45 fold, and fructose 120 fold while the root concentrations increased 1.13 and 1.10 folds, respectively. Under SD, the increases were 1.26 and 1.27 folds in the leaves but only fructose increased (1.37 fold) in the roots. Conversely, sucrose and starch decreased by 16 and 22% in the leaves and by 34 and 15% in the roots under MD stress. The SD stress reduced their concentrations by 35- and 34% in the leaves and by 30 and 22% in the root (Tables 2 and 3). Glucose-6-phosphate (G6P) and citric acid (CA) in the leaves increased by 1.40, and 1.27 folds under MD, and by 1.29, and 1.21 folds under SD stress. In roots, CA increased significantly under MD (1.19 fold) stress, and G-6-P showed little changes under both stress (Tables 2 and 3).
Moreover, sucrose synthase (SuSy), α-amylase (AMY), and β-amylase (BAM) increased in the leaves by 1.33, 1.28 and 1.23 folds under MD and by 1.27, 1.12 and 1.19 folds under SD stress. In root, their concentration increased by 1.32, 1.32, and 1.25 folds under MD stress. Also, SD stress increased SuSy and AMY (1.14 and 1.29-fold) in roots but had little effect on BAM. Sucrose phosphate phosphatase (SPP) decreased by 19 and 30% in the leaves and 5 and 9% in the roots under MD and SD stress (Tables 2 and 3). Sucrose phosphate phosphatase (SPP) showed little changes in the leaves under both stresses but decreased in the roots by 19% under SD stress. Moreover, glucose-6-phosphate dehydrogenase (G6PDH), and hexokinase (HK) increased by 1.11 and 1.18 folds in the leaves and by 1.24 and 1.21 folds in the roots under MD stress. Their activities increased by 1.12 and 1.23 folds in the leaves and by 1.12 1.24 folds in the roots under SD stress. Also, 6-phosphogluconate dehydrogenase (6PGDH) enhanced in the leaves under both MD (1.19 fold) and SD stress (1.09 fold) but showed little changes in the roots. During drought fructokinase (FRK), and invertase (INV) showed little changes in both leaves and roots (Tables 2 and 3).
In leaves, N application increased glucose, fructose, G-6-P, and ST by 1.15, 1.17, 1.10, and 1.06 folds under MD and sucrose by 1.25 and 1.36 folds under both MD and SD stress. In roots, CA, fructose, and sucrose increased by 1.03, 1.27, and 1.43 folds under MD stress, ST by 1.18 folds under SD, and glucose by 1.25 and 1.33 folds under both MD and SD stress.
N application increased PEPC by 1.20 and 1.33 folds and FRK by 1.68 and 1.80 folds in the leaves under MD and SD stress. Also, SPP enhanced by 1.17 and 1.09 folds SPS by 1.12 and 1.09 folds in the roots and G-6-PDH increased in the leaves by 1.16 and 1.22 folds and in the roots by 1.12 and 1.08 folds under MD and SD stress. SPS increased in the leaves by 1.14-fold under MD and HK in the roots by 1.05-fold under SD in N-supplied seedlings (Tables 2 and 3). Additionally, N supplementation had little effects on INV and 6-P-GDH in both the leaves and roots and on SPP and HK in the leaves and FRK in the roots. Conversely, N addition reduced AMY in the leaves by 18 and 13% and in the roots by 15 and 7% under MD and SD stress. Also, SuSy reduced by 15 and 9% and BAM by 15 and 17% in the leaves under MD and SD stress. In roots, SuSy reduced by 11% under SD and BAM by 28% under MD stress (Tables 2 and 3).
4.3 Oxidative Stress Indicators and Anti-Oxidant Enzymes
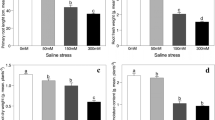
MDA, O2•−, and H2O2 increased by 1.58, 1.38, and 1.45 folds in the leaves, and by 1.41, 1.18, and 1.02 folds in the roots under MD stress. Also, their concentrations increased by 2.03, 1.55, and 1.56 folds in the leaves and, 1.64, 1.24, and 1.14 folds in the roots under SD stress (Fig. 1a-c). However, N application reduced MDA, O2•−, and H2O2 in the leaves by 20, 12, and 17% under MD stress, and by 22, 25, and 172% under SD stress. A similar pattern was observed in the roots for MDA and O2•−, but had little effect on H2O2 (Fig. 1a-c).
Changes in the concentration of (a) superoxide anion (b) hydrogen peroxide (c) malondialdehyde, and enzyme activity of (d) superoxide dismutase (e) catalase (f) peroxidases (g) glutathione peroxidase, and (h) polyphenol oxidase under drought stress and N addition. Bars represent means ± SD (n = 3). different letters indicate treatment differences at p < 0.05 (Duncan’s method). The green and brown bars represent leaves and root samples. – N and + N stand for nitrogen applied and non-applied treatments. WW: well-watered; MD: medium drought; severe drought
Leaves increased SOD by 1.21 and 1.18 folds, and CAT by 1.73 and 1.36 folds but reduced POD by 23 and 30% under MD and SD stress (Fig. 1d-f). GPX showed little changes under MD but increased by 1.09 fold under SD stress (Fig. 1g). Roots increased SOD, CAT, POD, and GPX under MD (1.34, 1.23, 1.06, and 1.24 folds) and SD stress (1.32, 1.39- and 1.17, and 1.34 folds). Conversely, PPO was reduced by 9 and 11% in the leaves and by 4 and 11% in the roots under MD and SD stress (Fig. 1h). N application increased CAT, POD, and SOD in leaves by 1.19, 1.19, and 1.26 folds under MD stress and by 1.28, 1.32, and 1.23 folds under SD stress. In roots, their activities increased by 1.21, 1.37, and 1.13 folds under MD stress. CAT and SOD increased by 1.24, and 1.11 folds under SD stress (Fig. 1d-f). However, N had little effect on GPX in the leaves and roots under both stress and PPO under MD stress. In root, N application increased PPO by 1.08 and 1.19 folds under both MD and SD stress (Fig. 1gh).
4.4 Responses of the Ascorbate–Glutathione Cycle to Drought Stress and N Addition
The concentrations of AsA decreased by 7 and 36% and GSH by 11- and 34% in the leaves under MD and SD stress. In roots, AsA under both stress and GSH under MD stress showed little changes. However, GSH reduced significantly in roots under SD stress (Fig. 2ab). Conversely, DHA and GSSG increased by 1.56 and 1.24 folds in the leaves and by 1.55 and 1.28 folds in the roots under MD stress. Also, their concentrations increased by 1.37 and 1.31 folds in the leaves and by 1.65 and 1.46 folds in the roots under SD stress (Fig. 2cd). Both MD and SD stress significantly increased APX, MDHAR in the leaves and roots, and GR in the roots (Fig. 3a-c). However, GR and DHAR were reduced in the leaves. In addition, SD stress reduced DHAR in the roots (Fig. 3d).
