Abstract
Upregulation of the antioxidant enzyme system in plants provides protection against various abiotic stresses. Transgenic potato plants overexpressing the strawberry d-galacturonic acid reductase (GalUR) gene with enhanced accumulation of ascorbate (AsA) were used to study the antioxidant system involving the ascorbate–glutathione cycle in order to understand the tolerance mechanism in plants in response to various abiotic stresses under in vitro conditions. Transgenic potato tubers subjected to various abiotic stresses induced by methyl viologen, sodium chloride and zinc chloride showed enhanced activities of superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.1.1.1.6) and enzymes of the ascorbate–glutathione cycle such as ascorbate peroxidase (APX, EC 1.11.1.11), dehydroascorbate reductase (DHAR, EC 1.8.5.1) and glutathione reductase (GR, EC 1.8.1.7), as well as increased levels of ascorbate, glutathione (GSH) and proline when compared to untransformed tubers. The increased enzyme activities correlated with the mRNA transcript levels in the stressed transgenic tubers. Significant differences in redox status of AsA and GSH were also observed in stressed transgenic potato tubers that showed increased tolerance to abiotic stresses compared to untransformed tubers. This study suggests that the increased accumulation of AsA could upregulate the antioxidant system which imparts improved tolerance against various abiotic stresses in transgenic tubers compared to untransformed tubers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ascorbate (vitamin C, l-ascorbic acid) is an abundant molecule in plant cells (Smirnoff and Wheeler 2000), present in all subcellular compartments, including the apoplast (cell wall), chloroplasts, cytosol, vacuoles, mitochondria and peroxisomes (Rautenkranz et al. 1994; Foyer and Lelandais 1996; Jimenez et al. 1997). It functions as an antioxidant, an enzyme cofactor, and also as a precursor for oxalate and tartrate synthesis (reviewed in Loewus 1999). It participates in a variety of cellular processes, including cell wall growth and cell expansion, resistance to environmental stress and senescence (Smirnoff and Wheeler 2000; Conklin and Barth 2004; Pavet et al. 2005). In plants, ascorbate interacts enzymatically and non-enzymatically to detoxify damaging reactive oxygen species (ROS) as well as in the regeneration of vitamin E (Thomas et al. 1992).
ROS, including the superoxide anion radical (O2 −), the hydroxyl radical (OH•) and hydrogen peroxide (H2O2), tend to increase in plants exposed to different stress conditions. Injuries associated with ROS, collectively referred to as oxidative stresses, are among the most profoundly damaging factors in plants. Under conditions of environmental stress such as high temperatures, low temperatures, salinity and drought, the ROS levels tend to increase in plant cells, which ultimately affects the growth and productivity of the plants. Therefore, plants have developed a number of antioxidant defense mechanisms to protect themselves against these ROS, including both enzymatic and non-enzymatic detoxification reactions and damage repair. Many antioxidant enzymes act jointly to keep a healthy cell redox status in different cellular compartments. Among these, superoxide dismutases (SOD, EC 1.15.1.1) constitute a frontline defense, by removing the superoxide (O2 −) radicals. The resulting hydrogen peroxide (H2O2) is further detoxified by catalases (CAT, EC 1.11.1.6). The H2O2 is also removed by the action of peroxidases, which require a reducing substrate as an electron donor (Noctor and Foyer 1998). In plants, ascorbate peroxidases (APX, EC 1.11.1.11) are the major enzymes among the reducing substrate-dependent peroxidases involved in intracellular H2O2 removal. These enzymes utilize ascorbate as an electron donor (Noctor and Foyer 1998). The APX catalyzes the first step of the ascorbate–glutathione cycle that plays a major antioxidant role in plant cells (Electronic Supplementary Material Fig. A). In this cycle, ascorbate and glutathione are employed as reducing agents to detoxify H2O2. The oxidized ascorbate and glutathione are not expended, but recovered at the expense of ATP and NAD(P)H. Besides APX, monodehydroascorbate reductase (MDHAR, EC 1.6.5.4), dehydroascorbate reductase (DHAR, EC 1.8.5.1) and glutathione reductase (GR, EC 1.8.1.7) also catalyze important steps of this cycle (Alscher et al. 1997; Noctor and Foyer 1998). Protection against ROS by ascorbate together with glutathione and several other enzymatic antioxidants during the Mehler reaction (Asada 1999) and photorespiration is also reported in plants (Noctor and Foyer 1998). Ascorbate is believed to detoxify the superoxide anion radical and the hydroxyl radical (Smirnoff 1996; Noctor and Foyer 1998; Asada 1999) and also acts under excess light as a cofactor of violaxanthin, which is involved in the non-photochemical quenching of excess excited energy in photosystem II (PSII) (Demmig-Adams 1990; Eskling et al. 1997). Ascorbate also plays a crucial role both in scavenging ROS produced in photosynthesis and in dissipating excess photons (Demmig-Adams and Adams 1992; Niyogi 1999).
