Abstract
Blossom-end rot (BER) is a physiological disorder believed to be triggered by low Ca2+ content in the distal fruit tissue. However, many other factors can also determine fruit susceptibility to BER. It is possible that during fruit growth, Ca2+ imbalance can increase membrane leakiness, which may trigger the accumulation of reactive oxygen species, leading to cell death. Brassinosteroids are a class of plant hormones involved in stress defenses, specially increasing the activity of antioxidant enzymes and the accumulation of antioxidant compounds, such as ascorbic acid. The objective of this study was to understand the mechanisms by which 24-epibrassinolide (EBL) reduces fruit susceptibility to BER. Tomato plants ‘BRS Montese’ were cultivated in a greenhouse and were weekly sprayed with water (control) or EBL (0.01 µM) after full bloom. Plants and fruits were evaluated at 15 days after pollination (DAP). According to the results, EBL treatment inhibited BER development, increased fruit diameter, length, and fresh weight. EBL-treated fruit showed higher concentrations of soluble Ca2+ and lower concentrations of cell wall-bound Ca2+. EBL-treated fruit also had higher concentrations of ascorbic acid and lower concentrations of hydrogen peroxide, compared to water-treated fruit. EBL treatment increased the activity of the three main antioxidant enzymes known as ascorbate peroxidase, catalase, and superoxide dismutase. According to the results, EBL treatment maintained higher soluble Ca2+ and antioxidant capacity, reducing fruit susceptibility to BER.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blossom-end rot (BER) is a physiological disorder that develops at the distal fruit tissues, leading to softening and subsequent necrosis. Although calcium (Ca2+) deficiency has been considered for a long time the main cause of the disorder, recent studies have shown more complex mechanisms regulating BER incidence in tomato fruit, like oxidative stress, loss of xylem functionality, and expression of some genes related to Ca2+ transport in fruit cells (De Freitas et al. 2017; Ikeda et al. 2017).
Calcium deficiency is highly affected by environment factors such as high temperatures, low air relative humidity, and soil salinity, as well as by genetic predisposition of the genotypes (Ho and White 2005). In this case, a few studied factors such as fruit shape (Riboldi et al. 2018b) and loss of xylem functionality in the distal portion (Riboldi et al. 2018a) have emerged as key factors determining fruit susceptibility to BER.
Brassinosteroids (BRs) have been shown to induce stress tolerance in plants (Maia et al. 2018), and to increase cell viability under stress conditions by increasing the cellular capacity to scavenge reactive oxygen species (ROS) (Liu et al. 2009). Studies using exogenous applications of BRs have also shown that it can promote a better adaptation of plants to stresses (Wu et al. 2017; Maia et al. 2018; Riboldi et al. 2018c).
In some studies, it is possible to verify that the mechanism of BRs action is different from abscisic acid action, a traditional hormone studied in plants under stress. The use of BRs has been reported to activate plant antioxidant defenses such as a generation of compounds such as ascorbic acid or even to activate enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT) that inactivate ROS (Yadav et al. 2012; Maia et al. 2018). Thus, stress tolerance conferred through the exogenous application of this hormone is different from all the mechanisms already analyzed in studies on Ca2+ disorders (Saure 2001, 2014).
One of the main effects of Ca2+ in plants is maintenance of membrane function and stability (Hepler and Winship 2010). Thus, Ca2+ deficiency results in membrane degradation, which can be evidenced by the peroxidation of lipids and generation of reactive oxygen species (ROS). Indeed, previous studies have shown high levels of ROS, such as superoxide radicals, hydroxyl radicals, and singlet oxygen (O2−), in fruit tissue with BER (Aktas et al. 2005; Turhan et al. 2006; Mestre et al. 2012). In this context, BR could increase fruit tissue capacity to scavenge ROS, which could prevent or reduce fruit susceptibility to Ca2+ deficiency (Turhan et al. 2006). However, an extensive study focusing on the relationship between oxidative metabolism and BER development revealed that reducing fruit Ca2+ concentration also reduced the activity of the main enzymes responsible for ROS detoxification, leading to H2O2 accumulation, lipid peroxidation, and BER symptom development in the fruit (Mestre et al. 2012).