Changes in the concentration of (a) ascorbic acid, (b) glutathione, (c) dehydroascorbic acid (e) oxidized glutathione (e) ascorbic acid/dehydroascorbic acid ratio, and (f) glutathione/oxidized glutathione ratio under drought stress and N addition. Bars represent means ± SD (n = 3). different letters indicate treatment differences at p < 0.05 (Duncan’s method). The green and brown bars represent leaves and root samples. – N and + N stand for nitrogen applied and non-applied treatments. WW: well-watered; MD: medium drought; severe drought
Changes in the enzyme activity of (a) ascorbate peroxidase (b) monodehydroascorbate reductase (c) glutathione reductase and (d) dehydroascorbate reductase under drought stress and N addition. Bars represent means ± SD (n = 3). different letters indicate treatment differences at p < 0.05 (Duncan’s method). The green and brown bars represent leaves and root samples. – N and + N stand for nitrogen applied and non-applied treatments. WW: well-watered; MD: medium drought; severe drought
Compared to their non-treated peers, N application increased AsA by 1.19 and 1.32 folds in the leaves by 1.09 and 1.10 folds in the roots and GSH by 1.11 and 1.09 folds in the leaves under MD and SD stress. In roots, GSH increased under MD stress and showed little changes under SD stress. In contrast, N addition decreased DHA in the leaves by 16 and 6% and in the roots by 17 and 10% under MD and SD stress. Also, GSSG decreased by 17% in the leaves and by 12% in the roots under MD stress. N application significantly improved APX and DHAR in the leaves by 1.04 and 1.40 folds under MD, and by 1.27 and 1.32 folds under SD stress. In roots, their activities increased by 1.01 and 1.10 folds under MD, and by 1.18 and 1.19 folds under SD stress whereas GR increased in the leaves by 1.31 and 1.32 folds under both stresses. MDHAR increased in the leaves by 1.23 fold and in the roots by 1.33 folds and GR increased in the roots by 1.15 fold under MD stress. However, they showed little changes under SD stress supplied with N application (Fig. 3a-d).
4.5 Responses of Phytohormones Production to Drought Stress and N Addition
The concentrations of ABA, JA, SLs and increased significantly in both parts under MD and SD stress (Fig. 4a-c). Also, SA increased in the leaves under MD and SD and the roots under SD stress (Fig. 4d). Conversely, ZR and BR decreased in both parts under MD and SD stress (Fig. 4ef) and IAA, GA, and CTK decreased in the leaves under both drought stress (Fig. 5a-c). Moreover, IAA, GA, and CTK showed little changes in the roots under MD but reduced significantly under SD stress.
Changes in the concentration of (a) abscisic acid, (b) jasmonic acid (c) strigolactones (d) salicylic acid (e) zeatin riboside, and (f) brassinosteroids under drought stress and N addition. Bars represent means ± SD (n = 3). different letters indicate treatment differences at p < 0.05 (Duncan’s method). The green and brown bars represent leaves and root samples. – N and + N stand for nitrogen applied and non-applied treatments. WW: well-watered; MD: medium drought; severe drought
Changes in the concentration of (a) indole acetic acid ratio, (b) gibberellic acid, and (c) cytokinin in leaf and root of A. sparsifolia seedlings in response to drought stress and nitrogen (N) supplementation. Bars represent means ± SD (n = 3). different letters indicate treatment differences at p < 0.05 (Duncan’s method). The green and brown bars represent leaves and root samples. – N and + N stand for nitrogen applied and non-applied treatments. WW: well-watered; MD: medium drought; severe drought
N application increased ABA by 1.48 and 2.21 folds, SA by 1.12 and 1.109 folds, and GA by 1.08 and 1.03 folds in the leaves under MD and SD stress. Also, N addition increased GA in the roots in roots by 1.06 and 1.09 folds under MD and SD and ABA by 1.13 fold, and SA by 1.19 fold under MD stress (Figs. 4 and 5). CTK and BR increased in the leaves by 1.14 and 1.06 folds under MD and by 1.02 and 1.14 folds under SD stress. Further, IAA increased in the leaves by 1.15 fold under MD and in the roots by 1.06 and 1.10 folds under both MD and SD stress (Fig. 5). Meanwhile, ZR increased in the leaves and roots by 1.21 and 1.12 folds and SLs in the leaves by 1.25 fold under MD stress.
4.6 Relationships Between Growth and Physiology
We found that LRWC was positively correlated with shoot growth metrics (SDW and PH), chlorophyll pigments, the phytohormones (IAA, ZR, GA), and anti-oxidant mechanism (POD, DHAR, PPO, AsA, GSH, AsA/DHA, and GSH/GSSG), SPS, sucrose, INV while it was negatively correlated JA, ABA, SLs, G6P, SS, BAM, fructose, citrate, Chl-a/Chl-b and oxidative stress indicators (O2•−, H2O2, MDA, DHA, GSSG) (Fig. 5ab). RWM was positively correlated with sucrose metabolism (sucrose, SPSS, and SPS), AsA-GSH metabolites (GSH, AsA, and their ratios) and DHAR, as well as hormones (ZR, IAA, and GA), and negatively correlated with the oxidative stress (O2•−, H2O2, MDA, DHA, GSSG), antioxidant enzymes APX, GR, SOD, GPX, fructose, AMY, SS, PEPC, and RSR (Fig. 6cd).
4.7 Principle Component Analysis
The principal component analysis (PCA) was conducted to investigate the response of shoot and root sugar metabolism, hormones, and antioxidant enzymes (Fig. 7A-D) under varying levels of drought stress and N addition. The first two principal components (PCs) described 75% of the phytohormones and osmolytes variations in shoot under well-watered with nitrogen (WW + N) and severe drought (SD + N) with N (Fig. 7A). However, the PCA results of roots were 63% of the first two axes displaying that, SPP and FRK were negatively associated with BAM and AMY under SD (severe drought) stress (Fig. 7B). On the other hand, the PCA of antioxidants and hormones showed 84% of the first two axes in shoot and root 84% under drought and N treatments.
Specifically, in the shoot, phytohormones were closely correlated with each other under WW + N conditions, while antioxidant activities showed a positive correlation under SD + N conditions (Fig. 7c). Conversely, in roots, phytohormones exhibited a positive correlation with antioxidant activities under both SD + N and WW + N conditions. However, under SD condition, markers of oxidative stress such as MDA, DHA, GSSG, O2−, and H2O2 depicted a negative correlation with DHAR, GSH, IAA, and GA (Fig. 7D). Briefly, the PCA analysis unveiled a close and positive association between antioxidant enzymes, hormones, sugar accumulation, and enzymes of the pentose phosphate pathway under drought with N addition treatments (Fig. 7). Conversely, they exhibited a negative correlation with ROS, lipids peroxidation, and oxidized pools of GSH and GSSG, and sugar metabolism enzymes. This indicates that the enhanced antioxidant mechanisms, hormonal production, and coordinated regulation of sugar metabolism are pivotal factors exclusively contributing to the nitrogen-mediated enhancement of drought tolerance.
5 Discussion
5.1 Changes in Biomass and Chlorophyll Pigments
Drought stress reduced the LRWC and biomass production in A. sparsifolia seedlings, which is a common adverse effect of water deficit (Liu et al. 2015; Tariq et al. 2019a). Plant biomass is produced by the division and enlargement of cells, involving a complex interaction between physio-biochemical and molecular changes, which are susceptible to drought stress (Farooq et al. 2009). Thus, the growth response to drought in our study is likely to involve a variety of physiological and biochemical responses (Valdés et al. 2013). Water deficit reduces cellular turgidity and increases oxidative stress, thus impeding photosynthesis, which in turn reduces the supply of photoassimilates required for new cell growth, resulting in reduced biomass and growth (Tariq et al. 2019b; Li et al. 2020). Our correlation analysis indicates a strong negative correlation between oxidative stress markers (ROS, MDA, GSSG, DHA) with various growth and biomass parameters (Fig. 6). This suggests that the elevated levels of ROS, coupled with increased MDA levels, are associated with inhibited metabolism and impaired growth and biomass under drought stress (Gill and Tuteja 2010).
In our study, N addition increased the LRWC and biomass production. Adequate N supply promotes the synthesis of proteins and structural carbohydrates, leading to accelerated cell division, growth, and increased biomass production (Fig. 7) (Huang et al. 2018). The improved biomass production due to N supplementation has also been associated with enhancements in hydraulic conductance (Xu et al. 2015b), N uptake and assimilation (Tariq et al. 2019a), growth hormone levels, cell wall elasticity, and increased meristematic cell numbers (Lawlor 2002).