The beneficial properties of ascorbate for plant health were already exploited 50 years ago: spraying plants with ascorbate solutions prevented plant damage by air-borne oxidizing agents (Freebairn and Taylor 1960). Plants with high ascorbate concentrations in their tissues proved to be more resistant to oxidative damage by ozone than plants with low ascorbate concentrations (Lee et al. 1984; Lee 1991). Particular cultivars of common bean maintaining higher ascorbate concentrations in the leaf apoplast were less affected by ozone treatment (Burkey and Eason 2002). Also, the higher stress sensitivity of the ascorbate-deficient Arabidopsis mutant soz1 confirms a close relationship between the ascorbate status of the plant tissue and its tolerance of environmental stresses (Conklin et al. 1996). The activity of the antioxidant enzymes associated with the detoxification of ROS can be increased via appropriate gene transfer, and the possible effects of such transformations on plant resistance to environmental stresses have been investigated (Inzé and Van Montagu 1995; Noctor and Foyer 1998; McKersie et al. 1999; Sen-Gupta et al. 1993; Allen et al. 1997; Payton et al. 1997; Chen et al. 2003). Transgenic tobacco plants expressing both CuZnSOD and APX in chloroplasts showed elevated tolerance to oxidative stresses induced by methyl viologen (MV), a ROS-generating herbicide (Kwon et al. 2002), and transgenic plants expressing human DHAR in the chloroplasts with high ascorbate levels showed increased tolerance to both MV-induced oxidative stress and salt stress (Kwon et al. 2003). However, there appear to be no investigations on the effect of elevated levels of ascorbate in in vitro tuberizing transgenic potato plants treated with various stress-inducing agents. In this study we investigated the effect of increased levels of ascorbate on the antioxidant enzyme system and the ascorbate and glutathione metabolic flux in in vitro transgenic potato tubers produced under stress conditions induced by MV, NaCl and ZnCl.
Materials and methods
Transformation and development of transgenic potato plants
Transgenic potato (Solanum tuberosum L. cv. Taedong Valley) plants overexpressing the strawberry d-galacturonic acid reductase (GalUR) gene were generated via Agrobacterium tumefaciens-mediated transformation. The putative T0 transformants were screened by PCR and Southern blotting for the integration of GalUR gene as described (Hemavathi et al. 2009, data not shown here). The sprouted T0 tubers were planted in pots (25 cm) to obtain T1 plants as well as T1 tubers. Single node cuttings from the Southern-positive T1 transgenic line were used for the tuber induction and in vitro stress analyses.
Plant material, treatments and in vitro tuberization
The Southern-positive T1 transgenic potato line overexpressing GalUR with elevated ascorbate (Hemavathi et al. 2009) was used for these experiments. The transgenic and untransformed plants grown in vitro were maintained on MS basal medium. To investigate the effect of various abiotic stresses on in vitro tuberization, single node cuttings (1.0 cm long) from T1 transgenic potatoes were sub-cultured in 25 × 150 mm Pyrex glass culture tubes containing 15 ml of MS (Murashige and Skoog 1962) medium supplemented with 9% sucrose, 10 μM MV (methyl viologen), 100 mM sodium chloride, or 20 mM zinc chloride. Tubes containing MS medium supplemented with sucrose without any stress agents served as control. The tubes with nodal cuttings were maintained in the dark at 18°C for ca. 3 weeks. Ten T1 transgenic as well as untransformed nodal segments were used for each stress treatment.
Assay of ROS scavenging enzymes
The frozen tuber samples (0.5 g) were ground in liquid nitrogen to a fine powder and homogenized in 50 mM phosphate buffer (pH 7.0) and 100 mM triethanolamine buffer (TEA, pH 7.4). The homogenates were centrifuged at 16,000g for 20 min at 4°C and the supernatants were collected for assay of CAT, APX, DHAR and GR activities. The protein content of the crude extract was quantified by the protein dye binding assay with bovine serum albumin as the standard (Bradford 1976).
SOD activity was determined according to the protocol described by Paoletti et al. (1986) based on the inhibition of superoxide-driven NADH oxidation. The assay mixture contained 100 mM triethanolamine (TEA, pH 7.4), 100/50 mM EDTA/MnCl2, 7.5 mM NADPH and 10 mM mercaptoethanol in a total volume of 1.0 ml. The reaction was initiated by adding mercaptoethanol solution and the oxidation of NADH was measured at 340 nM (extinction coefficient 6.2 mM−1 cm−1). One unit of SOD was defined as the amount of enzyme oxidizing 1 nmol of NADPH per min.