Production of ROS can also be increased under exposure to many abiotic stresses (Wu et al. 2017). Under stress conditions, such as exposure to salt or water deficit, increasing H2O2 production is the major contributor to cell and tissue damage in plants (Choudhury et al. 2017). Plant cells have a large number of protective mechanisms to eliminate or reduce ROS (Cuypers et al. 2016). The activation of the enzymatic antioxidant system, which operates by the sequential and simultaneous actions of enzymes including SOD, CAT, and APX, is one of them (Gratão et al. 2015; Alves et al. 2017). The accumulation of H2O2 is prevented in plant cells either by CAT and peroxidases or by the ascorbate–glutathione cycle where APX reduces it to H2O (Gratão et al. 2005). 24-Epibrassinolide (EBL) was shown to increase the activity of enzymes such as APX, CAT, SOD in plant cells, as well as enhance the synthesis of antioxidant substances such as ascorbic acid, resulting in lower contents of ROS, and lipid peroxidation in the cells (Maia et al. 2018). Ascorbic acid (AsA) can also directly scavenge ROS produced during aerobic metabolic processes such as photosynthesis or respiration; however, the extent to which the direct reduction of ROS occurs in plants remains to be determined (Gallie 2013).
According to the previous studies, it is possible that neither total Ca2+ nor ROS alone can fully explain BER development in tomato fruit, but rather the interaction between Ca2+ and ROS levels in the tissue. Therefore, the objective of this study was to understand the mechanisms through which EBL inhibits BER development in tomato fruit.
Materials and Methods
Plant Material, Growth Conditions, and Application of Treatments
This study was carried out using the ‘BRS Montese’ (EMBRAPA) tomato, which has long-shaped fruit. The seeds used in the study were donated by Agrocinco Seeds of Value (Monte Mor, São Paulo, Brazil). The experiment was performed in a greenhouse with average solar radiation at midday of 1000 µmol m−2 s−1, average temperature of 18.3 °C, and relative humidity of 77% at night and 28.2 °C and 52% during daytime, respectively. Seeds were seeded separately in trays with a 1:1 (v/v) peat-based substrate mixture (Plantmax HT, Eucatex Brazil) and expanded vermiculite, and supplemented with 1 g L−1 of NPK (10:10:10) fertilizer and 4 g L−1 of dolomitic limestone, supplementing calcium and magnesium.
Thirty days after planting, the seedlings were transplanted into individual 30 L pots containing substrate mixture. Plants were fertilized every 20 days, during growing and fructification time, with 10 g of slow release fertilizer containing N (43 kg ha−1), P2O5 (21.2 kg ha−1), K2O (32 kg ha−1), MgO (5.3 kg ha−1), S (13.3 kg ha−1), Fe (1.1 kg ha−1), Cu (0.13 kg ha−1), Mn (0.16 kg ha−1), Zn (0.05 kg ha−1), B (0.05 kg ha−1), Mo (0.04 ka ha− 1), but without Ca (Basacote Plus, Agricultural Soil Fertilizer; Compo Expert) to stimulate BER incidence.
When plants started blooming, they were sprayed weekly with a 125-mL solution per plant containing water (control) or 24-epibrassinolide—EBL (Sigma-Aldrich, Saint Louis, MO, USA) (0.01 µM). The EBL concentration was determined based on the level that has been shown to trigger plant responses in other studies (Ogweno et al. 2008; Jiang et al. 2012; Yadav et al. 2012; Zheng et al. 2016; Riboldi et al. 2018c). Fruit samples were harvested on the first cluster at 15 days after pollination (DAP).
BER Incidence, Growth Parameters, and Xylem Functionality
The incidence of BER was calculated by multiplying the number of fruits with BER symptoms by 100 and dividing by the total number of fruit in the first cluster. The plant dry weight was determined by drying the samples (65 °C) until constant weight. Leaf area was determined through a leaf area meter (LI-3100C Area Meter, LI-COR, Lincoln, USA), using all the leaves. Both plant dry weight and leaf area were determined at full bloom. Fruit length and diameter were determined using a caliper. Fruit weight was determined using the average of fruits.