In conditions of low soil moisture, maintaining optimal plant-water relations is crucial for normal growth and metabolism. Previous studies have demonstrated that LRWC tends to increase following N application, likely attributed to the greater accumulation of osmolytes responsible for osmotic adjustment mechanisms (Tariq et al. 2019a; Zhang et al. 2021b). Osmolytes indirectly enhance LRWC by promoting improved water retention within leaf tissue, thereby maintaining cell turgor pressure. This mechanism ultimately contributes to the maintenance or increase of LRWC, particularly under drought conditions (Taiz and Zeiger 2010). The strong response of N in the hyperarid Taklimakan Desert supports the hypothesis that N application increases drought tolerance, thereby increasing hydration, biomass, and seedling survival. Similar effects of N have been reported in other arid ecosystems (Hooper and Johnson 1999; Zhou et al. 2011; Zhang et al. 2021b) as confirmed by our data.
Dry-land species may cope with their habitats by adapting to their morphologies (Patterson et al. 1997). In dry conditions, plants often reduce their growth rate and biomass production, making more biomass available to roots, to conserve water and increase water absorption. Prior studies on drought reported this phenomenon for desert species (Zhang et al. 2020; Ullah et al. 2022) which agrees with our findings. However, the N application significantly reduced the R/S ratio under MD stress. Generally, plants face a trade-off between protein allocation to photosynthesis and nutrient uptake (Patterson et al. 1997). According to Broadley et al. (Broadley et al. 2000), plants often use N primarily to produce and maintain leaves, and to optimize C fixation.
The increase in shoot biomass with N addition under MD stress in this study further supports this idea. Hence, N application enhanced shoot growth in Alhagi significantly under MD (Table 1), resulting in a decrease in the R/S ratio (Wu et al. 2008). Furthermore, our findings support the general theory that low soil nutrients lead to greater root growth (Tan and Hogan 1997), while high soil nutrients result in greater above-ground biomass partitioning (Li and Kang 2002).
5.2 Chlorophyll Concentration
In our study, drought stress significantly reduced the concentration of chlorophyll pigments which could be attributed to oxidative stress, reduced pigment synthesis, or increased chlorophyll degradation, all of which adversely affect photosynthesis and plant growth. There are previous reports of similar effects of drought on chlorophyll pigments in perennial xerophytes (Ullah et al. 2022; Zhang et al. 2021b, 2020). The concentration of photosynthetic pigments appears to be directly related to the concentration of N, which is an essential component of the photosynthetic apparatus (Farooq et al. 2009). Compared to non-applied, N application significantly increased Chl a under MD and SD stress (Table 1). Previous studies have shown that N-application promotes chlorophyll synthesis by increasing the concentration of proteins in stroma and thylakoids (Cooke et al. 2005) and the synthesis of chloroplasts during leaf development (Li et al. 2012) and ultimately improves net photosynthesis (Tariq et al. 2019a).
The ratio of Chl-a/Chl-b, serves as a valuable indicator to assess chlorophyll synthesis function, especially under stress conditions. In our study, we observed that the Chl-a/Chl-b ratios increased under SD stress regardless of N availability. This suggests that stress has a more pronounced impact on Chl-b compared to Chl-a (Gomes et al. 2017). The resulting elevation in the Chl-a/Chl-b ratio can be attributed to the preferential breakdown of Chl-b into Chl-a under stress conditions (Upadhyay and Panda 2005). Under stress, an increase in the Chl-a/Chl-b ratio enhances the plant's ability to capture and utilize light energy, thereby sustaining photosynthetic activity. Additionally, it promotes plant resilience against stress by optimizing the balance between light absorption and photoprotection (Nahakpam 2017). The adjustment in chlorophyll ratios is indicative of the plant's adaptive response to stress, which is intended to maintain essential physiological functions despite adverse environmental conditions.
5.3 Sugar Metabolism
A coordinated supply and utilization of carbon is critical for optimal plant growth and development under both normal and controlled conditions. As a result of this coordination, plants can efficiently utilize carbon resources to support essential physiological processes and accomplish optimal growth (Muller et al. 2011; Sami et al. 2016). Due to drought stress, photosynthetic products are inhibited and carbon balance is disrupted, resulting in growth reductions and physiological and metabolic disturbances (Du et al. 2020). Compared to well-watered seedlings, drought-stressed seedlings had higher glucose and fructose in both parts; N application increased glucose in leaves under MD stress and in roots under both stresses, while fructose levels increased in both parts under MD stress. Plants utilize soluble sugars in many ways in response to drought stress, including maintaining cell swelling pressure, and osmotic balance, preventing osmotic damage, and providing energy (Kaur et al. 2007; Sami et al. 2016).
Aside from serving as key substrates for energy metabolism sucrose, glucose, and fructose are also required to regulate signal transduction and stress responses during plant growth and development (Wingler and Roitsch 2008). Furthermore, under drought stress, plant cells often accumulate soluble sugars, which serve as osmoprotectants and energy sources. We suggest that N addition improves drought resistance of both species by increasing soluble sugar levels, as depicted in the PCA analysis (Fig. 7), where higher levels of soluble sugars are associated with drought stress and N addition (Van den Ende and Valluru 2009). In addition, sucrose and starch levels decreased in drought conditions, possibly as a result of an increase in the activities of enzymes responsible for degrading these compounds such as SuSy, AMY, and BAM. As illustrated in Fig. 6, there is a negative correlation between the activity of these enzymes and the level of these carbohydrates, which supports this inference. Previous studies have shown that drought stress increases the activity of sucrose metabolic enzymes to balance the sucrose level in cells (Du et al. 2020; Xu et al. 2015a). Additionally, the sugar status of a plant cell can down- or up-regulate enzymes that metabolize carbohydrates.
There was a significant increase in enzymes involved in sucrose metabolism under drought (Fig. 4A and B), suggesting that A. sparsifolia continuously improves its metabolic capacity under drought. Sucrose conversion into glucose and fructose is an effective way of improving osmotic regulation during drought. Sucrose is converted into glucose and fructose during glycolysis, which might explain the lower sucrose and higher glucose and fructose in our study. However, N application decreased the SuSy, resulting in sucrose accumulation. In plants, starch serves as an energy source and carbon storage compound, and it is affected by abiotic stresses such as drought and salinity (Sami et al. 2016; Thalmann et al. 2016). This study showed that drought stress decreased starch levels in both leaves and roots, which was in line with the upregulation of α-amylase and β-amylase activities in both parts. Starch degradation accelerated under drought stress by upregulating the expression of starch-degrading genes in Glycine max (GmAMY3 and GmBAM1) (Du et al. 2020) and Arabidopsis (Thalmann et al. 2016).
Starch degradation appeared to have accelerated in Alhagi, resulting in the conversion of starch into soluble sugars. However, nitrogen (N) addition has been reported to increase starch synthesis by regulating the expression of starch metabolism genes under drought (Lv et al. 2021) which agrees with our findings. The accumulation of starch and sucrose may indeed be correlated with lower activities of their degrading enzymes, as indicated by the negative correlation shown in Fig. 6. Further phosphorylation of the hexoses is carried out by HK and FRK (Renz and Stitt 1993). A recent study reported that the activity of HK and FRK decreases under drought stress (Shokat et al. 2020), which contradicts our findings. However, our results are in line with those of Whittaker et al. (2001), who noted that increased HK activity in Sporobolus stapfianus leaves might enhance drought tolerance. Fulda et al. (2011) reported that drought-tolerant sunflower plants under water deficit upregulated SlFRK3, a protein responsible for FK activity. Consequently, FRK increases following N supplementation appear to contribute to enhancing the drought resistance of A. sparsifolia seedlings.