CAT activity was measured according to the method described by Aebi (1984). The assay mixture contained 3.125 mM H2O2 in 50 mM phosphate buffer (pH 7.0) and 100 μl of enzyme extract in a total volume of 3.0 ml. CAT activity was estimated by the decrease in absorbance of H2O2 at 240 nm. One unit of CAT was defined as the amount of enzyme dismuting 1.0 nmol of H2O2 per min.
APX activity was determined according to the method of Nakano and Asada (1981) in 3 ml of a reaction mixture containing 50 mM potassium phosphate (pH 7.0), 2 mM ascorbate, 2 mM H2O2 and 100 μl of enzyme extract. Oxidation of ascorbate was determined by monitoring the decrease in absorbance at 300 nm (extinction coefficient 0.74 mM−1 cm−1). One unit of APX was defined as the amount of enzyme oxidizing 1 nmol of ascorbate per min.
DHAR activity was determined as described by Nakano and Asada (1981). The reaction mixture (1.0 ml) contained 50 mM phosphate buffer (pH 7.0), 20 mM reduced glutathione, 2 mM dehydroascorbate and 100 μl crude enzyme. DHAR activity was assayed at 25°C by following the increase in absorbance at 265 nm (extinction coefficient 6.2 mM−1 cm−1) due to the GSH-dependent production of AsA.
Glutathione reductase activity was determined according to the method of Foyer and Halliwell (1976). The assay mixture (1.0 ml) contained 1 mM oxidized glutathione, 2 mM NADPH and 50 μl crude enzyme at 25°C. Oxidation of NADPH was determined by monitoring the decrease in absorbance at 340 nm (extinction coefficient 6.2 mM−1 cm−1).
RT–PCR and real-time PCR
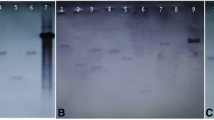
Quantitative real-time PCR and semi-quantitative RT–PCR analyses were also performed (data not shown) at mRNA expression levels to establish a correlation with the expression of these antioxidant proteins. Total RNA was isolated from the transgenic as well as from the untransformed tubers grown under stress condition using TRI reagent (Sigma, USA). RT–PCR analysis was carried out for the evaluation of transcript levels of various stress-related genes (SOD, CAT, APX, DHAR and GR). First-strand cDNA was synthesized using SuperScript™ Reverse Transcriptase (Invitrogen, USA) from 2 μg of total RNA in 20 μl reaction volume. The reaction mixture (1 μl) was subjected to subsequent RT–PCR in 25 μl PCR reaction volume. Sequences of primers used for the various genes are shown in Table 1. Actin served as loading control. The amplification products were separated on 1% agarose gel stained with ethidium bromide and visualized with UV light.
Real-time PCR was carried out using RNA treated with DNase I before use in reverse transcription. Random hexamer primers and SuperScript-II reverse transcriptase (Invitrogen, USA) were used to generate a first-strand cDNA template. Samples were amplified using an ABI Prism 7700 sequence detector (Applied Biosystems). The real-time PCR amplification of ROS pathway genes (SOD, CAT, APX, DHAR and GR) were carried out using cDNA specific primers as mentioned in Table 1. The PCR was performed using a SYBR green PCR kit (Qiagen, Hilden, Germany). Actin was used as an internal control. Comparative threshold (Ct) values were normalized to actin control and compared to obtain relative expression levels.
Ascorbate and glutathione assay
Ascorbate and DHA were assayed according to the method of Kampfenkel et al. (1995). Tissue samples (0.5 g) were homogenized in 8 ml 3% (w/v) metaphosphoric acid containing 1 mM EDTA on ice and centrifuged at 12,000g for 20 min. The assay is based on the reduction of Fe3+ to Fe2+ by AsA in acidic solution. Total ascorbate was determined with 50 μl of extracted solution by initially incubating for 20 min in 200 mM phosphate buffer solution (pH 7.4) and 1.5 mM dithiothreitol (DTT) to reduce all DHA to AsA. After incubation, 200 μl of 0.5% (w/v) N-ethylmaleimide (NEM) was added to remove excess DTT. AsA was assayed in a similar manner except that 400 μl of deionized H2O was substituted for DTT and NEM. Color was developed in both the series of reaction mixtures with the addition of 10% trichloroacetic acid, 42% phosphoric acid, 65 mM 2,2′-dipyridyl in 70% (v/v) ethanol and 3% (w/v) FeCl3. The reaction mixtures were then incubated at 42°C for 1 h and quantified at 525 nm using a spectrophotometer. Standard curves of ascorbate and DHA were established and used for quantification.
