Abstract
Although gibberellins (GAs) have been shown to induce development of the physiological disorder blossom-end rot (BER) in tomato fruit (Solanum lycopersicum), the mechanisms involved remain largely unexplored. BER is believed to result from calcium (Ca) deficiency, but the relationship between Ca content and BER incidence is not strong. Our objectives were to better understand how GAs and a GA biosynthesis inhibitor affect BER development in tomato fruit. Tomato plants of two BER-susceptible cultivars, ‘Ace 55 (Vf)’ and ‘AB2,’ were grown in a greenhouse environment and subjected to Ca-deficiency conditions. Plants were treated weekly during fruit growth and development with 300 mg L−1 GA4+7, 300 mg L−1 prohexadione-calcium (Apogee®, a GA biosynthesis inhibitor), or water beginning 1 day after flower pollination. GA4+7 treatment induced an increase in BER incidence in both cultivars up to 100%, whereas ‘Ace 55 (Vf)’ and ‘AB2’ plants treated with Apogee did not show BER incidence. The number of functional xylem vessels was higher in the placental and pericarp tissue of tomato fruit treated with Apogee at the early stages of fruit growth. Treatment with Apogee also increased fruit pericarp Ca concentration. GA4+7 treatment enhanced the expression of the putative CAX and Ca-ATPase genes, that code for proteins involved in Ca movement into storage organelles. The lowest water-soluble apoplastic Ca concentration and the highest membrane leakage values were observed in the pericarp of GA4+7-treated fruit. These results suggest that GAs consistently reduced fruit Ca uptake and water-soluble apoplastic Ca concentration, leading to leakier plasma membranes and an increase in BER development in fruit tissue of both tomato cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanisms responsible for calcium (Ca)-deficiency development in fruit are among the most complex and challenging processes in plants. In tomato fruit, blossom-end rot (BER), considered to be a Ca-deficiency disorder, has been studied for more than 100 years and the mechanisms that trigger this disorder are still not well understood (Ho and White 2005). The complexity is based on the fact that the total level of Ca in the tissue cannot be used to predict BER development, and fruit with the same or even greater levels of total Ca can have higher BER incidence (Castro 1980; Nonami and others 1995).
Microscopic studies have shown that plasma membrane breakdown is the first visual symptom of BER, followed by tissue oxidation. The tissue eventually becomes dark and corky at the blossom end of the fruit (Suzuki and others 2003; Ho and White 2005). Peripheral cells around the necrotic tissue of BER have a normal internal structure, but the plasma membrane is detached from the cell wall, suggesting increased membrane permeability, leakage of solutes to the apoplast, and plasmolysis of the cell (Suzuki and others 2000). Other studies have shown an increase in Ca bound to the plasma membrane as the distance from collapsed cells increased (Suzuki and others 2003). Calcium is known to bind to phospholipids and proteins on the membrane surface, which is required to maintain proper membrane structure and integrity (White and Broadley 2003). Because cytosolic levels of Ca are extremely low, plasma membrane structure and stability are dependent on the apoplastic pool of free Ca. Previous studies have shown that low levels of free Ca in the apoplast negatively affect membrane structure and integrity, resulting in leaky membranes (Hanson 1960; Kirby and Pilbeam 1984; Picchioni and others 1998; Suzuki and others 2003). Therefore, mechanisms that favor a reduction in the levels of Ca in the apoplast also favor BER development in fruit tissue. The symptoms of BER appear during the early stages of cell and fruit expansion when high rates of gibberellin (GA) biosynthesis occur (El-Beltagy and others 1976). Gibberellin has been suggested to limit fruit Ca uptake and affect Ca partitioning and distribution at the cellular level (Saure 2001, 2005; Ho and White 2005).
Calcium movement to the fruit is exclusively xylemic (Ferguson and Watkins 1989). Inside the fruit, functional xylem vessels and Ca content are greater at the peduncle end, decreasing toward the blossom end, which implies that Ca movement along the fruit tissue is also dependent on the abundance of functional xylem vessels (Nonami and others 1995; Ho and White 2005). The mechanisms involved in functional xylem development and maintenance are still not well understood, but growth regulators are suggested to play a role (Nelson and Dengler 1997). Physiologically active GAs are known to inhibit tissue differentiation (Sachs 2000; Aloni 2001), which may have a negative effect on functional xylem vessel development in the fruit. Alternatively, GAs are known to trigger vigorous cell expansion, which has been suggested to induce obstruction of xylem vessels in the fruit (Drazeta and others 2004; Ho and White 2005; Saure 2005). Whether inhibition of differentiation and obstruction of xylem vessels results in a restriction in xylemic movement of water and Ca into the fruit and an increase in BER incidence remains to be examined.
Tomato fruit with similar Ca content have been reported to have different incidences of BER, implying that fruit with adequate Ca uptake can still develop the disorder (Nonami and others 1995). In this case, cellular Ca partitioning and distribution can be the limiting factor, and an imbalance in Ca partitioning and distribution in the cell can eventually trigger BER development in the fruit. Calcium partitioning and distribution is controlled by complex cellular mechanisms, including the expression and activity of Ca-ATPases, Ca2+/H+ exchangers (CAXs), and Ca channels (White and Broadley 2003). Calcium storage organelles are strong sinks for Ca and evidence supports the idea that an increase in Ca movement into these organelles can induce BER development in tomato fruit. Previous studies have shown that in tomato plants, high expression of an Arabidopsis thaliana Ca 2+ /H + exchanger (sCAX1) tonoplast protein without the N-regulatory domain not only increased the total amount of Ca in the fruit, but also increased the incidence of BER by 90% (Park and others 2005a). The sCAX1 tomato plant phenotypes, in conjunction with the biochemical properties of sCAX1 in yeast (Hirschi and others 1996; Hirschi 1999), suggest that expression of this transporter potentially altered cellular Ca partitioning and distribution by increasing organellar and decreasing apoplastic pools of free Ca which eventually resulted in leaky membranes and BER development.
The involvement of GA in cellular Ca partitioning and distribution has been reported for many physiological processes (Bush and others 1989; Gilroy and Jones 1992; Chen and others 1997). Gibberellins have been shown to increase the expression of Ca-ATPase and Vacuolar-ATPase (V-ATPase) in the membrane of Ca storage organelles of rice and tomato (Chen and others 1997; Cooley and others 1999). The V-ATPases and the H+-pyrophosphatases (H+-PPases) are electrogenic proton pumps that create an electrochemical potential across the membrane of storage organelles, which is used by other proteins such as the CAXs to drive Ca uptake into these organelles (White and Broadley 2003). Consequently, V-ATPases and H+-PPases can also play an important role in driving an increase in stored Ca at the expense of other pools of Ca in the cell. GA treatment has consistently been reported to trigger an increase in Ca content in storage organelles and an increase in membrane permeability (Wood and Paleg 1972; Bush and others 1989). However, it is not clear whether high expression and/or activity of Ca-ATPases and CAX genes and/or proteins induced by high GA levels could trigger depletion of free Ca in the apoplastic pool, resulting in leaky membranes and BER development.
Our hypothesis is that GA triggers BER development through mechanisms that are also involved in cell and fruit expansion. The objectives of this work were to better understand the mechanisms involved in BER development in tomato fruit in response to GA treatment.