Furthermore, G6PDH and 6PGDH increased in leaves under MD and SD stress, whereas in roots, G6PDH increased and 6PGDH remained unchanged. At both stress levels, N application further increased G6PDH in leaves and roots, as depicted by PCA analysis (Fig. 7). It is clear from our findings that stressed plants exhibit a high demand for the reduced form of NADPH, a demand that has continued to grow even after N application, satisfying a need for this nucleotide. The level of cytosolic G6PDH activity and transcripts may increase under stress to facilitate the synthesis of cofactors or intermediates related to tolerance mechanisms (Naliwajski and Skłodowska 2018). This enzyme controls the flow of carbon in the pentose phosphate pathway and produces NADPH, which is used in antioxidant pathways (Fig. 7) (Corpas and Barroso 2018), N metabolism, and amino acids synthesis (Sharkey and Weise 2016).
5.4 Changes in Oxidative Indicators and Antioxidant Enzymes
In plants under stress, ROS accumulation damages cell membranes and disrupts normal cell function, resulting in a reduction in growth (Gill and Tuteja 2010). In our study, significant ROS molecules (H2O2, and O2−) were detected under drought, regardless of N fertilization, which may represent higher lipid oxidation and changes in physiological responses resulting in lower growth, as corroborated by correlation analysis (Apel and Hirt 2004; Gill and Tuteja 2010; Tariq et al. 2018).
Antioxidant enzymes are produced by plants as a general mechanism to defend against drought stress, thereby preventing oxidative stress from damaging their tissues (Wahab et al. 2022). In our study, leaves exhibited significantly higher SOD and CAT in both MD and SD stress and GPX in SD-stressed seedlings. In roots, the activity of CAT, POD, and GPX increased at both stress levels. Several studies have shown that drought stress strengthens the antioxidant defense mechanisms in plants (Tariq et al. 2018; 2019a,b; 2022), which is consistent with our findings, suggesting that young A. seedlings can reduce drought-induced oxidative damage by upregulating the mechanisms of scavenging O2•− and H2O2 (Fig. 8).
However, this was accompanied by a substantial decline in biomass production. In plants, nutrients are crucial for preventing adverse effects of drought, as they maintain enzyme activity, charge balance, and osmotic balance (Li et al. 2020). A PCA analysis showed that drought-stressed seedlings supplied with N had a greater positive correlation with antioxidant-enzyme levels compared to seedlings solely exposed to drought stress, indicating that N upregulates antioxidant potential (Fig. 7). For instance, N addition resulted in significantly higher CAT, POD, and SOD activity in leaves and roots under both stress levels. In roots, the PPO activity is upregulated under both MD and SD stress. Previous studies suggest that N application has no consistent impact on antioxidant enzyme regulation. According to our findings, N application under drought stress has a positive effect on antioxidant enzymes and likely improves membrane stability by reducing ROS accumulations (Figs. 7 and 8). Thus, N supply reduced oxidative damage to A. sparsifolia seedlings under drought stress, which agrees with the findings of previous studies (Zhong et al. 2017; Iqbal et al. 2020; Zhang et al. 2020).
In summary N application enhanced antioxidant enzyme activities and reduced ROS (H2O2, and O2−) and MDA levels, which indicates an increased redox defense system in response to drought (Fig. 7). In this regard, N application could improve drought resistance in A. sparsifolia seedlings through the production of antioxidant enzymes that help prevent cell membrane damage and promote effective dissipation of energy. In contrast, a recent study suggested that N application reduces antioxidant enzymes (Tariq et al. 2019a), which contradicts our findings. Perhaps these differences are a result of species-specific responses to N addition.
5.5 Changes in the AsA-GSH Cycle
AsA and GSH play a crucial role in scavenging ROS as part of the AsA-GSH cycle. Within this cycle, AsA is oxidized to MDHA by APX, and then further converted to DHA by removing H2O2. MDHA can be reduced back to AsA by MDHAR, and DHA can be regenerated into AsA through DHAR. Hence, the ratio of AsA/DHA serves as an indicator of the oxidation and reduction states within cells. In our study, MD and SD stress levels significantly AsA but increased DHA, resulting in a reduced ratio of AsA/DHA ratio. These findings are consistent with previous research (Jiang et al. 2022), suggesting that drought stress leads to decreased AsA and more oxidized AsA-GSH pools. This can be attributed to the increased activity of APX, which facilitated the H2O2 scavenging using AsA. Conversely, the observed decrease in DHAR activity may hinder the regeneration of AsA (Jiang et al. 2022).
The activities of APX, MDHAR, and DHAR are closely associated with increased stress tolerance (Jiang et al. 2022), while their low activity is linked to more severe membrane lipid peroxidation (Shao et al. 2008). N application significantly improved APX and DHAR in both parts and GR in leaves under both stress levels and MDHAR in leaves and roots under MD stress. Furthermore, GR upregulated in roots following N addition under MD stress. Hence, stabilizing the redox state of cells and increasing drought resistance (Jiang et al. 2022).
The ASA/DHA ratio, which represents the amount of ASA available for APX enzyme, increased in N-treated seedlings under drought stress. This may be attributed to the significant increase in MDHAR and DHAR activity. Moreover, the redox state of GSH influences the response of plants to stress, and the increase of GSH in this response determines the effectiveness of AsA. Adding N to A. sparsifolia restored the high GSH/GSSG ratio. Transforming GSSG to GSH enhances plants' ability to resist environmental stress (Verma et al. 2015). This mechanism may be a result of increased GR activity which increases GSH biosynthesis and reduces GSH degradation (Szalai et al. 2009), which is consistent with the findings in this study. Under stress, increased GR activity promotes H2O2 removal by GSH and maintains a high GSH/GSSG ratio (Szalai et al. 2009). It has been reported that H2O2 and GSH interact with stress signals in soybeans (Xing et al. 2020). This may explain why, in our experiment, N application reduced H2O2 by upregulating GR activity to increase the GSH and GSH/GSSG ratio.
The N application has therefore stabilized the redox state of A. sparsifolia cells by regulating the antioxidant enzymes and reducing H2O2 and MDA, resulting in improved drought resistance (Lukić et al. 2020). The PCA outcomes in our study, strongly suggest that N additions enhance drought tolerance in both species by bolstering their AsA-GSH cycle, leading to decreased H2O2 (Fig. 7). Several studies suggested that N application significantly reduces O2•− and H2O2 levels due to increased enzymatic and nonenzymatic antioxidant mechanisms (Chang et al. 2016; Tariq et al. 2019a; Zhang et al. 2021b). This reduction in oxidative stress contributes to enhanced membrane stability. Hence, our findings suggest that N application can substantially alleviate the detrimental effects of drought on membrane integrity and stability in A. sparsifolia cells.
5.6 Production of Phytohormones
In both normal and stressful conditions, phytohormones modulate several physiological processes. In our study, the concentration of ABA, JA, and SLs increased in leaves and roots under drought. In contrast, ZR decreased in both leaves and roots whereas IAA and GA decreased in leaves. Although IAA and GA did not exhibit significant changes in roots under MD, both declined under SD stress. These phytohormonal imbalances may cause marked growth and biomass reductions (Li et al. 2018). The growth features and ABA, JA, SA, and SLs were negatively correlated, suggesting that plants produced many anti-stress hormones to cope with drought (Fig. 6B, D). Given the interplay between hormones and drought, our findings suggest a potential strategy for coping with drought by adjusting the regulation of endogenous hormones (Li et al. 2018).
Phytohormones also modulate the response to oxidative stress by interacting with ROS and generating distinct transcriptomic and physiological responses resulting in plant acclimation. Plant respiratory burst oxidase homologs (RBOHs) play a critical role in this interaction. It has been shown that stress affects several hormones that act as regulators of ROS production and RBOH activity via various mechanisms (Devireddy et al. 2021) (Fig. 7). In drought, increasing ABA and JA modulate antioxidant mechanisms and osmolytes, thereby contributing to drought tolerance (Dhakarey et al. 2017; Iqbal et al. 2022). This confirms our findings, where a positive correlation was found between these hormones, antioxidant enzymes, and osmolytes (glucose and fructose) (Fig. 6). In addition, JA has been found to increase rapidly in Citrus (de Ollas et al. 2013) and Arabidopsis plants (Balbi and Devoto 2008). Drought-induced increases in ABA contribute to diverse mechanisms of stress tolerance including antioxidant activity and protection from photooxidative damage (Iqbal et al. 2022).