Xylem function was measured in developing fruit as previously described by Ho et al. (1993) and De Freitas et al. (2011b). Fruits were harvested 15 days after pollination and held in sealed plastic bags for 20 min with 100 mL of free water to reduce transpiration until the peduncle of each fruit was immersed in a solution of 1% Safranin-O at 20 °C under ≤ 20% relative humidity. After 24 h, fruit were cut into three equal sections at a 90° angle to the peduncle axis. The number of stained vascular bundles (xylem vessels) was counted in the placenta and pericarp tissues at the cut surfaces at the blossom and peduncle end regions of each fruit.
Determination of Leaf Stomatal Conductance, Leaf Transpiration Rate, and Leaf Water Potential
An infrared gas analyzer (IRGA) model LCpro+ (ADC BioScientific LTD., Hertfordshire, UK) was used to determine stomatal conductance (gs, mol H2O m−2 s−1) and transpiration rate (E, mmol H2O m−2 s−1). The evaluations were accomplished between 9 and 11 am in fully expanded leaves close to the first cluster at 15 DAP. For the determination of leaf water potential, we used the equilibrium vapor pressure method by means of a psychrometric technique using a microvoltmeter model HR-33T (Wescor, Logan, UT, USA) coupled to Wescor C-52 chambers (Wescor, Logan, UT, USA).
Total Tissue Ca2+, Mg2+, and K+ Contents in Leaf and Fruit and Ca2+ Bound to the Cell Wall
Nutrient analysis was accomplished in proximal and distal fruit tissues, as well as in fully expanded leaves close to the first fruit cluster. Samples were oven dried at 65 °C until constant weight. About 500 mg of dry material was added to 6 mL of nitroperchloric acid (2:1). The digestion was performed in a plaster block at 240 °C with 15 g of distilled water. Nutrient quantification was performed by atomic absorption, according to Malavolta et al. (1997). The results were expressed as g of Ca2+, Mg2+, and K+ per kg of tissue dry weight.
Calcium bound to the cell wall was determined in fruit distal tissue after extracting cell-wall material following the protocol described by Campbell et al. (1990). The quantification of Ca2+ was carried out using the same method described above.
Apoplastic and Cytoplasmic Electrolytic Leakage and Soluble Ca2+ Content in Fruit Tissue
Fruit electrolyte leakage was performed according to the protocol described by De Freitas et al. (2011a). Three fruit pericarp discs with 1 cm in diameter and 0.7 cm thickness were collected in each replication. The discs were then added to 50 mL tubes containing a 0.4 M mannitol solution, which were placed on a rotary shaker (CT-165, Cientec). The conductivity in the mannitol solution was recorded for 6 h at 1 h intervals. Subsequently, the samples were frozen and thawed three times to determine the total conductivity (Saltveit 2002).
Apoplastic electrolyte leakage was considered to be the leakage of ions during the first 3 h of increasing solution conductivity, representing the ions leaking from the apoplast space in the tissue (Saltveit 2002). Cytoplasmic electrolyte leakage was considered to be the last 3 h of increasing solution conductivity, representing the ions leaking through the membranes in the tissue (Saltveit 2002). The results were expressed as the percentage increase of electrolyte leakage per gram of tissue per hour relative to total tissue conductivity.
At the end of the 6 h, 1 mL solution of the samples was collected to determine soluble Ca2+, Mg2+, and K+ in fruit tissue according to the approach described above. The results were expressed as g of Ca2+, Mg2+, and K+ per kg of tissue dry weight.
Ascorbic Acid, MDA, H2O2, and Enzymatic Assays
The determination of ascorbic acid (AsA) was based on the method described by Carvalho et al. (1990), of reducing 2,6-dichlorophenol indophenol sodium (DCIP) by ascorbic acid, which has a strong reducing action. Distal fruit tissue was used, obtained from fruits after 15 DAP.