Materials and Methods
The fresh market tomato (Solanum lycopersicum) cultivar ‘Ace 55 (Vf)’ and the processing tomato cultivar ‘AB2’ were grown from transplants in 9.5-L pots containing organic substrate (33.3% peat, 33.3% sand, 33.3% redwood compost with 2.6 kg dolomite lime m−3) in a greenhouse environment during the spring season at about 20°C day and 18°C night temperatures, without supplemental light. At full bloom, fully opened flowers were selected, tagged, and manually pollinated on each plant to monitor the chronological age of the fruit. The treatments started 1 day after pollination (DAP) of the selected flowers. The plants were sprayed with a solution (200 mL plant−1) containing water (control) or 300 mg L−1 GA4+7 (Typrus, Nufarm Americas, Burr Ridge, IL) or 300 mg L−1 prohexadione-calcium (GA biosynthesis inhibitor, Apogee®, BASF Corporation, Research Triangle Park, NC). Each solution contained 0.05% polysorbate 20 (Tween® 20) as a surfactant. There were four single-plant replications per treatment and evaluation time. Prohexadione-calcium has a molecular weight of 250.2 g mol−1 that contains 40.0 g mol−1 of Ca2+. Therefore, the 300 mg L−1 prohexadione-calcium solution sprayed on each plant contained 0.0048% (w/v) of Ca2+. Considering the low amount and frequency of application, and the fact that Ca2+ is not mobile in the plant through the phloem (Ho and White 2005), we believe that Ca2+ present in the prohexadione-calcium treatment had no significant effect on the results obtained.
The treatments were applied every week to the same plants, and the same treatments were applied to each cultivar. Before initiating the treatments, the plants were irrigated once a day until the substrate in each plant was saturated with a nutrient solution containing N (7.2 mmol L−1), P (0.84 mmol L−1), K (3.1 mmol L−1), Ca (2.2 mmol L−1), Mg (1 mmol L−1), S (0.5 mmol L−1), Fe (0.03 mmol L−1), Mn (0.005 mmol L−1), Cu (0.003 mmol L−1), Zn (0.002 mmol L−1), B (0.024 mmol L−1), and Mo (0.00016 mmol L−1). On the day of manual pollination, 20 g of slow-release fertilizer containing N (10.7 mol kg−1), P (2.9 mol kg−1), K (3 mol kg−1), Mg (0.41 mol kg−1), S (0.71 mol kg−1), Fe (0.08 mol kg−1), Mn (0.01 mol kg−1), Cu (0.008 mol kg−1), Zn (0.008 mol kg−1), B (0.018 mol kg−1), and Mo (0.002 mol kg−1), but not Ca (Osmocote Plus®, Scotts-Sierra Horticultural Products Co., Marysville, OH) was added to each plant pot. From this point on, the plants were irrigated once a day with deionized water only, as described previously.
Tomato plants and fruit were analyzed 3 days after each weekly treatment application. A separate set of four plants per treatment were destructively sampled at each evaluation time. Both tomato cultivars were analyzed for BER incidence, membrane permeability, total fruit tissue Ca content, number of stained xylem vessels in the fruit, changes in the expression of genes involved in Ca movement into storage organelles, plant weight, and root weight. Comparison of the two tomato cultivars was used to analyze if the mechanisms involved in BER development in response to GA are conserved in different tomato genotypes, and to show the consistency and repeatability of the results. Details of these methods are described below.
Relative BER incidence was calculated by multiplying the number of tagged fruit with BER symptoms by 100 and dividing that number by the total number of tagged fruit per plant.
Electrolyte leakage was determined in three pericarp fruit discs of 1-cm diameter and 0.5-cm thickness (approximately 3 g weight) cut from the pericarp tissue at the blossom end of the fruit with a stainless-steel cork borer and sectioned with a double-bladed knife beginning 1 mm under the skin. Each disc was cut from a different fruit and a total of three discs per plant represented one replication. Discs from each replication were placed into a 50-mL conical tube with an isotonic mannitol solution (0.2 M), and the conductivity was recorded periodically during 6 h. After 6 h, the samples were frozen and thawed twice to determine total conductivity. The values were expressed as a percentage of the total conductivity (Saltveit 2002). After all measurements, each replication was filtered and the solution collected was used to determine the total soluble Ca in the tissue.
Xylem function was measured in developing tomato fruit. Fruit were harvested with the peduncle attached and held in sealed plastic bags for about 20 min with 100 mL of free deionized water to maintain high relative humidity (RH) and reduce transpiration until the peduncle of each fruit was immersed in a solution of 1% Safranin-O at 20°C under 20% or less RH for 24 h. Fruit were then cut horizontally into three equal sections at a 90° angle to the peduncle axis. The number of stained xylem vessels was counted in the placenta and pericarp tissues at the blossom and peduncle end regions of each fruit.
Plant weight was determined by cutting the plants at the substrate surface level and weighing the fresh stems and leaves (without fruit). Root weight was determined by washing, drying at room temperature for 30 min, and weighing the root systems.
Mineral analysis was done using approximately 10 g of pericarp tissue cut from the blossom-end half of six fruit per replication. After being cut, the samples were immediately frozen in liquid N2 and freeze-dried. Samples were subjected to microwave acid digestion/dissolution and analyzed for Ca by inductively coupled plasma atomic emission spectrometry (Meyer and Keliher 1992).
Water-soluble apoplastic Ca content in pericarp tissue was measured in 12 fruit discs of 1-cm diameter and 0.7-cm thickness (~32 g fresh weight) cut from the blossom end of the fruit with a stainless-steel cork borer and sectioned with a double-bladed knife beginning 1 mm under the skin. Each sample of 12 discs, two discs from each of six fruit, represented one replication. Each disc was individually placed in a 15-mL funnel (1.2-cm diameter.) containing a flat acrylic membrane with pore size of 10–16 μm (Kimax®, Kimble, Vineland, NJ). The funnel was then placed and sealed in a kitasato flask (Pyrex®, Lowell, MA), and 10–15 mmHg of vacuum was applied with a vacuum pump. An isotonic mannitol solution (0.2 M) was slowly dripped over the entire disc surface (500 μL) and collected in the kitasato flask. After repeating the same procedure for all 12 fruit discs, the mannitol solution accumulated in the kitasato flask was used for Ca quantification, representing the water-soluble apoplastic Ca content. The entire procedure was accomplished at 4°C. Cell damage was not detected under a light microscope (SZH10, Olympus America Inc., Lake Success, NY) by analyzing samples before and after the extraction of water-soluble apoplastic Ca extraction.
Real-time polymerase chain reaction (RT-PCR) was performed with total RNA extracted from the blossom-end pericarp plus skin tissues as described in the RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA). The RNA concentration and purity were determined at 260 and 280 nm using a UV spectrophotometer (NanoDrop 2000, Thermo Scientific, Wilmington, DE, USA). For all samples, equal amounts of total RNA (3 μg) were reverse transcribed using SuperScript III (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RT-PCR was then performed with the addition of 1× SYBR® Green (Applied Biosystems, Foster City, CA, USA) to each sample containing about 100 ng of the synthesized cDNA. The data obtained were normalized based on the expression of the housekeeping tomato 18S rRNA (Martinelli and others 2009). All primers designed were 20 nucleotides long and with melting point temperature of 58°C (±3°C).