Also, GA has been reported to alter the regulatory functions of several genes within tomato plants exposed to drought, leading to reduced cell size, internode number, shoot length, and dry weight (Litvin et al. 2016). The interaction of drought with other hormones can also affect the activity, synthesis, metabolism, and transport of IAA in a variety of plants (Iqbal et al. 2022). Further, CTK concentration can increase or decrease under drought stress. Several physiological responses are regulated by CTK, including cell division, apical meristem support, and several physiological responses, which allow plants to respond and adapt to rapid environmental changes (Yadav et al. 2021). Growth hormones were negatively correlated with ROS and MDA (Fig. 6). Hence, their under drought could explain the sensitivity of plants to and severe decline in shoot and root growth and biomass, in our study.
N application significantly increased ABA in leaves under both stresses and JA and ABA in both leaves and roots under MD and SD. The increased ABA concentration, for instance, regulates a wide range of stress-related mechanisms (Danquah et al. 2014), for example, improvement in turgor pressure (Iqbal et al. 2022), and protects the xanthophyll cycle and the photosynthetic machinery from photooxidative stress (Zhu et al. 2011) as well as modulation of the osmotic and antioxidant potentials (Iqbal et al. 2022), leading to improved drought tolerance and growth responses. The N-treated MD-stressed seedlings had higher GA, IAA, and ZR in leaves; and IAA and GA in roots under MD and SD stress levels. Since both IAA and GA are principal growth stimulants in plants (Tiwari et al. 2017), higher concentrations under drought could result in increased shoot and root development and biomass in the N-fertilized A. sparsifolia seedlings.
Moreover, a growing body of evidence indicates that SLs, carotenoid-derived phytohormones, can play an important role in regulating plant response to environmental stress (Tariq et al. 2023). In our study, N application had no significant impact on SLs in roots under drought stress but increased in leaves under MD stress. However, regardless of N supply, SL concentration was still higher in seedlings. Dry soils are nutrient-limited particularly P availability, and drought further exacerbated their limitation (Tariq et al. 2022). There is evidence that SLs play an important role in modulating root and shoot development in response to nutrient deficiencies, particularly phosphorus, which promotes the synthesis of SLs (Andreo-Jimenez et al. 2015; Tariq et al. 2023). In Arabidopsis, Ruyter-Spira et al. (2011) reported that SLs enhance the growth of the lateral roots under limited P conditions by facilitating the uptake of P. Therefore, the upregulation of SLs in drought-stressed seedlings, particularly in roots, may have contributed to improved root growth and facilitated P uptake under drought. Therefore, SLs upregulation appears to be an important strategy for A. sparsifolia seedlings in the hyper-arid Taklamakan Desert to improve drought tolerance and facilitate root growth and nutrient uptake. Moreover, the PCA revealed that antioxidant enzymes, hormones, sugar accumulation, and enzymes of the pentose phosphate pathway were closely and positively associated with the drought and N addition treatments (Fig. 7), and negatively correlated with ROS, MDA, GSSG, and DHA and sugar degrading enzymes which suggests that enhanced antioxidant mechanisms, hormonal production and coordinated regulation of sugar metabolism exclusively confer the N-mediated improvement in drought tolerance.
6 Conclusion
Our study revealed that A. sparsifolia seedlings responded to N nutrition and performed better under drought stress, due to the coordinated regulations of hormones, sugar metabolism, and antioxidant mechanism. As compared to untreated seedlings, N supplementation increased the biomass and Chl-a production and improved the LRWC of drought-stressed seedlings. The N-mediated upregulation of H2O2 and O2-scavenging enzymes led to lower ROS and lipid peroxidation and improved AsA and GSH redox state. They also accumulated higher IAA, GA, CTK, BR, and ZR, which may account for their better growth. Hence, our findings provided evidence that N addition to drought-stressed seedlings promotes physiological and phytohormone response mechanisms that mitigate the effects of drought stress and this strategy could be adopted in programs for the restoration of hyperarid ecosystems. These findings have improved our understanding of the morpho-physiological plasticity of young xerophytes seedlings and their responses to N addition and water deficit before accessing groundwater resources, in the nutrient-deficient hyperarid desert–oasis ecotone of northwest China. However, likely variation in species responses to drought and nutrient supply necessitates further studies to determine the biochemical and molecular mechanisms of xerophyte seedlings for wider vegetation restoration and mitigation programs.
Data Availability
All data can be provided upon request to the corresponding author.
References
Abid M, Tian Z, Ata-Ul-Karim ST, Liu Y, Cui Y, Zahoor R, Jiang D, Dai T (2016) Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and sensitive wheat cultivars. Plant Physiol Biochem 106:218–227. https://doi.org/10.1016/j.plaphy.2016.05.003
Aksenova NP, Konstantinova TN, Golyanovskaya SA, Sergeeva LI, Romanov GA (2012) Hormonal regulation of tuber formation in potato plants. Russ J Plant Physl 59:451–466. https://doi.org/10.1134/S1021443712040024
Andreo-Jimenez B, Ruyter-Spira C, Bouwmeester HJ, Lopez-Raez JA (2015) Ecological relevance of strigolactones in nutrient uptake and other abiotic stresses, and in plant-microbe interactions below-ground. Plant Soil 394:1–19. https://doi.org/10.1007/s11104-015-2544-z
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signaling transduction. Annu Rev Plant Biol 55:373. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Arndt SK, Arampatsis C, Foetzki A, Li X, Zeng F, Zhang X (2004) Contrasting patterns of leaf solute accumulation and salt adaptation in four phreatophytic desert plants in a hyperarid desert with saline groundwater. J Arid Environ 59:259–270. https://doi.org/10.1016/j.jaridenv.2004.01.017
Balbi V, Devoto A (2008) Jasmonate signaling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177:301–318. https://doi.org/10.1111/j.1469-8137.2007.02292.x
Broadley MR, Escobar-Gutierrez AJ, Burns A, Burns IG (2000) What are the effects of nitrogen deficiency on growth components of lettuce? The New Phytol 147:519–526. https://doi.org/10.1046/j.1469-8137.2000.00715.x
Canham CA, Froend RH, Stock WD (2015) Rapid root elongation by phreatophyte seedlings does not imply tolerance of water table decline. Trees 29:815–824. https://doi.org/10.1007/s00468-015-1161-z
Chang Z, Liu Y, Dong H, Teng K, Han L, Zhang X (2016) Effects of cytokinin and nitrogen on drought tolerance of creeping bentgrass. PLoS ONE 11:e0154005. https://doi.org/10.1371/journal.pone.0154005
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osório ML, Carvalho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field? Photosynthesis and growth. Ann Bot 89:907–916. https://doi.org/10.1093/aob/mcf105