Determination of glutathione in the reduced (GSH) and the oxidized (GSSG) form was carried out according to the method of Griffith (1980). Non-protein thiols were extracted by homogenizing the samples (1 g) in 6 ml 5% (w/v) sulfosalicylic acid on ice and then centrifuged at 10,000g for 20 min. The supernatant was collected and used for analysis. Total glutathione was determined at 412 nm with a spectrophotometer using yeast-GR, 5,5′-dithio-bis-nitrobenzoic acid and NADPH. GSSG was determined by the same method in the presence of 2-vinylpyridine at 27°C for 1 h to derivatize GSH before adding GR. The GSH content was calculated from the difference between total GSH and GSSG. A standard curve of GSH was used for quantification.
Proline determination
Extraction and colorimetric determination of proline was carried out according to the method described by Bates et al. (1973). Tuber samples (0.5 g) were ground in a mortar after the addition of a small amount of quartz sand and 3 ml of a 3% (w/v) aqueous sulfosalicylic acid solution. The homogenate was filtered through layers of glass-fiber filter and the clear filtrate was then used for assay. To 0.2 ml of the supernatant, 0.4 ml of distilled water and 2 ml of reagent mixture consisting of 30 ml glacial acetic acid, 20 ml distilled water and 0.5 g ninhydrin was added. The closed test tubes with the reaction mixture were kept in a boiling water bath for 1 h, cooled and extracted with 6 ml of toluene. Readings were taken immediately at a wavelength of 546 nm. The proline concentration was determined from a standard curve and calculated on a fresh weight basis (μmol proline g−1 FW).
Lipid peroxidation
The levels of malondialdehyde (MDA), a measure of lipid peroxidation, were assessed as described by Heath and Packer (1968) by measuring thiobarbituric acid reactive substances (TBARS). Tuber samples (0.5 g) from T1 transgenic and untransformed plants were homogenized in a solution of 0.5% (w/v) TBA in 20% (w/v) TCA. The homogenate was incubated at 95°C for 30 min, the reaction was stopped on ice, and samples were centrifuged at 12,000g for 10 min. The absorbance of the resultant supernatant was measured at 532 and 600 nm. The non-specific absorbance at 600 nm was subtracted from the absorbance at 532 nm and the MDA concentration was calculated using its extinction coefficient of 155 mM−1 cm−1.
Statistical analysis
Three replicates of each sample were used for statistical analysis and the means were analyzed using Statistical Analysis Software package program 9.1 (SAS, USA). Statistical differences were determined using one-way analysis of variance (ANOVA) and means were considered significantly different at P < 0.01.
Results
Effect of abiotic stress on in vitro tuber production
Both transgenic as well as untransformed nodal segments of microtubers were observed growing under different abiotic stress conditions with delayed tuber formation and reduced tuber fresh weight (Fig. 1) compared to unstressed control conditions. The transgenic tubers experienced a minor decrease in fresh weight under the stress conditions compared to the untransformed control tubers.
Enzymatic activities of microtubers growing under stress conditions
The enzymatic activities of the major ROS scavengers were analyzed in order to understand the effect of abiotic stress treatments such as oxidative, salt and heavy metal stresses on the antioxidant capacity of tuberizing transgenic potato. The specific activity of SOD significantly increased in the transgenic tubers compared to untransformed tubers growing under these stress conditions. The activity of SOD in the transgenic tubers was ca. 2.1-fold higher under MV stress and 1.9-fold higher under salt and metal stresses (Fig. 3a). Under unstressed control conditions, no significant difference was seen in the SOD activity in both transgenic and untransformed tubers (Fig. 2a).
Specific activity of enzymes in the antioxidant system in transformed (T) and untransformed (UT) potato tubers subjected to oxidative (MV), salt (NaCl) and heavy metal (ZnCl) stresses. SOD, CAT, APX, DHAR and GR activities (a–e). The values are presented as the mean ± SEM of three replicates. *, Means were significantly different at P < 0.01
A significant increase in the specific activity of CAT was also observed in the transgenic and untransformed tubers under stress conditions compared to the unstressed control conditions (Fig. 2b). The specific activity of APX increased up to 2.3-fold in the transgenic tubers under stress conditions and its activity was highest in transgenic tubers growing under MV stress (Fig. 2c). Similarly, the specific activity of DHAR also increased significantly (ca. 2–1.6-fold) in the transgenic tubers growing under the stress conditions compared to untransformed tubers (Fig. 2d). The specific activity of GR also showed a similar trend, with its maximum expression in transgenic tubers grown under salt stress when compared to control tubers (Fig. 2e). The specific activities of these antioxidant enzymes showed positive correlation with their mRNA expression levels as measured by quantitative real-time PCR and semi-quantitative RT–PCR analyses (data shown as Electronic Supplementary Material Fig. B).