Genes Involved in Ca Transport into Storage Organelles
Ca is known to be transported into storage organelles directly by the activity of Ca/proton exchangers and Ca-ATPases and indirectly by the activity of vacuolar hydrogen pumps (V-ATPases) and H+-pyrophosphatases (PPases) (White and Broadley 2003). Previous studies have shown that high expression of genes involved in Ca transport into storage organelles can lead to higher fruit susceptibility to BER development (Park and others 2005a; De Freitas and others 2011). With the exception of one V-ATPase gene, no other genes involved in Ca movement into storage organelles have been identified and characterized in tomato fruit. Therefore, to analyze the expression and assess the role of these other genes in BER development in response to GA, a Blast search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) for nucleotide sequences of putative genes involved in Ca movement into storage organelles was accomplished using gene sequences available for other plant species. Nucleotide sequences for four putative Ca 2+ /H + exchangers and one Ca 2+ /Na + exchanger were obtained in the tomato (Solanum lycopersicum) expressed sequence tags (EST) database in the NCBI GenBank (http://www.ncbi.nlm.nih.gov/Genbank/). Among these ESTs, only three Ca 2+ /H + exchangers and the Ca 2+ /Na + exchanger were found to be expressed in the targeted fruit tissue. The expressed nucleotide sequences were named CAX3 (NCBI: BP884739), 83% similarity to vacuolar Ca 2+ /H + exchanger (NCBI: AF461691); CAX4 (NCBI: EF647616), 86% similarity to vacuolar Ca 2+ /H + exchanger (NCBI: XM_002528054); CAX6 (NCBI: AK326847), 75% similarity to vacuolar Ca 2+ /H + exchanger (NCBI: NM_001158996); and CAX7 (NCBI: AW221661), 69% similarity to Ca 2+ /Na + exchanger (NCBI: NM_104200). CAX3, CAX6, and CAX7 nucleotides are part of unigene sequences. The gene sequence for one tomato vacuolar hydrogen pump (V-ATPase) (GeneID: 543862), and the nucleotide sequence for a putative tomato H + -pyrophosphatase (PPase) (NCBI: AK320752), 84% similar to H + -pyrophosphatase (XM_002530709), were also obtained from the NCBI GenBank. Seven putative Ca-ATPase unigenes obtained from the Sol Genomics Network (SGN) database (http://solgenomics.net/index.pl) were also used for expression analysis, and all seven sequences were found to be expressed in the targeted fruit tissues. The unigene sequences were named Ca-ATPase1 (SGN-U581856), 75% similar to endoplasmic reticulum Ca-ATPase (NCBI: NM_100655); Ca-ATPase2 (SGN-U581346), 72% similar to tonoplast Ca-ATPase (NCBI: NM_115593); Ca-ATPase3 (SGN-U571409), 75% similar to endoplasmic reticulum Ca-ATPase (NCBI: NM_119927); Ca-ATPase4 (SGN-U568889), 78% similar to endoplasmic reticulum Ca-ATPase (NCBI: XM_002320177); Ca-ATPase5 (SGN-U603702), 77% similar to endoplasmic reticulum Ca-ATPase (NCBI: NM_100640); Ca-ATPase6 (SGN-U563935), 80% similar to endoplasmic reticulum Ca-ATPase (NCBI: XM_002320646); and Ca-ATPase7 (SGN-U568808), 74% similar to endoplasmic reticulum Ca-ATPase (NCBI: NM_100887). The primers used for the expression analysis are given in Table 1.
Virus-induced Gene Silencing (VIGS)
Previous studies showed that high expression of an Arabidopsis tonoplast Ca 2+ /H + exchanger in tomato increased fruit susceptibility to BER (Park and others 2005a; De Freitas and others 2011). In our study, GA treatment also increased the expression of endogenous Ca 2+ /H + exchangers in fruit tissue and the fruit’s susceptibility to BER development. Based on these findings, we silenced the three Ca 2+ /H + exchangers (CAX3, CAX4, and CAX6) that showed the highest expression in GA-treated fruit to further assess the role of these genes in ‘Ace 55 (Vf)’ tomato fruit susceptibility to BER development in response to GA treatment. In this study, nucleotide sequences of CAX3, CAX4, and CAX6 and the marker gene phytoene desaturase (PDS) were amplified by PCR, cloned into pDrive (Qiagen), and then ligated into the tobacco rattle virus (TRV) RNA2 (VIGS vector pTRV2) (Liu and others 2002). The primer sequences used for PCR amplification are given in Table 1. Another pTRV2 vector containing only the PDS nucleotide sequence was used as the control vector (Fu and others 2005, 2006; Jiang and others 2008). The pTRV2 vectors were then electroporated into the Agrobacterium strain GV3101. The Agrobacterium strain GV3101 containing TRV-VIGS vectors was grown at 28°C in Luria–Bertani medium containing 10 mM MES and 20 mM acetosyringone with appropriate antibiotics. After 24 h, Agrobacterium cells were harvested and resuspended in the Agrobacterium infiltration buffer (10 mM MgCl2, 10 mM MES, pH 5.6, 150 mM acetosyringone) to a final absorbance at 600 nm of 2.0 OD (for pTRV1 or pTRV2) and shaken for 4–6 h at room temperature before infiltration (Liu and others 2002; Fu and others 2005).
At full bloom, fully opened flowers were tagged and manually pollinated. Starting 1 week after pollination, 100 μL of Agrobacterium containing pTRV1 and pTRV2 mixed in a 1:1 (v/v) ratio was infiltrated into the peduncle of tagged fruit using a 1-mL needleless syringe (Liu and others 2002; Fu and others 2005). Fruit infection was accomplished every week until fruit reached the red ripe stage (52 DAP). Tomato fruit infected with TRV containing only the PDS nucleotide sequence were used as the control. Tomato plants were grown in the spring of 2010 under the same conditions described in the previous experiment. Before infection, the plants were irrigated with a nutrient solution containing Ca. On the day of fruit infection, plants were exposed to Ca-limited conditions, as previously described. Twelve plants were used for fruit infection with pTRV2 vector-containing CAXs or PDS (control) nucleotide sequences. Each plant represented one replication. Starting 2 weeks after the time of first fruit infection, all plants were sprayed weekly with a solution (200 mL plant−1) containing 300 mg L−1 GA4+7 (Typrus, Nufarm Americas, Burr Ridge, IL) and 0.05% polysorbate 20 (Tween® 20) as a surfactant to induce BER development. The elapsed time between fruit infection and GA4+7 treatment was required for the viral vector to reach a proper level of fruit tissue silencing. Six CAXs- or PDS- (control) infected plants were used for fruit analysis at 38 and 52 DAP. Silenced fruit tissue was visually identified as white, photo-bleached tissue due to the low levels of PDS protein. Silenced fruit were analyzed for BER incidence, and silenced blossom-end pericarp with skin tissue was analyzed for PDS, CAX3, CAX4, CAX6, and CAX7 gene expression. Silenced pericarp with skin tissue was also analyzed for the expression of Ca-ATPase1, Ca-ATPase3, and Ca-ATPase5, which showed increases in expression in response to GA4+7 treatment in the previous experiment. Uninfected, tagged green fruit were used as wild-type fruit samples.
The experimental design of both experiments was completely randomized and the data were subjected to analysis of variance. Treatments were compared by Duncan’s test (p < 0.05) using the Statistical Analysis System (SAS) software (SAS Institute, Cary, NC).
Results
This work was accomplished in two consecutive years, 2007 and 2008, using tomato cultivars ‘Ace 55 (Vf),’ a fresh market tomato, and ‘AB2,’ a processing tomato, respectively. In the first year, ‘Ace 55 (Vf)’ tomato fruit were evaluated five times during fruit growth and development. In the second year, ‘AB2’ tomato fruit were evaluated three times at the most relevant times observed in the previous year with ‘Ace 55 (Vf)’ (early at 12 DAP, at the time of first BER development in GA4+7-treated plants at 26 DAP, and later at 40 DAP, prior to the ripening stages). This approach was used to analyze if the mechanisms involved in BER development in response to GA are conserved in different tomato genotypes and to show the consistency and repeatability of the results.