Cooke JE, Martin TA, Davis JM (2005) Short-term physiological and developmental responses to nitrogen availability in hybrid poplar. New Phytol 167:41–52. https://doi.org/10.1111/j.1469-8137.2005.01435.x
Corpas FJ, Barroso JB (2018) Peroxisomal plant metabolism–an update on nitric oxide, Ca2+, and the NADPH recycling network. J Cell Sci 131:jcs202978. https://doi.org/10.1242/jcs.202978
Danquah A, De Zélicourt A, Colcombet J, Hirt H (2014) The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv 32:40–52. https://doi.org/10.1016/j.biotechadv.2013.09.006
de Ollas C, Hernando B, Arbona V, Gómez-Cadenas A (2013) Jasmonic acid transient accumulation is needed for the abscisic acid increase in citrus roots under drought stress conditions. Physiol Plant 147:296–306. https://doi.org/10.1111/j.1399-3054.2012.01659.x
Devireddy AR, Zandalinas SI, Fichman Y, Mittler R (2021) Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J 105:459–476. https://doi.org/10.1111/tpj.15010
Dhakarey R, Raorane ML, Treumann A, Peethambaran PK, Schendel RR, Sahi VP, Hause B, Bunzel M, Henry A, Kohli A (2017) Physiological and proteomic analysis of the rice mutant cpm2 suggests a negative regulatory role of jasmonic acid in drought tolerance. Front Plant Sci 8:1903
Du Y, Zhao Q, Chen L, Yao X, Zhang W, Zhang B, Xie F (2020) Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol Biochem 146:1–12. https://doi.org/10.1016/j.plaphy.2019.11.003
Fàbregas N, Fernie AR (2019) The metabolic response to drought. J Exp Bot 70:1077–1085. https://doi.org/10.1093/jxb/ery437
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212. https://doi.org/10.1051/agro:2008021
Fleta-Soriano E, Munné-Bosch S (2016) Stress memory and the inevitable effects of drought: a physiological perspective. Front Plant Sci 7:143. https://doi.org/10.3389/fpls.2016.00143
Fleta-Soriano E, Pinto-Marijuan M, Munné-Bosch S (2015) Evidence of drought stress memory in the facultative CAM, Aptenia cordifolia: possible role of phytohormones. PLoS ONE 10:e0135391. https://doi.org/10.1371/journal.pone.0135391
Fulda S, Mikkat S, Stegmann H, Horn R (2011) Physiology and proteomics of drought stress acclimation in sunflower (Helianthus annuus L.). Plant Biol 13:632–642. https://doi.org/10.1111/j.1438-8677.2010.00426.x
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gomes MA da C, Pestana IA, Santa-Catarina C, Hauser-Davis RA, Suzuki MS (2017) Salinity effects on photosynthetic pigments, proline, biomass and nitric oxide in Salvinia auriculata Aubl. Acta Limnol Bras 29: https://doi.org/10.1590/S2179-975X4716
Gui D, Zeng F, Liu Z, Zhang B (2013) Characteristics of the clonal propagation of Alhagi sparsifolia Shap. (Fabaceae) under different groundwater depths in Xinjiang. China Rangel J 35:355–362. https://doi.org/10.1071/RJ13004
Hansen J, Møller IB (1975) Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal Biochem 68:87–94. https://doi.org/10.1016/0003-2697(75)90682-x
Hooper DU, Johnson L (1999) Nitrogen limitation in dryland ecosystems: responses to geographical and temporal variation in precipitation. Biogeochemistry 46:247–293. https://doi.org/10.1007/BF01007582
Huang L, Li M, Zhou K, Sun T, Hu L, Li C, Ma F (2018) Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol Biochem 127:185–193. https://doi.org/10.1016/j.plaphy.2018.03.031
Iqbal A, Dong Q, Wang X, Gui H, Zhang H, Zhang X (2020) Song M (2020) High nitrogen enhance drought tolerance in cotton through antioxidant enzymatic activities, nitrogen metabolism and osmotic adjustment. Plants 9:178. https://doi.org/10.3390/plants9020178
Iqbal WX, Mubeen I, Kamran M, Kanwal I, Díaz GA, Abbas A, Parveen A, Atiq MN, Alshaya H (2022) Phytohormones trigger drought tolerance in crop plants: outlook and future perspectives. Front Plant Sci 12:3378. https://doi.org/10.3389/fpls.2021.799318
Jiang K, Asami T (2018) Chemical regulators of plant hormones and their applications in basic research and agriculture. Biosci Biotechnol Biochem 82:1265–1300. https://doi.org/10.1080/09168451.2018.1462693
Jiang Z, Zhu H, Zhu H, Tao Y, Liu C, Liu J, Yang F, Li M (2022) Exogenous ABA enhances the antioxidant defense system of maize by regulating the AsA-GSH cycle under drought stress. Sustainability 14:3071. https://doi.org/10.3390/su14053071
Johnson G, Lambert C, Johnson DK, Sunderwirth SG (1964) Plant tissue analysis, colorimetric determination of glucose, fructose, and sucrose in plant materials using a combination of enzymatic and chemical methods. J Agric Food Chem 12:216–219. https://doi.org/10.1021/jf60133a007
Kaur K, Gupta AK, Kaur N (2007) Effect of water deficit on carbohydrate status and enzymes of carbohydrate metabolism in seedlings of wheat cultivars. Indian J Biochem Biophys 44:223–230
Lawlor DW (2002) Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J Exp Bot 53:773–787. https://doi.org/10.1093/jexbot/53.370.773
Li FS, Kang SZ (2002) Effects of atmospheric CO2 enrichment, applied nitrogen and soil moisture on dry matter accumulation and nitrogen uptake in spring wheat. Pedosphere 12:207–218. https://doi.org/10.1023/A:1025521701732
Li CR, Liang YH, Hew CS (2002) Responses of Rubisco and sucrose-metabolizing enzymes to different CO2 in a C3 tropical epiphytic orchid Oncidium Goldiana. Plant Sci 163:313–320. https://doi.org/10.1016/S0168-9452(02)00100-0
Li H, Li M, Luo J, Cao X, Qu L, Gai Y, Jiang X, Liu T, Bai H, Janz D (2012) N-fertilization has different effects on the growth, carbon and nitrogen physiology, and wood properties of slow-and fast-growing Populus species. J Exp Bot 63:6173–6185. https://doi.org/10.1093/jxb/ers271
Li L, Gu W, Li J, Li C, Xie T, Qu D, Meng Y, Li C, Wei S (2018) Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol Biochem 129:35–55. https://doi.org/10.1016/j.plaphy.2018.05.017
Li S, Zhou L, Addo-Danso SD, Ding G, Sun M, Wu S, Lin S (2020) Nitrogen supply enhances the physiological resistance of Chinese fir plantlets under polyethylene glycol (PEG)-induced drought stress. Plant Sci 10:7509. https://doi.org/10.1038/s41598-020-64161-7
Li J, Yu B, Ma C, Li H, Jiang D, Nan, J, ... Li, H (2022) Functional characterization of sugar beet M14 antioxidant enzymes in plant salt stress tolerance. Antioxidants, 12(1), 57. https://doi.org/10.3390/antiox12010057
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Ltd. https://doi.org/10.1042/bst0110591
Litvin AG, van Iersel MW, Malladi A (2016) Drought stress reduces stem elongation and alters gibberellin-related gene expression during vegetative growth of tomato. J Am Soc Hortic Sci 141:591–597. https://doi.org/10.21273/JASHS03913-16
Liu F, Jensen CR, Andersen MN (2004) Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: its implication in altering pod set. Field Crops Res 86:1–13. https://doi.org/10.1016/S0378-4290(03)00165-5
Liu C, Wang Y, Pan K, Jin Y, Li W, Zhang L (2015) Effects of phosphorus application on photosynthetic carbon and nitrogen metabolism, water use efficiency and growth of dwarf bamboo (Fargesia rufa) subjected to water deficit. Plant Physiol Biochem 96:20–28. https://doi.org/10.1016/j.plaphy.2015.07.018