Biochemical characterization of stressed tubers
The ratios of reduced to oxidized ascorbate (AsA:DHA) and reduced to oxidized glutathione (GSH:GSSG) were calculated in the transgenic and untransformed microtubers growing under stressed and controlled conditions. An increase in the ratio of ascorbate (AsA:DHA) was observed under stressed conditions in the case of both transgenics and untransformed control tubers. A significant increase in the redox state of ascorbate (up to 2.0-fold) was observed in transgenic tubers under these stress conditions compared to untransformed tubers (Fig. 3a). The transgenic tubers grown under stress conditions also showed an altered ratio of reduced to oxidized glutathione. The levels of GSH increased significantly in stressed transgenic tubers, thus increasing the glutathione redox status (GSH:GSSG) up to 2–2.2-fold in stressed transgenic tubers (Fig. 3b).
Biochemical characterization of transformed (T) and untransformed (UT) potato tubers subjected to oxidative (MV), salt (NaCl) and heavy metal (ZnCl) stresses. Ascorbate (a) and glutathione (b) contents in transgenic and untransformed tubers grown under various stresses. Values are mean ± SEM of three replicates. The values are presented as the mean ± SEM of three replicates. *, Means were significantly different at P < 0.01
Proline accumulation in potato tubers
Proline is a common osmolyte in higher plants which accumulates in response to stress. The change in the proline content was measured in the transgenic and untransformed tubers growing under stress conditions. The proline content increased in both untransformed and transformed tubers under stress conditions. However, a more dramatic increase in proline content was recorded in the transgenic tubers (ca. 2–2.8-fold) grown under stress conditions compared to untransformed tubers (Fig. 4a). The increase in proline content was greater in the transgenic tubers grown under salt stress than under MV and metal stress.
MDA content
A reduction in membrane permeability during abiotic stress could be due to peroxidation of polyunsaturated fatty acids in the membranes, which results in the formation of MDA. The level of MDA was therefore measured as an indicator of lipid peroxidation. The level of MDA as a consequence of MV, salt and heavy metal stresses was increased in both transgenic and untransformed tubers. However, in the transgenic tubers, a mild increase (33–47%) in MDA level was detected compared to a dramatic increase (215–300%) in the untransformed tubers under stress conditions (Fig. 4b).
Discussion
Plants subjected to various abiotic stresses undergo impairment of electron transport systems in the membranes, resulting in increased ROS production (Smirnoff 1993; Navari-Izzo and Rascio 1999). Antioxidant mechanisms operate to effectively remove ROS such as H2O2, free active oxygen, or hydroxyl free radicals (Noctor and Foyer 1998; Smirnoff 2005). An efficient approach to overcome these stresses in plants is to manipulate the genes in order to enhance the levels of antioxidants such as ascorbate, glutathione, glyoxalase, or tocopherols. Studies from different plant systems suggested the involvement of ascorbate in providing resistance to various stresses such as ozone (Sanmartin et al. 2003; Chen and Gallie 2005), high temperature (Larkindale et al. 2005), low temperature (Kwon et al. 2002, 2003), excess light (Ma and Cheng 2004; Bartoli et al. 2006), salt (Shalata and Neumann 2001), oxidative (Tokunaga et al. 2005) and pathogen stress (Barth et al. 2004; Pavet et al. 2005).
It is well known that abiotic stress in plants induces the accumulation of ROS species. In order to alleviate the risk of ROS accumulation, plants respond by activating different ROS scavenging pathways (Levine et al. 1994; Apel and Hirt 2004; Fujita et al. 2006). In this study we analyzed the transcript levels as well as specific activities of five important ROS-scavenging enzymes in transgenic potato tubers over-expressing the GalUR gene growing under various stress treatments. The transcript levels shown by real-time PCR and the enzyme activities demonstrated positive correlation. As expected, a significant increase in SOD and APX activity was observed under each stress condition in the transgenic tubers compared to untransformed controls. MV and salt stress could induce relatively higher SOD and APX activity than the metal stress treatment. The increase in SOD activity often results in cytotoxic conditions due to the formation of H2O2 and subsequent generation of free hydroxyl radicals from H2O2 through a Fenton-type reaction (Finazzi-Agro and Di Giulio 1986; Scott et al. 1987). Free hydroxyl radicals can damage virtually all types of macromolecules such as carbohydrates, nucleic acids, lipids and amino acids. As APX is a major H2O2-scavenging enzyme in plants (Nakano and Asada 1981), its activity significantly increased with increased levels of H2O2, confirming the earlier reports (Benavides et al. 2000; del Rio et al. 2002). Similar results were reported with in vitro potato tubers exposed to freezing stress (Mora-Herrera and Lopez-Delgado 2007) and potato seedlings exposed to salt stress (Rahnama and Ebrahimzadeh 2005). However, the increase in specific activity of CAT in stressed transgenic tubers was moderate compared to SOD and APX, suggesting an important role of APX in the detoxification of H2O2.