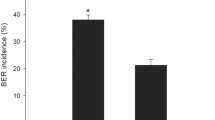
Both ‘Ace 55 (Vf)’ and ‘AB2’ were highly susceptible to BER development under the experimental conditions. Although the response to the treatments was very similar between the two cultivars, BER developed later in ‘AB2’ compared with ‘Ace 55 (Vf)’ water-treated plants. ‘Ace 55 (Vf)’ and ‘AB2’ water-treated tomato plants reached about 35 and 40% BER incidence, respectively, whereas there was about 95 and 100% BER incidence in GA4+7-treated plants at 45 and 40 DAP, respectively (Fig. 1a, c). Gibberellin4+7 treatment induced a faster rate of increase in BER incidence in both ‘Ace 55 (Vf)’ and ‘AB2.’ Tomato plants treated with the GA biosynthesis inhibitor Apogee had 0% BER development in both cultivars under our experimental conditions (Fig. 1a, c).
Blossom-end rot incidence determined as the percentage of ‘Ace 55 (Vf)’ (a) or ‘AB2’ (c) fruit with visual BER symptoms in each replication. Electrolyte leakage of pericarp tissue calculated based on the change in conductivity per hour as a percentage of the total conductivity in ‘Ace 55 (Vf)’ (b) or ‘AB2’ (d) fruit. Mean values with different letters at each evaluation time are significantly different according to Duncan’s test (p = 0.05). Apogee is prohexadione-Ca, a GA biosynthesis inhibitor
Gibberellin4+7 treatment increased and Apogee treatment decreased membrane permeability of fruit tissue during growth and development of both ‘Ace 55 (Vf)’ and ‘AB2’ tomato. The lowest values for electrolyte leakage were observed in Apogee-treated fruit at 12 DAP in ‘Ace 55 (Vf)’ and at 26 and 40 DAP in ‘AB2’ fruit (Fig. 1b, d). Electrolyte leakage increased with fruit ripening in ‘Ace 55 (Vf)’ but not in ‘AB2.’
The number of stained xylem vessels in the placenta and pericarp tissues at the peduncle and blossom ends decreased during growth and development of both tomato fruit cultivars (Fig. 2). ‘Ace 55 (Vf)’ tomato fruit had the highest number of stained xylem vessels in the placental and pericarp tissues at the peduncle and blossom ends in response to Apogee treatment during the early stages of fruit growth and development (Fig. 2a–d). However, at 12 DAP, the number of stained xylem vessels in placental tissue did not differ statistically between Apogee and water treatments. There were no statistically significant differences between water and GA treatments for stained xylem vessels (Fig. 2a–d). ‘AB2’ tomato fruit showed a higher number of stained xylem vessels in the pericarp tissue at the peduncle end and blossom end in response to Apogee treatment at 12 and 26 DAP (Fig. 2f, h). Although the same trend was also observed in the placenta tissue at the peduncle and blossom ends of ‘AB2’ tomato fruit, the Apogee treatment was statistically equal to all other treatments (Fig. 2e, g).
Number of stained xylem vessels in the placenta (a, c, e, g) and pericarp (b, d, f, h) tissue at the peduncle (a, b, e, f) and blossom (c, d, g, h) end regions of ‘Ace 55 (Vf)’ (a–d) or ‘AB2’ (e–h) fruit. Xylem vessels were stained by immersing the peduncle of each fruit in a solution of 1% Safranin-O (red color) that moved into the fruit through functional xylem vessels in response to fruit transpiration. Fruit were then sliced and the number of stained xylem vessels counted in the placenta and pericarp tissues at the blossom and peduncle end regions of each fruit. Statistical analysis and Apogee description as in Fig. 1
Plant weight was negatively affected by GA4+7 treatment in both tomato cultivars, beginning approximately 26–31 DAP. The lowest plant weight values were observed at 31, 38, and 45 DAP for ‘Ace 55 (Vf)’ treated with GA4+7 (Fig. 3a). In ‘AB2,’ the lowest plant weights were observed at 26 and 40 DAP in plants treated with GA4+7 (Fig. 3c). The root weight was higher in both tomato cultivars in response to GA4+7 treatment beginning at the first measurement at 12 DAP and continuing during fruit growth and development (Fig. 3b, d).
Plant and root fresh weight of ‘Ace 55 (Vf)’ (a, b), or ‘AB2’ (c, d) tomato, respectively. Statistical analysis and Apogee description as in Fig. 1
The pattern of Ca accumulation among the treatments in the blossom-end tissue of tomato fruit was similar in both ‘Ace 55 (Vf)’ and ‘AB2’ at 45 and 40 DAP, respectively (Fig. 4). These cultivars showed equal Ca content in fruit treated with water and GA4+7, but higher Ca content in response to Apogee treatment (Fig. 4). During growth and development of ‘Ace 55 (Vf)’ fruit, the apoplastic Ca content in pericarp tissue was consistently lower in GA4+7-treated fruit and higher in Apogee-treated fruit (Fig. 5a). In water-treated ‘Ace 55 (Vf)’ fruit, the levels of apoplastic Ca content started lower and increased during fruit growth and development (Fig. 5a). The total soluble Ca content did not differ among treatments, but decreased during growth and development of ‘Ace 55 (Vf)’ fruit (Fig. 5b).
Calcium content in the blossom end pericarp of ‘Ace 55 (Vf)’ and ‘AB2’ tomato fruits at 45 and 40 DAP, respectively. Mean values with different letters on each cultivar are significantly different according to Duncan’s test (p = 0.05). Apogee description as in Fig. 1
Apoplastic water-soluble Ca extracted by vacuum infiltrating an isotonic mannitol solution (0.68 MPa) through ‘Ace 55 (Vf)’ tomato fruit pericarp discs (a). Total water-soluble Ca extracted after freezing and thawing pericarp samples (b). Statistical analysis and Apogee description as in Fig. 1
The expression analysis of genes coding for proteins involved in Ca movement into storage organelles showed that GA4+7 treatment increased the expression of CAX3, CAX4, CAX6, CAX7, VATPase, VPPase, Ca-ATPase1, and Ca-ATPase5 in ‘Ace 55 (Vf)’ tomato fruit at 31 DAP (Figs. 6a, 7a). In ‘AB2’ tomato fruit at 40 DAP, GA4+7 increased the expression of CAX3, Ca-ATPase1, Ca-ATPase2, Ca-ATPase3, Ca-ATPase5, and Ca-ATPase6 (Figs. 6b, 7b). The expression of CAX4 and CAX7 were not detected by RT-PCR, and CAX6, VATPase, and VPPase showed similar expression levels in ‘AB2’ tomato fruit for all treatments (Fig. 6b). Apogee treatment reduced the expression of seven and four Ca-ATPases in ‘Ace 55 (Vf)’ (Fig. 7a) and ‘AB2’ (Fig. 7b) fruit tissue, respectively.