Liu L, Liu D, Wang Z, Zou C, Wang B, Zhang H, Gai Z, Zhang P, Wang Y, Li C (2020) Exogenous allantoin improves the salt tolerance of sugar beet by increasing putrescine metabolism and antioxidant activities. Plant Physiol Biochem 154:699–713. https://doi.org/10.1016/j.plaphy.2020.06.034
Liu L, Cao X, Zhai Z, Ma S, Tian Y, Cheng J (2022) Direct evidence of drought stress memory in mulberry from a physiological perspective: Antioxidative, osmotic and phytohormonal regulations. Plant Physiol Biochem 186:76–87. https://doi.org/10.1016/j.plaphy.2022.07.001
Lukić N, Kukavica B, Davidović-Plavšić B, Hasanagić D, Walter J (2020) Plant stress memory is linked to high levels of anti-oxidative enzymes over several weeks. EEB 178:104166. https://doi.org/10.1016/j.envexpbot.2020.104166
Lv X, Ding Y, Long M, Liang W, Gu X, Liu Y, Wen X (2021) Effect of foliar application of various nitrogen forms on starch accumulation and grain filling of wheat (Triticum aestivum L.) under drought stress. Front Plant Sci 12:645379. https://doi.org/10.3389/fpls.2021.645379
Mahieu S, Germon F, Aveline A, Hauggaard-Nielsen H, Ambus P, Jensen ES (2009) The influence of water stress on biomass and N accumulation, N partitioning between above and below ground parts and on N rhizodeposition during reproductive growth of pea (Pisum sativum L.). Soil Biol Biochem 41:380–387. https://doi.org/10.1016/j.soilbio.2008.11.021
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739. https://doi.org/10.1111/j.1469-8137.2008.02436.x
Miura K, Tada Y (2014) Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci 5:4. https://doi.org/10.3389/fpls.2014.00004
Moellering H, Gruber W (1966) Determination of citrate with citrate lyase. Anal Biochem 17:369–376. https://doi.org/10.1016/0003-2697(66)90172-2
Muller B, Pantin F, Génard M, Turc O, Freixes S, Piques M, Gibon Y (2011) Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J Exp Bot 62:1715–1729. https://doi.org/10.1093/jxb/erq438
Nahakpam S (2017) Chlorophyll stability: a better trait for grain yield in rice under drought. Indian J Ecol 44:77–82
Naliwajski MR, Skłodowska M (2018) The relationship between carbon and nitrogen metabolism in cucumber leaves acclimated to salt stress. PeerJ 6:e6043. https://doi.org/10.7717/peerj.6043
Nazir F, Peter P, Gupta R, Kumari S, Nawaz K, Khan MIR (2024) Plant hormone ethylene: A leading edge in conferring drought stress tolerance. Physiol Plant 176:e14151. https://doi.org/10.1111/ppl.14151
Neves DM, Almeida LADH, Santana-Vieira DDS, Freschi L, Ferreira CF, Soares Filho WDS, Costa MGC, Micheli F, Coelho Filho MA, Gesteira ADS (2017) Recurrent water deficit causes epigenetic and hormonal changes in citrus plants. Sci Rep 7:13684
Patterson TB, Guy RD, Dang QL (1997) Whole-plant nitrogen- and water-relations traits, and their associated trade-offs, in adjacent muskeg and upland boreal spruce species. Oecologia 110:160–168. https://doi.org/10.1007/s004420050145
Pospisilova J, Vagner M, Malbeck J, Travnickova A, Batkova P (2005) Interactions between abscisic acid and cytokinins during water stress and subsequent rehydration. Biol Plant 49:533–540. https://doi.org/10.1007/s10535-005-0047-0
Rai GK, Kumar P, Choudhary SM, Singh H, Adab K, Kosser R, Magotra I, Kumar RR, Singh M, Sharma R (2023) Antioxidant Potential of Glutathione and Crosstalk with Phytohormones in Enhancing Abiotic Stress Tolerance in Crop Plants. Plants 12:1133. https://doi.org/10.3390/plants12051133
Ramawat KG, Merillon J-M (2013) Bulbous plants: biotechnology. CRC Press
Renz A, Stitt M (1993) Substrate specificity and product inhibition of different forms of fructokinases and hexokinases in developing potato tubers. Planta 190:166–175. https://doi.org/10.1007/BF00196608
Ruyter-Spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, De Ruijter N, Cardoso C, Lopez-Raez JA, Matusova R, Bours R (2011) Physiological effects of the synthetic strigolactones analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol 155:721–734. https://doi.org/10.1104/pp.110.166645
Sami F, Yusuf M, Faizan M, Faraz A, Hayat S (2016) Role of sugars under abiotic stress. Plant Physiol Biochem 109:54–61. https://doi.org/10.1016/j.plaphy.2016.09.005
Shao H-B, Chu L-Y, Lu Z-H, Kang C-M (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4:8. https://doi.org/10.7150/ijbs.4.8
Sharkey TD, Weise SE (2016) The glucose 6-phosphate shunt around the Calvin-Benson cycle. J Exp Bot 67:4067–4077. https://doi.org/10.1093/jxb/erv484
Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot 55:2343–2351. https://doi.org/10.1093/jxb/erh276
Shokat S, Großkinsky DK, Roitsch T, Liu F (2020) Activities of leaf and spike carbohydrate-metabolic and antioxidant enzymes are linked with yield performance in three spring wheat genotypes grown under well-watered and drought conditions. BMC Plant Biol 20:1–19. https://doi.org/10.1186/s12870-020-02581-3
Sreenivasulu N, Harshavardhan VT, Govind G, Seiler C, Kohli A (2012) Contrapuntal role of ABA: does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 506:265–273. https://doi.org/10.1016/j.gene.2012.06.076
Sun M, Li S, Yu H, Gong Q, Zhang B, Liu G, Xiao Y, Peng F (2023) Effects of valine and urea on carbon and nitrogen accumulation and lignin content in Peach trees. Plants 12:1596. https://doi.org/10.3390/plants12081596
Szalai G, Kellős T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul 28:66–80. https://doi.org/10.1007/s00344-008-9075-2
Taiz L, Zeiger E (2010) Plant physiology, 5th edn. Sinauer associates Inc., Sunderland, p 782
Tan W, Hogan GD (1997) Physiological and morphological responses to nitrogen limitation in jack pine seedlings: potential implications for drought tolerance. New Forests 14:19–31. https://doi.org/10.1023/A:1006546014858
Tariq A, Pan K, Olatunji OA, Graciano C, Li Z, Sun F, Zhang L, Wu X, Chen W, Song D (2018) Phosphorous fertilization alleviates drought effects on Alnus cremastogyne by regulating its antioxidant and osmotic potential. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-24038-2
Tariq A, Pan K, Olatunji OA, Graciano C, Li N, Li Z, Song D, Sun F, Justine MF, Huang D (2019a) Role of nitrogen supplementation in alleviating drought-associated growth and metabolic impairments in Phoebe zhennan seedlings. J Plant Nutr Soil Sc 182:586–596. https://doi.org/10.1002/jpln.201800435
Tariq A, Pan K, Olatunji OA, Graciano C, Li Z, Li N, Song D, Sun F, Wu X, Dakhil MA (2019b) Impact of phosphorus application on drought resistant responses of Eucalyptus grandis seedlings. Physiol Plant 166:894–908. https://doi.org/10.1111/ppl.12868
Tariq A, Graciano C, Sardans J, Zeng J, Hughes AC, Ahmed Z, Ullah A, Ali S, Gao Y, Penuelas J (2024) Plant root mechanisms and their effects on carbon and nutrient accumulation in desert ecosystems under changes in land-use and climate. New Phytol 242:916–934. https://doi.org/10.1111/nph.19676
Tariq A, Ullah A, Sardans J, Zeng F, Graciano C, Li X, Wang W, Ahmed Z, Ali S, Zhang Z (2022) Alhagi sparsifolia: An ideal phreatophyte for combating desertification and land degradation. Sci Total Environ 157228. https://doi.org/10.1016/j.scitotenv.2022.157228