The increase in DHAR activity ensures efficient regeneration of ascorbate, which can scavenge increased levels of H2O2 under stress conditions. Increases in the activity of DHAR and GR (another ascorbate–glutathione cycle enzyme) in stressed transgenic tubers also demonstrated improved tolerance to various oxidative stresses (Foyer et al. 1991, 1995; Aono et al. 1995). The peroxidase activity also increased, along with the activities of other antioxidant enzymes like CAT, SOD and GR in response to various environmental stresses, suggesting that various components of ROS-scavenging systems were co-regulated (Shigeoka et al. 2002). These enzymes have a possible synergy to jointly resist oxidative damage caused by MV, salt and metal stresses.
In the present study, the ascorbate redox state (AsA:DHA) and ratio of reduced to oxidized glutathione (GSH:GSSG) were found to be significantly higher (ca. 2–2.2-fold) in stressed transgenic tubers compared to stressed untransformed tubers. Similar results with increased AsA:DHA and GSH:GSSG ratios were reported in the transgenic tobacco plants expressing the DHAR gene (Kwon et al. 2003) and tobacco plants expressing CuZnSOD, APX and DHAR (Lee et al. 2007). Increased redox status due to the increase in AsA and glutathione content was accomplished by the elevated activities of DHAR and GR (Foyer and Noctor 2003), suggesting the important role of these enzymes in recycling and maintaining AsA–glutathione content under stress conditions. In plant cells, GSH is also a key non-enzymatic antioxidant that scavenges ROS either directly or indirectly by participating in the ascorbate–glutathione cycle (Noctor and Foyer 1998; Polle 2001). The increased level of GSH is regarded as a protective response against oxidative stress (May and Leaver 1993). It regenerates AsA through the Halliwell–Asada pathway (Jimenez et al. 1997; Noctor and Foyer 1998; Asada 1999) and maintains ascorbate redox status (Shalata et al. 2001).
In general, ROS accumulation in plants caused by abiotic stresses can result in considerable damage to the membrane lipids and proteins (Apel and Hirt 2004). Increased levels of proline accumulation in stressed transgenic tubers correlated with enhanced stress toleranceMunns, 2005 R. Munns, Genes and salt tolerance: bringing them together, New Phytol (2005), pp. 645–663. Also, the levels of MDA were lower in transgenic tubers, suggesting low levels of membrane damage by lipid peroxidation in transgenic tubers. Similarly, enhanced accumulation of proline and reduced levels of MDA imparted salt and drought tolerance in transgenic tall fescue overexpressing Arabidopsis AtHDG11 (Cao et al. 2009). Elevated levels of AsA in stressed transgenic tubers might have reduced the accumulation of TBARS and protected the cellular membranes by inhibiting lipid peroxidation. In conclusion, the transgenic potato tubers with elevated AsA levels could tolerate and survive multiple abiotic stresses remarkably well. The increase in plant resistance to ROS induced by these stresses was due to the de-novo synthesis of antioxidant enzymes accompanied by subsequent increase in the pool of antioxidants, possibly required to maintain the flux of reduced metabolites through the AsA–glutathione cycle and to reduce membrane damage by inhibiting lipid peroxidation.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- DHAR:
-
Dehydroascorbate reductase
- GalUR:
-
d-galacturonic acid reductase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- MDA:
-
Malondialdehyde
- MDHAR:
-
Monodehydroascorbate reductase
- MV:
-
Methyl viologen
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- ZnCl:
-
Zinc chloride
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:21–126
Allen RD, Webb RP, Schake SA (1997) Use of transgenic plants to study antioxidant defenses. Free Radicals BioMed 23:473–479
Alscher RG, Donahue JL, Crammer CL (1997) Reactive oxygen species and antioxidants: relationship in green cells. Plant Physiol 100:224–233
Aono M, Saji H, Sakamoto A, Tanaka K, Kondo N (1995) Paraquat tolerance of transgenic Nicotiana tabacum with enhanced activities of glutathione reductase and superoxide dismutase. Plant Cell Physiol 36:1687–1691
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Ann Rev Plant Physiol Plant Mol Biol 50:601–639
Barth C, Moeder W, Klessig DF, Conklin PL (2004) The timing of senescence and response to pathogens is altered in the ascorbate deficient Arabidopsis mutant vitamin c-1. Plant Physiol 134:1784–1792
Bartoli CG, Yu JP, Gómez F, Fernández L, McIntosh L, Foyer CH (2006) Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot 57:1621–1631