Expression of CAX, VATPase, and VPPase genes involved in Ca movement into cellular organelles at the initial time of BER development in ‘Ace 55 (Vf)’ tomato fruit 31 DAP (a) and in ‘AB2’ tomato fruit 40 DAP (b). *Expression not detected. Expression analysis was accomplished by RT-PCR. The data were normalized based on the expression of the tomato 18S rRNA. The water-treated (control) fruit tissue was used as the reference (expression level = 1) for the expression analysis. Mean values with different letters on each gene are significantly different according to Duncan’s test (p = 0.05). Apogee description as in Fig. 1
Expression of Ca-ATPase genes involved in Ca movement into cellular organelles at the initial time of BER development in ‘Ace 55 (Vf)’ tomato fruit 31 DAP (a) and ‘AB2’ tomato fruit 40 DAP (b). Expression and statistical analyses were accomplished as described for Fig. 6. Apogee description as in Fig. 1
In the silencing experiment, ‘Ace 55 (Vf)’ tomato fruit silenced for CAX3, CAX4, and CAX6 genes showed the same BER incidence in silenced fruit tissue as PDS-silenced and wild-type fruit tissue in response to weekly GA4+7 treatment (data not shown). After treatment with GA, all plants in the silencing experiment reached rates of BER development similar to the rate observed in the previous experiment with ‘Ace 55 (Vf)’ tomato treated with GA (Fig. 1a). The area of silenced fruit tissue decreased from 38 DAP (Fig. 8b) to 52 DAP (Fig. 8c). The VIGS was effective in downregulating the expression of target genes in silenced fruit tissue at 38 DAP (Fig. 9a) and at 52 DAP (Fig. 9b). Fruit tissue silenced for CAX3, CAX4, and CAX6 genes had lower expression of CAX7 and higher expression of Ca-ATPase1, Ca-ATPase3, and Ca-ATPase5 genes at 38 DAP compared with PDS-silenced and wild-type fruit tissue (Fig. 10a). At 52 DAP, fruit tissue silenced for CAX3, CAX4, and CAX6 genes had higher expression of CAX7, Ca-ATPase3, and Ca-ATPase5 genes compared with PDS-silenced and wild-type fruit tissue (Fig. 10b).
Wild-type (a) and CAX3-4-6-PDS-silenced ‘Ace 55 (Vf)’ tomato fruit at 38 DAP (b). CAX3-4-6-PDS-silenced tomato fruit at 52 DAP (c). Gene silencing was accomplished with the virus-induced gene-silencing (VIGS) technique. Starting at full bloom, Agrobacterium containing the viral vectors pTRV1 and pTRV2 mixed in a 1:1 (v/v) ratio were infiltrated into the peduncle of the fruit with a 1-mL needleless syringe. Fruit infection was accomplished every week until fruit reached the red ripe stage (52 DAP)
Expression of PDS, CAX3, CAX4, and CAX6 genes in wild-type, PDS-silenced, and CAX3-4-6-PDS-silenced ‘Ace 55 (Vf)’ tomato fruit at 38 (a) and 52 (b) DAP. Expression and statistical analyses were accomplished as described in Fig. 6. Wild-type fruit tissue was used as the reference (expression level = 1) for the expression analysis
Expression of CAX7, Ca-ATPase1, Ca-ATPase3, and Ca-ATPase5 genes in wild-type, PDS-silenced, and CAX3-4-6-PDS-silenced ‘Ace 55 (Vf)’ tomato fruit at 38 (a) and 52 (b) DAP. Expression and statistical analyses were accomplished as described in Fig. 6. Wild-type fruit tissue was used as the reference (expression level = 1) for the expression analysis
Discussion
Gibberellins Trigger BER Development in Tomato Fruit
Early studies have reported that tomato plants treated with GAs have higher BER incidence than untreated control plants (Bangerth 1973; Castro 1980). It has also been suggested that GAs are the primary cause of Ca-deficiency development in fruit (Saure 2001, 2005). However, few studies have explored the possible mechanisms involved in BER development in response to GA. We showed that BER could be induced or prevented by treating tomato plants with GA4+7 or with the GA biosynthesis inhibitor Apogee, respectively. Our results also show consistency and repeatability in the GA effects on mechanisms that can lead to BER development in the two tomato cultivars used in our study. These effects include a reduction in fruit Ca uptake, altered cellular Ca partitioning and distribution resulting in decreased apoplastic Ca, and an increase in electrolyte leakage of fruit pericarp cells.
Fruit Pericarp Electrolyte Leakage and BER Development
Our results indicate a high correlation among BER incidence, electrolyte leakage of fruit pericarp tissue, and expected GA levels on each evaluation day. Both ‘Ace 55 (Vf)’ and ‘AB2’ GA-treated fruit had the highest and Apogee-treated fruit the lowest electrolyte leakage and rates of BER incidence at each evaluation day. In water-treated fruit, the pattern of electrolyte leakage of fruit pericarp tissue and the rate of BER incidence were likely influenced by the endogenous levels of GA in the fruit. In ‘Ace 55 (Vf)’, water-treated fruit had electrolyte leakage values as high as those of GA-treated fruit at 12 and 24 DAP, which preceded the start of BER development at 24 DAP. After 24 DAP, water-treated ‘Ace 55 (Vf)’ fruit had electrolyte leakage values as low as those of Apogee-treated fruit, which had a high correlation with a reduction in the rate of increase in BER incidence (~0%). These results suggest that GA levels in water-treated fruit were higher from 12 to 24 DAP, resulting in similar electrolyte leakage and rate of BER incidence as GA-treated fruit. After 24 DAP, the GA levels in water-treated fruit likely decreased, resulting in similar electrolyte leakage and little to no increase in BER incidence, as observed in Apogee-treated fruit.
In ‘AB2’ tomatoes at 12 DAP, water-treated fruit showed electrolyte leakage values equal to those of Apogee-treated fruit, whereas GA-treated fruit had higher electrolyte leakage. At 26 and 40 DAP, water-treated fruit showed higher electrolyte leakage values than Apogee-treated fruit. Similar to ‘Ace 55 (Vf)’, the results obtained in ‘AB2’ suggest that a peak in GA biosynthesis in water-treated fruit triggered an increase in electrolyte leakage, which preceded the start of BER development. The peak in GA biosynthesis likely takes place earlier in ‘Ace 55 (Vf)’ (12–24 DAP) and later in ‘AB2’ (26–40 DAP). Previous studies have shown that GA biosynthesis increases in fruit tissue at early stages of growth and development, but the exact timing of this increase is cultivar-dependent (Sjut and Bangerth 1983; Gillaspy and others 1993). In addition, previous studies also supported the idea that GAs can increase membrane permeability (Pauls and others 1982).
Although there was a high correlation between BER incidence and electrolyte leakage of fruit pericarp tissue on each evaluation day, a low correlation was observed between evaluation days. In ‘Ace 55 (Vf),’ increases in electrolyte leakage at 45 DAP were not correlated with increases in BER incidence in water- and Apogee-treated fruit. The increase in electrolyte leakage observed at 45 DAP in ‘Ace 55 (Vf)’ could be explained by the fact that all the fruit started ripening at this stage. Previous studies have shown that ripening of fruit tissue is followed by changes in membrane lipid composition, which can lead to observed increases in membrane permeability (Bergevin and others 1993). In ‘AB2,’ however, electrolyte leakage decreased while BER incidence increased during fruit growth and development. As a firm processing tomato, ‘AB2’ may not show the same changes in electrolyte leakage with ripening as ‘Ace 55 (Vf).’ Further differences may result from cellular changes that affect membrane structure and integrity independent of GA concentration, such as changes in turgor pressure and membrane composition during fruit growth and development (Bergevin and others 1993; Saure 2001; Welti and others 2002; White and Broadley 2003; Saure 2005). These changes in membrane structure and integrity could lead to changes in the threshold of membrane leakage that triggers BER development in fruit tissue.