Tariq A, Ullah I, Sardans J, Graciano C, Mussarat S, Ullah A, Zeng F, Wang W, Al-Bakre DA, Ahmed Z (2023) Strigolactones can be a potential tool to fight environmental stresses in arid lands. Environ Res 115966. https://doi.org/10.1016/j.envres.2023.115966
Thalmann M, Pazmino D, Seung D, Horrer D, Nigro A, Meier T, Kölling K, Pfeifhofer HW, Zeeman SC, Santelia D (2016) Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 28:1860–1878. https://doi.org/10.1105/tpc.16.00143
Tiwari S, Lata C, Singh Chauhan P, Prasad V, Prasad M (2017) A functional genomic perspective on drought signaling and its crosstalk with phytohormone-mediated signaling pathways in plants. Curr Genomics 18:469–482. https://doi.org/10.2174/1389202918666170605083319
Ullah A, Tariq A, Zeng F, Sardans J, Graciano C, Ullah S, Chai X, Zhang Z, Keyimu M, Asghar MA (2022) Phosphorous Supplementation Alleviates Drought-Induced Physio-Biochemical Damages in Calligonum mongolicum. Plants 11:3054. https://doi.org/10.3390/plants11223054
Upadhyay RK, Panda SK (2005) Salt tolerance of two aquatic macrophytes, Pistia stratiotes, and Salvinia molesta. Biol Plant 49:157–159. https://doi.org/10.1007/s10535-005-7159-4
Valdés AE, Irar S, Majada JP, Rodríguez A, Fernández B, Pagès M (2013) Drought tolerance acquisition in Eucalyptus globulus (Labill.): a research on plant morphology, physiology, and proteomics. J Proteom 79:263–276. https://doi.org/10.1016/j.jprot.2012.12.019
Van den Ende W, Valluru R (2009) Sucrose, sucrosyl oligosaccharides, and oxidative stress: scavenging and salvaging? J Exp Bot 60:9–18. https://doi.org/10.1093/jxb/ern297
van Handel E (1968) Direct micro determination of sucrose. Anal Biochem 22:280–283. https://doi.org/10.1016/0003-2697(68)90317-5
Verma G, Mishra S, Sangwan N, Sharma S (2015) Reactive oxygen species mediate axis-cotyledon signaling to induce reserve mobilization during germination and seedling establishment in Vigna radiata. J Plant Physiol 184:79–88. https://doi.org/10.1016/j.jplph.2015.07.001
Vrede T, Dobberfuhl DR, Kooijman S, Elser JJ (2004) Fundamental connections among organism C: N: P stoichiometry, macromolecular composition, and growth. Ecol 85:1217–1229. https://doi.org/10.1890/02-0249
Wahab A, Abdi G, Saleem MH, Ali B, Ullah S, Shah W, Mumtaz S, Yasin G, Muresan CC, Marc RA (2022) Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants 11:1620. https://doi.org/10.3390/plants11131620
Whittaker A, Bochicchio A, Vazzana C, Lindsey G, Farrant J (2001) Changes in leaf hexokinase activity and metabolite levels in response to drying in the desiccation-tolerant species Sporobolus stapfianus and Xerophyta viscosa. J Exp Bot 52:961–969. https://doi.org/10.1093/jexbot/52.358.961
Wingler A, Roitsch T (2008) Metabolic regulation of leaf senescence: interactions of sugar signaling with biotic and abiotic stress responses. Plant Biol 10:50–62. https://doi.org/10.1111/j.1438-8677.2008.00086.x
Wu F, Bao W, Li F, Wu N (2008) Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ Exp Bot 63:248–255. https://doi.org/10.1016/j.envexpbot.2007.11.002
Wu H, Zhang Y, Zhang W, Pei X, Zhang C, Jia S, Li W (2015) Transcriptomic analysis of the primary roots of Alhagi sparsifolia in response to water stress. PLoS ONE 10:e0120791. https://doi.org/10.1371/journal.pone.0120791
Wu L, Lan J, Xiang X, Xiang H, Jin Z, Khan S, Liu Y (2020) Transcriptome sequencing and endogenous phytohormone analysis reveal new insights in CPPU controlling fruit development in kiwifruit (Actinidia chinensis). PLoS ONE 15:e0240355. https://doi.org/10.1371/journal.pone.0240355
Xing X, Xu Z, Tong F, Qi Y, Sun D, Bian N, Wang X (2020) Involvement of triadimefon induced early ABA-dependent H2O2 accumulation in soybean against water stress. Oil Crop Science 5:41–47. https://doi.org/10.1016/j.ocsci.2020.03.002
Xu Z, Zhou G, Shimizu H (2009) Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J Exp Bot 60:3737–3749. https://doi.org/10.1093/jxb/erp216
Xu G, Wang H, Zhai Z, Sun M, Li Y (2015) Effect of water and nitrogen coupling on root morphology and physiology, yield and nutrition utilization for rice. Transactions of CSAE 31:132–141. https://doi.org/10.11975/j.issn.1002-6819.2015.10.018
Xu W, Cui K, Xu A, Nie L, Huang J, Peng S (2015b) Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol Plant 37:1–11. https://doi.org/10.1007/s11738-014-1760-0
Yadav B, Jogawat A, Gnanasekaran P, Kumari P, Lakra N, Lal SK, Pawar J, Narayan OP (2021) An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene 25:100264. https://doi.org/10.1016/j.plgene.2020.100264
Zhang Z, Tariq A, Zeng F, Graciano C, Zhang B (2020) Nitrogen application mitigates drought-induced metabolic changes in Alhagi sparsifolia seedlings by regulating nutrient and biomass allocation patterns. Plant Physiol Biochem 155:828–841. https://doi.org/10.1016/j.plaphy.2020.08.036
Zhang L, Sun S, Liang Y, Li B, Ma S, Wang Z, Ma B, Li M (2021a) Nitrogen levels regulate sugar metabolism and transport in the shoot tips of crabapple plants. Front Plant Sci 12:626149. https://doi.org/10.3389/fpls.2021.626149
Zhang Z, Tariq A, Zeng F, Chai X, Graciano C (2021b) Involvement of soluble proteins in growth and metabolic adjustments of drought-stressed Calligonum mongolicum seedlings under nitrogen addition. Plant Biol 23:32–43. https://doi.org/10.1111/plb.13190
Zhong C, Cao X, Hu J, Zhu L, Zhang J, Huang J, Jin Q (2017) Nitrogen metabolism in adaptation of photosynthesis to water stress in rice grown under different nitrogen levels. Front Plant Sci 8:1079. https://doi.org/10.3389/fpls.2017.01079
Zhou X, Zhang Y, Ji X, Downing A, Serpe M (2011) Combined effects of nitrogen deposition and water stress on growth and physiological responses of two annual desert plants in northwestern China. Environ Exp Bot 74:1–8. https://doi.org/10.1016/j.envexpbot.2010.12.005
Zhu S-Q, Chen M-W, Ji B-H, Jiao D-M, Liang J-S (2011) Roles of xanthophylls and exogenous ABA in protection against NaCl-induced photodamage in rice (Oryza sativa L) and cabbage (Brassica campestris). J Exp Bot 62:4617–4625. https://doi.org/10.1093/jxb/err170
Acknowledgements
The present work was supported by the National Natural Science Foundation of China (No. 32250410301, 42271071, 41977050); the Ministry of Science and Technology, China (No. QN2022045005L; WGXZ2023078L); and the National Key Research and Development Project of China (No. 2022YFF1302504). Josep Peñuelas and Jordi Sardans were funded by the Spanish Government grants PID2020115770RB-I, TED2021-132627 B–I00, and PID2022-140808NB-I00, funded by MCIN, AEI/10.13039/ 501100011033 European Union Next Generation EU/PRTR, the Fundación Ramón Areces grant CIVP20A6621, and the Catalan Government grant SGR 2021–1333.
The first author is very thankful to the Alliance of International Science Organizations (ANSO) for providing financial support for his Ph.D. program through their scholarship for young talent.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ullah, A., Tariq, A., Zeng, F. et al. Insights into Alhagi sparsifolia Seedlings Adaptations to Drought Stress under Nitrogen Addition: Regulation of Sugar Metabolism, Hormones, and Anti-Oxidant Potential. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01814-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01814-w