Bates LE, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Benavides MP, Marconi PL, Gallego SM, Comba ME, Tomaro ML (2000) Relationship between antioxidant defence systems and salt tolerance in Solanum tuberosum. Aust J Plant Physiol 27:273–278
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 72:248–254
Burkey KO, Eason G (2002) Ozone tolerance in snap bean is associated with elevated ascorbic acid in the leaf apoplast. Physiol Plant 114:387–394
Cao YJ, Wei Q, Liao Y, Song HL, Li X, Xiang CB, Kuai BK (2009) Ectopic overexpression of AtHDG11 in tall fescue resulted in enhanced tolerance to drought and salt stress. Plant Cell Rep 28:579–588
Chen Z, Gallie DR (2005) Increasing tolerance to ozone by elevating foliar ascorbic acid confers greater protection against ozone than increasing avoidance. Plant Physiol 138:1673–1689
Chen Z, Todd E, Ling YJ, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100:3525–3530
Conklin PL, Barth C (2004) Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ 27:959–970
Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA 3:9970–9974
del Rio LA, Corpas FJ, Sandalio LM, Palme JM, Gómez M, Barroso JB (2002) Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J Exp Bot 53:1255–1272
Demmig-Adams B (1990) Carotenoids and photoprotection: a role for the xanthophylls zeaxanthin. Biochim Biophys Acta 1020:1–24
Demmig-Adams B, Adams WW III (1992) Photoprotection and other responses to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43:599–626
Eskling M, Arvidsson PO, Akerlund HE (1997) The xanthophyll cycle, its regulation and components. Physiol Plant 100:806–816
Finazzi-Agro A, Di Giulio A, Amicosante G, Crifo C (1986) Photohemolysis of erythrocytes enriched with superoxide dismutase, catalase, and glutathione peroxidae. Photochem Photobiol 43:409–412
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Lelandais M (1996) A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cells. J Plant Physiol 148:391–398
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisome and mitochondria. Physiol Plant 119:355–364
Foyer C, Lelandais M, Galap C, Kunert KJ (1991) Effect of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. Plant Physiol 97:863–872
Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C (1995) Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance in photoinhibition in poplar trees. Plant Physiol 109:1047–1057
Freebairn HT, Taylor OC (1960) Prevention of plant damage from airborne oxidising agents. Proc Am Soc Hort Sci 76:693–699
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Ann Biochem 106:207–212
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hemavathi, Upadhyaya CP, Ko EY, Nookaraju A, Kim HS, Heung JJ, Oh MO, Reddy AC, Chun SC, Kim DH, Park SW (2009) Over-expression of strawberry D-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci 177:659–667
Inzé D, Van Montagu M (1995) Oxidative stress in plants. Curr Opin Biotechnol 6:153–158
Jimenez A, Hernandez JA, del Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Kampfenkel K, Van Montagu M, Inze D (1995) Effect of iron excess on Nicotiana plumbaginifolia plants. Implications to oxidative stress. Plant Physiol 107:725–735
Kwon SY, Jeong YJ, Lee HS, Kim JS, Cho KY, Allen RD (2002) Enhanced tolerance of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen mediated oxidative stress. Plant Cell Environ 25:873–882
Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, Park YM, Kwak SS (2003) Enhanced stress-tolerance of transgenic plants expressing a human dehydroascorbate reductase gene. J Plant Physiol 160:347–353
Larkindale J, Mishkind M, Vierling E (2005) Plant responses to high temperature. In: Jenks MA, Hasegawa PM (eds) Plant abiotic stress. Blackwell Scientific Publications, Oxford
Lee EH (1991) Plant resistance mechanisms to air pollutants: rhythms in ascorbic acid production during growth under ozone stress. Chronobiol Int 8:93–102
Lee EH, Jersey JA, Gifford C, Bennett J (1984) Differential ozone tolerance in soybean and snapbeans: analysis of ascorbic acid in O3-susceptible and O3-resistant cultivars by high performance liquid chromatography. Environ Exp Bot 24:331–341
Lee YP, Kim SH, Bang JW, Lee HS, Kwak SS, Kwon SY (2007) Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Rep 26:591–598
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Loewus FA (1999) Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry 52:193–210
Ma FW, Cheng LL (2004) Exposure of the shaded side of apple fruit to full sun leads to upregulation of both xanthophyll cycle and the ascorbate-glutathione cycle. Plant Sci 166:1479–1486
May M, Leaver CJ (1993) Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension-cultures. Plant Physiol 103:621–627
McKersie BD, Bowley SR, Jones KS (1999) Winter survival of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol 119:839–847