Gibberellins Reduce Ca Accumulation in Fruit Tissue During Early Stages of Growth and Development
We observed the lowest Ca content in the blossom-end tissue of tomato fruit treated with water and GA4+7 and the highest Ca content in Apogee-treated fruit, indicating that higher levels of GA during the early stages of fruit growth and development can restrict fruit Ca uptake, thereby increasing the probability of BER incidence. These results are highly correlated with the lower abundance of stained xylem vessels observed during the early stages of fruit growth and development in water- and GA4+7-treated fruit and the higher abundance observed in Apogee-treated fruit. The results obtained suggest that externally applied GA4+7 or internally synthesized GA may inhibit the formation and/or maintenance of functional xylem vessels in the fruit during the early stages of growth and development. Although the mechanisms by which GAs reduce the abundance of stained xylem vessels is not well understood, it has been suggested that GAs are suppressors of tissue differentiation (Sachs 2000; Aloni 2001) and may act by inhibiting xylem vessel development in fruit tissue. Other studies have also suggested that high GA levels may trigger rapid cell expansion and induce breakage and obstruction of xylem vessels, restricting the xylemic movement of water and nutrients into the fruit (Drazeta and others 2004; Ho and White 2005; Saure 2005). Calcium concentration is consistently lowest where growth is most vigorous in the fruit, especially at the blossom end (Saure 2005). In addition, the reduction in Ca accumulation by the fruit during the course of development occurs when fruit growth by cell division is substituted by fruit growth by cell expansion, which coincides with increased levels of GA in the fruit (El-Beltagy and others 1976; Sjut and Bangerth 1983; Ebert and Bangerth 1985; Gillaspy and others 1993). It has also been proposed that associated with a reduction in Ca uptake, vigorous cell expansion may result in dilution of fruit Ca content, enhancing the susceptibility of tomato fruit to BER incidence (Ho and White 2005; Saure 2005). Alternatively, high GA levels have also been shown to increase cuticle deposition on tomato fruit (Knoche and Peschel 2007), which can potentially decrease transpiration rates, reducing water and Ca uptake into the fruit and increasing the probability of BER development. Although water- and GA-treated fruit had similar pericarp Ca content and similar total water-soluble Ca concentration, GA-treated fruit showed the highest susceptibility to BER development. The greater incidence of BER in GA-treated fruit may be explained by the lower apoplastic water-soluble Ca concentration and the higher electrolyte leakage of fruit pericarp observed in response to GA treatment.
Gibberellins Change Shoot/Root Ratio of Tomato Plants Under Ca-deficit Conditions
Gibberellins are known to be involved in cell division and trigger cell expansion, which has been linked to its role in shoot and root growth (Butcher and Street 1960; Barlow and others 1991; Ubeda-Tomas and others 2009; Bidadi and others 2010). Our results show that under Ca-deficit conditions, applying GA to the shoot reduced shoot weight and increased root weight. Previous studies have proposed that a functional equilibrium exists between the size and activity of the shoot (which supplies carbohydrates) and the size and activity of the root (which supplies water and essential nutrients) (Reynolds and Thornley 1982). Our results show that high GA levels in the shoot triggered high levels of Ca deficiency in the fruit and inhibited shoot growth. The reduction in shoot growth may have resulted from multiple applications of GA resulting in a supraoptimal GA concentration in the shoot, which has been suggested to inhibit growth (Butcher and Street 1960). The lower shoot growth in GA-treated plants may have reduced carbohydrate consumption in the shoot and increased carbohydrate translocation to the roots, favoring root growth in an attempt to increase plant Ca uptake and reestablish the functional equilibrium between shoot and root. However, in our study, tomato plants were exposed to limited Ca in the soil, and the increased root growth observed in GA-treated plants was likely not enough to supply sufficient Ca to the shoot. In addition, a portion of the Ca taken up by the root would be used to maintain proper cell function in the root, and only the remaining Ca then is translocated to the shoot through the xylem. Accordingly, our results also showed that roots are able to satisfy their basic Ca requirements for growth, even with limited Ca availability, whereas the organs most distant from the source of Ca, that is, the apical shoot meristem, young leaves, and blossom end of fruit, are damaged at much higher Ca concentrations in the soil than the root (Ericsson 1995; Marschner and others 1996; White and Broadley 2003).
Gibberellins May Trigger an Increase in Ca Movement into Storage Organelles
Besides fruit Ca uptake, proper Ca partitioning and distribution is also important for normal cell metabolism and control of BER incidence (Ho and White 2005). Our results show that tomato plants treated with water and GA4+7 had statistically equal levels of Ca in the fruit tissue when both treatments had greater than 20% BER incidence. However, GA4+7-treated plants showed a higher rate of increase in BER incidence than water-treated plants. GA4+7-treated fruit had higher expression of CAX and Ca-ATPase genes in both cultivars and higher expression of V-ATPase and VPPase genes in ‘Ace 55 (Vf).’ The increase in expression of these genes involved in Ca movement into storage organelles in response to GA4+7 treatment suggests a reduction in the Ca levels present in other cellular compartments. Because the cytosolic level of Ca is carefully maintained at extremely low levels (0.0001–0.0002 mM), it is possible that an influx of Ca from the apoplast, which contains about 0.1–1 mM, could supply the influx of Ca into storage organelles where Ca concentrations are as high as 1–10 mM (Hanson 1960; Almeida and Huber 1999; Plieth 2001). Early reports have suggested that apoplastic pools of free Ca must be higher than 0.1 mM to maintain the integrity and selective ion transport properties of the plasma membrane, and lower Ca values can result in leaky membranes (Hanson 1960; Epstein 1961).
These ideas are consistent with the lower levels of apoplastic Ca observed in GA4+7-treated fruit and higher levels in Apogee-treated fruit. GA4+7 treatment may have triggered a reduction in the apoplastic pool of free Ca to values lower than the threshold required for proper membrane structure and integrity, eventually leading to the observed leakier membranes and higher BER incidence (95–100%). Water-treated fruit consistently developed BER only at early stages of fruit growth and development when apoplastic Ca concentration was lower in the fruit. The water-treated plants may have increased BER incidence by the same mechanism observed in GA4+7-treated fruit, because tomato fruit are known to have an increase in GA biosynthesis at the early stages of fruit growth and development when fruit growth by cell division is replaced by cell expansion (El-Beltagy and others 1976; Gillaspy and others 1993; Saure 2005). These results are also supported by previous studies that showed that increased expression of CAX genes not only increased total tissue Ca content, but also the incidence of Ca-deficiency symptoms in tobacco (Hirschi 1999), carrots (Park and others 2004), tomatoes (Park and others 2005a; De Freitas and others 2011), and potatoes (Park and others 2005b). It is also important to note that in those experiments, the incidence and severity of Ca deficiency were always correlated with the level of expression of the CAX genes.
In Apogee-treated fruit, the pattern of CAXs, V-ATPase, and VPPase expression did not support our hypothesis of low GA biosynthesis and low expression of these genes, with the exception of V-ATPase in ‘Ace 55 (Vf)’ and VPPase in ‘AB2’ fruit tissue. In addition, ‘Ace 55 (Vf)’ fruit tissue exhibited an increase in CAX3, CAX4, and Ca-ATPase expression in response to Apogee treatment compared with water-treated fruit. As a signaling molecule, Ca is involved in many plant responses to biotic and abiotic stresses, and low levels of this ion in the cytosol are required for proper cellular responses and metabolism (White and Broadley 2003). Previous studies have shown that cells exposed to high levels of exogenous Ca increase the expression of CAX genes, possibly in an attempt to avoid the toxic effects of high levels of this signaling molecule in the cytosol (Hirschi 1999; Kamiya and others 2006). Accordingly, in Arabidopsis, CAX3-null alleles were modestly sensitive and CAX1/CAX3 double mutants were highly sensitive to exogenous Ca (Cheng and others 2005). Therefore, the higher expression pattern of CAX3, CAX4, and Ca-ATPase1 in ‘Ace 55 (Vf)’ and Ca-ATPase2 in ‘AB2’ observed in response to Apogee treatment may be the result of the higher fruit Ca uptake also observed in fruit from this treatment.