Mora-Herrera ME, Lopez-Delgado HA (2007) Freezing tolerance and antioxidant activity in potato microplants induced by abscisic acid treatment. Am J Potato Res 84:467–475
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Navari-Izzo F, Rascio N (1999) Plant response to water-deficit conditions. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker Inc, New York, pp 231–270
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Paoletti F, Aldinucci D, Mocali A, Caparrini A (1986) A sensitive spectrometric method for the determination of superoxide dismutase activity in tissue extracts. Ann Biochem 54:536–541
Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH (2005) Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiol 139:1291–1303
Payton P, Allen RD, Trolinder N, Holaday AS (1997) Over-expression of chloroplast-targeted Mn superoxide dismutase in cotton (Gossypium hirsutum L., cv. Coker 312) does not alter the reduction of photosynthesis after short exposures to low temperature and high light intensity. Photosyn Res 52:233–244
Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathione pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol 126:445–462
Rahnama H, Ebrahimzadeh H (2005) The effect of NaCl on antioxidant enzyme activities in potato seedling. Biol Planta 49:93–97
Rautenkranz AAF, Li L, Michler F, Mgxtinoia E, Oertli JJ (1994) Transport of ascorbic and dehydroascorbic acids across protoplast and vacuole membranes isolated from barley (Hordeum vulgate L. cv Gerbel) leaves. Plant Physiol 106:187–193
Sanmartin M, Drogoudi PA, Lyons T, Pateraki I, Barnes J, Kanellis AK (2003) Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta 216:918–928
Scott MD, Meshnick SR, Eaton JW (1987) Superoxide dismutase-rich bacteria. Paradoxical increase in oxidant toxicity. J Biol Chem 262:3640–3645
Sen-Gupta A, Heinen J, Holaday AS, Burke JJ, Allen RD (1993) Increased resistance to oxidative stress in transgenic plants that over-express chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA 90:1629–1633
Shalata A, Neumann PM (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52:2207–2211
Shalata A, Mittova V, Volokita M, Guy M, Tal M (2001) Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol Plant 112:487–494
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and functions of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1311
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Smirnoff N (2005) Ascorbate, tocopherol and carotenoids: metabolism, pathway engineering and functions. In: Smirnoff N (ed) Antioxidants and reactive oxygen species in plants. Blackwell Publishing Ltd, Oxford, pp 53–86
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci 19:267–290
Thomas CE, McLean LR, Parker RA, Ohlweiler DF (1992) Ascorbate and phenolic antioxidant interactions in prevention of liposomal oxidation. Lipids 27:543–550
Tokunaga T, Miyahara K, Tabata K, Esaka M (2005) Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for L-galactono-1, 4-lactone dehydrogenase. Planta 220:854–863
Acknowledgments
This research was supported by Konkuk University research fund. The research fellowship from Konkuk University to Hemavathi as research fellow is gratefully acknowledged. We thank Mayank A. Gururani and Shashank K. Pandey for experimental assistance and proof-reading of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hemavathi and Chandrama Prakash Upadhyaya contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11032_2010_9465_MOESM1_ESM.tif
Fig. A. Antioxidant defense system in plants (Halliwell-Asada pathway; modified from Bowler et al. 1992). The dismutation of superoxide radicals (O2 −) occurs in this pathway. APX-ascorbate peroxidase; DHAR-dehydroascorbate reductase; GR-glutathione reductase; GSH-glutathione (red.); GSSG-glutathione (ox.); SOD-superoxide dismutase (TIFF 176 kb)
11032_2010_9465_MOESM2_ESM.tif
Fig. B. Quantitative real time PCR analysis of antioxidant protein gene expression profiling during various stress treatments. The cDNA was normalized in dependence of the transcript level of actin mRNA. Antioxidant enzyme gene expression in transformed and untransformed potato tubers under control conditions (A), Methyl viologen i.e. Oxidative stress (B); NaCl i.e. salt stress (C) and ZnCl i.e. heavy metal stress (D). The values are presented as the mean ± SEM of three replicates (TIFF 201 kb)
Rights and permissions
About this article
Cite this article
Hemavathi, Upadhyaya, C.P., Akula, N. et al. Biochemical analysis of enhanced tolerance in transgenic potato plants overexpressing d-galacturonic acid reductase gene in response to various abiotic stresses. Mol Breeding 28, 105–115 (2011). https://doi.org/10.1007/s11032-010-9465-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-010-9465-6