Possible Functional Redundancy Among Ca Transport Proteins
Previous studies have shown that high expression of CAX genes can increase fruit susceptibility to BER development (Park and others 2005a; De Freitas and others 2011). In addition, our study also shows high expression of CAX genes in tomato fruit with high BER incidence in response to high GA levels. Based on these findings, we chose to downregulate the expression of the three CAX genes that were most highly expressed in response to GA to assess their role in fruit susceptibility to BER in response to high levels of GA. The downregulation of CAX3, CAX4, and CAX6 expression in fruit tissue had no effect on BER development in response to GA4+7 treatment. These results could be explained by the fact that other genes with similar function, such as CAX7, Ca-ATPase3, and Ca-ATPase5, increased expression possibly to compensate for the downregulation of the CAX3, CAX4, and CAX6 genes. In conclusion, the mechanisms by which GA regulates cellular Ca partitioning and distribution, resulting in increased fruit susceptibility to BER development, are likely complex, involving not only upregulation of CAX genes, but also upregulation of other genes to ensure a conserved response of Ca movement into cellular storage organelles. Based on the visual analysis of downregulated fruit tissue (white tissue), we concluded that ‘Ace 55 (Vf)’ tomato fruit became less susceptible to the VIGS vector as the fruit grew and developed, which may have reduced the effect of downregulation of gene expression on BER development. Future approaches to overcome this problem should include the use of stable transformation techniques or the use of tomato cultivars that are more susceptible to the VIGS vector.
Gibberellins as a Potential Factor in Tomato Fruit Susceptibility to BER
Gibberellins are growth regulators known to trigger cell and fruit expansion, but the mechanisms involved remain poorly understood (Ubeda-Tomas and others 2008; Zhang and others 2008). Our results show that high levels of GA can reduce fruit Ca uptake and potentially increase Ca movement into storage organelles, resulting in lower levels of free Ca in the apoplast. Low free Ca in the apoplast reduces Ca binding to de-esterified pectins and increases cell wall extensibility, potentially increasing the rates of cell expansion (Pelloux and others 2007), which can also dilute the limited amount of Ca that moves into the fruit. Although GA may trigger cell expansion through the mechanisms described above, these mechanisms can also increase fruit susceptibility to calcium-deficiency development. Accordingly, fruit susceptibility to BER has been reported to be higher during stages of cell expansion, when rates of GA biosynthesis are higher (El-Beltagy and others 1976; Saure 2001) and fruit Ca uptake is low (Ho and White 2005). Therefore, GA not only triggers a reduction in apoplastic free Ca, it may also decrease Ca binding to the expanding plasma membrane, which can result in plasma membrane Ca deficiency, increasing membrane leakage and eventually the observed water-soaked BER symptoms. Therefore, years of tomato fruit selection for size and weight may have contributed to enhancing mechanisms that favor low fruit Ca uptake and high influx of Ca into storage organelles, reducing the amount of Ca bound to the cell wall, increasing cell wall extensibility, and facilitating cell expansion and fruit growth. However, such intense selection may have also resulted in tomato fruit that can reach an imbalance in Ca partitioning and distribution at the cellular level in response to elevated GA levels, eventually resulting in plasma membrane breakdown and BER development. BER consistently appears to be related to fruit growth rate and/or potential fruit size among cultivars, and there is a clear genetic influence on the susceptibility of different cultivars to BER (Ho and others 1995; Sperry and others 1996; Cuartero and Fernández-Muñoz 1999). Plum tomatoes are more susceptible to BER than round tomatoes, and BER is never observed in cherry tomatoes (Ho and White 2005), which may potentially be linked to the ability of these different tomato types to biosynthesize and/or sense GAs.
In conclusion, elevated GA levels promote BER development in tomato fruit by decreasing fruit Ca uptake and by possibly increasing Ca concentration inside cellular organelles, which reduces apoplastic Ca concentration. Low apoplastic Ca leads to increased plasma membrane leakiness and eventually to water-soaked symptoms of BER on the fruit surface. New research should focus on the comparative analysis of genes involved in GA biosynthesis, Ca uptake, and Ca movement into storage organelles in fruit tissue of tomato cultivars with different susceptibilities to BER to further our understanding of the development of calcium-deficiency disorders and to facilitate the selection of new cultivars with reduced susceptibility to BER development.
References
Almeida DPF, Huber DJ (1999) Apoplastic pH and inorganic ion levels in tomato fruit: a potential means for regulation of cell wall metabolism during ripening. Physiol Plant 105:506–512
Aloni R (2001) Foliar and axial aspects of vascular differentiation: hypotheses and evidence. J Plant Growth Regul 20:20–22
Bangerth F (1973) Investigations upon Ca related physiological disorders. Phytopathol Z 77:20–37
Barlow PW, Brain P, Parker JS (1991) Cellular growth in roots of a gibberellin-deficient mutant of tomato (Lycopersicon esculentum Mill.) and its wild-type. J Exp Bot 42:339–351
Bergevin M, L’Heureux GP, Thompson JE, Willemot C (1993) Effect of chilling and subsequent storage at 20°C on electrolyte leakage and phospholipid fatty acid composition of tomato pericarp. Physiol Plant 87:522–527
Bidadi H, Yamaguchi S, Asahina M, Satoh S (2010) Effects of shoot-applied gibberellin/gibberellin-biosynthesis inhibitors on root growth and expression of gibberellin biosynthesis genes in Arabidopsis thaliana. Plant Root 4:4–11
Bush DS, Biswas AK, Jones RL (1989) Gibberellic-acid-stimulated Ca2+ transport and steady-state levels. Planta 178:411–420
Butcher DN, Street HE (1960) The effect of gibberellins on the growth of excised tomato roots. J Exp Bot 11:206–216
Castro PRC (1980) Plant growth regulators in tomato crop production. Acta Hortic 100:99–104
Chen X, Chang M, Wang B, Wu R (1997) Cloning of a Ca2+-ATPase gene and the role of cytosolic Ca2+ in the gibberellin-dependent signaling pathway in aleurone cells. Plant J 11:363–371
Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138:2048–2060
Cooley MB, Yang H, Dahal P, Mella RA, Downie AB, Haigh AM, Bradford KJ (1999) Vacuolar H-ATPase is expressed in response to gibberellin during tomato seed germination. Plant Physiol 121:1339–1347
Cuartero J, Fernández-Muñoz R (1999) Tomato and salinity. Sci Hortic 78:83–125
De Freitas ST, Padda M, Wu Q, Park S, Mitcham E (2011) Dynamic alterations in cellular and molecular components during blossom-end rot development in tomatoes expressing sCAX1, a constitutively active Ca2+/H+ antiporter from Arabidopsis. Plant Physiol 156(2):844–855
Drazeta L, Lang A, Hall AJ, Volz RK, Jameson PE (2004) Causes and effects of changes in xylem functionality in apple fruit. Ann Bot 93:275–282
Ebert A, Bangerth F (1985) Veränderungen im Phytohormongehalt und mögliche Beziehungen zur Fruchtentwicklung beim Apfel. Gartenbauwissenschaft 50:37–41
El-Beltagy AS, Patrick JP, Hewitt EW, Hall MA (1976) Endogenous plant growth regulator levels in tomato fruits during development. J Hortic Sci 51:15–30
Epstein E (1961) The essential role of calcium in selective cation transport by plant cells. Plant Physiol 36:437–444
Ericsson T (1995) Growth and shoot: root ratio of seedlings in relation to nutrient availability. Plant Soil 169:205–214
Ferguson IB, Watkins CB (1989) Bitter pit in apple fruit. Hortic Rev 11:289–355
Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB (2005) Virus-induced gene silencing in tomato fruit. Plant J 43:299–308
Fu DQ, Zhu BZ, Zhu HL, Zhang HX, Xie YH, Jiang WB, Zhao XD, Luo YB (2006) Enhancement of virus-induced gene silencing in tomato by low temperature and low humidity. Mol Cells 21:153–160
Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5:1439–1451
Gilroy S, Jones RL (1992) Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secretory activity in barley aleurone protoplasts. Proc Natl Acad Sci USA 89:3591–3595
Hanson JB (1960) Impairment of respiration, ion accumulation, and ion retention in root tissue treated with ribonuclease and ethylenediamine tetraacetic acid. Plant Physiol 35:372–379
Hirschi KD (1999) Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11:2113–2122
Hirschi KD, Zhen RG, Cunningham KW, Rea PA, Fink GR (1996) CAX1, an H+/Ca2+ antiporters from Arabidopsis. Proc Natl Acad Sci USA 93:8782–8786
Ho LC, White PJ (2005) A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Ann Bot 95:571–581
Ho LC, Adams P, Li XZ, Shen H, Andrews J, Xu ZH (1995) Responses of Ca-efficient and Ca-inefficient tomato cultivars to salinity in plant growth, calcium accumulation and blossom-end rot. J Hortic Sci 70:909–918
Jiang CZ, Lu F, Imsabai W, Meir S, Reid MS (2008) Silencing polygalacturonase expression inhibits tomato petiole abscission. J Exp Bot 59:973–979
Kamiya T, Akahori T, Ashikari M, Maeshima M (2006) Expression of the vacuolar Ca2+ exchanger, OsCAX1a, in rice: cell and age specificity of expression, and enhancement by Ca2+. Plant Cell Physiol 47:96–106
Kirby EA, Pilbeam DJ (1984) Calcium as a plant nutrient. Plant Cell Environ 7:397–405
Knoche M, Peschel S (2007) Gibberellins increase cuticle depositions in developing tomato fruit. Plant Growth Regul 51:1–10
Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31:777–786
Marschner H, Kirkby E, Cakmak I (1996) Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J Exp Bot 47:1255–1263
Martinelli F, Uratsu SL, Reagan RL, Chen Y, Tricoli D, Fiehn O, Rocke M, Gasser CS, Dandekar AM (2009) Gene regulation in parthenocarpic tomato fruit. J Exp Bot 60:3873–3890
Meyer GA, Keliher PN (1992) An overview of analysis by inductively coupled plasma-atomic emission spectrometry. In: Montaser A, Golightly DW (eds) Inductively coupled plasmas in analytical atomic spectrometry. VCH Publishers, New York, pp 473–516
Nelson T, Dengler N (1997) Leaf vascular pattern formation. Plant Cell 9:1121–1135
Nonami H, Fukuyama T, Yamamoto M, Yang L, Hashimoto Y (1995) Blossom-end rot of tomato plants may not be directly caused by calcium deficiency. Acta Hortic 396:107–114
Park S, Kim CK, Pike LM, Smith RH, Hirschi KD (2004) Increased calcium in carrots by expression of an Arabidopsis H+/Ca2+ transporter. Mol Breed 14:275–282
Park S, Cheng NH, Pittman JK, Yoo KS, Park J, Smith RH, Hirschi KD (2005a) Increasing calcium levels and prolonged shelf live in tomatoes expressing Arabidopsis H+/Ca2+ transporters. Plant Physiol 139:1194–1206
Park S, Kang TS, Kim CK, Han JS, Kim S, Smith RH, Pike LM, Hirschi KD (2005b) Genetic manipulation for enhancing calcium content in potato tuber. J Agric Food Chem 53:5598–5603
Pauls KP, Chambers JA, Dumbroff EB, Thompson JE (1982) Perturbation of phospholipid membranes by gibberellins. New Phytol 91:1–17
Pelloux J, Rusterucci C, Mellerowicz EJ (2007) New insights into pectin methylesterase structure and function. Trends Plant Sci 12:267–277
Picchioni GA, Watada AE, Conway WS, Whitaker BD, Sams CE (1998) Postharvest calcium infiltration delays membrane lipid catabolism in apple fruit. J Agric Food Chem 46:2452–2457
Plieth C (2001) Plant calcium signaling and monitoring: pros and cons and recent experimental approaches. Protoplasma 218:1–23
Reynolds JF, Thornley JHM (1982) A shoot:root partitioning model. Ann Bot 49:585–597
Sachs T (2000) Integrating cellular and organismal aspects of vascular differentiation. Plant Cell Physiol 41:649–656
Saltveit ME (2002) The rate of ion leakage from chilling-sensitive tissue does not immediately increase upon exposure to chilling temperatures. Postharvest Biol Technol 26:295–304
Saure MC (2001) Blossom-end rot of tomato (Lycopersicon esculentum Mill.)—a calcium- or a stress-related disorder? Sci Hortic 90:193–208
Saure MC (2005) Calcium translocation to fleshy fruit: its mechanism and endogenous control. Sci Hortic 105:65–89
Sjut V, Bangerth F (1983) Induced parthenocarpy—a way of changing the levels of endogenous hormones in tomato fruit (Lycopersicon esculentum Mill.) 1. Extractable hormones. Plant Growth Regul 1:243–251
Sperry WJ, Davis JM, Sanders DC (1996) Soil moisture and cultivar influence cracking, blossom-end rot, zippers, and yield of staked fresh-market tomatoes. HortTechnology 6:21–23
Suzuki K, Takeda H, Egawa Y (2000) Morphological aspects of blossom-end rot fruits of tomato. Acta Hortic 511:257–264
Suzuki K, Shono M, Egawa Y (2003) Localization of calcium in the pericarp cells of tomato fruits during the development of blossom-end rot. Protoplasma 222:149–156
Ubeda-Tomas S, Swarup R, Coates J, Swarup K, Laplaze L, Beemster GTS, Hedden P, Bhalerao R, Bennett MJ (2008) Root growth in Arabidopsis requires gibberellin/DELLA signaling in the endodermis. Nat Cell Biol 10:625–628
Ubeda-Tomas S, Federici F, Casimiro I, Beemster GTS, Bhalerao R, Swarup R, Doerner P, Haseloff J, Bennett M (2009) Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Curr Biol 19:1194–1199
Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X (2002) Profiling membrane lipids in plant stress responses. J Biol Chem 277:31994–32002
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
Wood A, Paleg LG (1972) The influence of gibberellic acid on the permeability of model membrane systems. Plant Physiol 50:103–108
Zhang C, Lee U, Tanabe K (2008) Hormonal regulation of fruit set, parthenogenesis induction and fruit expansion in Japanese pear. Plant Growth Regul 55:231–240
Acknowledgments
The authors recognize support from CAPES Foundation, a federal agency under the Ministry of Education of Brazil, and the Fulbright Program, which awarded a Scholarship to PhD student Sergio Tonetto de Freitas for his work on this project. We also thank the California League of Food Processors and the California Tomato Research Institute for partial funding of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Freitas, S.T., Jiang, CZ. & Mitcham, E.J. Mechanisms Involved in Calcium Deficiency Development in Tomato Fruit in Response to Gibberellins. J Plant Growth Regul 31, 221–234 (2012). https://doi.org/10.1007/s00344-011-9233-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-011-9233-9