The measurements of the malondialdehyde (MDA) and hydrogen peroxide (H2O2) contents were performed in the same extraction, using distal fruits tissue, collected 15 days after pollination and leaves, collected next to the clusters. The concentration of MDA was calculated from the absorbance at 535 nm by using the absorbance coefficient 155 mM−1 cm−1, following a correction for unspecific turbidity determined by the absorbance at 600 nm (Heath and Packer 1968).
H2O2 was measured spectrophotometrically after reaction with KI as described by Alexieva et al. (2001), using the same tissue used for MDA analysis. The reaction was developed for 1 h in darkness at room temperature and absorbance measured at 390 nm. The amount of H2O2 was calculated using a standard curve prepared with known concentrations of H2O2.
The total protein concentration was determined by the method of Bradford (1976) using bovine serum albumin as a standard (Bio-Rad Protein Dye Reagent), using distal fruits tissue, collected 15 days after pollination and leaves, collected next to the clusters. Superoxide dismutase (SOD, E.C. 1.15.1.1) activity was carried out as reported by Constantine and Ries (1977) by the inhibition of NBT chloride photoreduction. One unit of SOD activity was defined as the amount of the enzyme required to inhibit the reduction of NBT by 50% under the specified conditions. SOD activity of the extracts was expressed as U mg−1 protein.
Catalase (CAT, E.C. 1.11.1.6) activity was determined as described by Kraus (1995) with modifications as described by Azevedo et al. (1998). CAT activity was calculated using an extinction coefficient for H2O2 of 39.4 mM−1 cm−1 and results were expressed as µmol−1 min−1 mg protein. Ascorbate peroxidase (APX, E.C. 1.11.1.11) activity was determined by monitoring the rate of ascorbate oxidation at 290 nm at 30 °C. APX activity was expressed as µmol−1 min−1 mg protein (Nakano and Asada 1981).
Experimental Design
The experiment followed a randomized blocks design with five blocks per treatment and four plants per block. Therefore, each treatment was composed by a total of 20 plants. The data were subjected to analysis of variance (ANOVA) and the averages were compared by the t test with statistical significance at 5%, using SAS statistical software (Cary, North Carolina, USA). The data without normal distribution were analyzed using Friedman’s non-parametric test with statistical significance at 5%. Pearson’s correlation was also performed with statistical significance at 5%. Soluble Ca2++APX, Soluble Ca2++AsA, Soluble Ca2++SOD, Soluble Ca2++CAT, represent the sum of each respective average, whereas Soluble Ca2+-H2O2 represents the subtraction of each respective average. In this case, the data were centered on the respective average so that, the magnitude of the values of different parameters had the same weight at the time of adding or subtracting. Total correlation refers to all values added or subtracted, depending on its correlation, inhibiting or triggering BER.
Results
BER Incidence, Biometric, and Physiological Parameters
The incidence of BER was markedly different between water- and EBL-treated plants. BER levels in the control were higher than in EBL-treated plants, with 44.2% of BER incidence reduction (Fig. 1).
There were also no differences between leaf area and dry weight between treatments (Table 1). Fruit length, diameter, and weight were higher in EBL-treated plants (Table 1).
There were no differences in leaf stomatal conductance, leaf transpiration, and leaf water potential between treatments (Table 1). Xylem functionality was similar between treatments (Table 2).
Nutrient Concentration in Leaf and Fruit Tissues and Membrane Permeability
The Ca2+ partitioning between leaves and fruits varied with the treatments (Table 3). In leaves, Ca2+ concentration was higher in control plants, compared to EBL-treated plants. In fruits, the highest concentration of soluble Ca2+ was observed in EBL-treated plants. In proximal and distal fruit tissue, the highest Ca2+ concentration was observed in control plants. The highest ratio between proximal and distal part (P/D) was observed in EBL-treated plants. Ca2+ bound to the cell wall in distal fruit tissue was higher in the control treatment. The ratio between the wall-bound Ca2+ and the soluble Ca2+, both in the distal tissue (CW/D), was higher in control plants (Table 3).
In another way, there were no differences in Mg2+ concentration between treatments in leaves and fruit proximal and distal tissue (Table 4). EBL-treated plants had higher concentrations of soluble Mg2+ in comparison to control plants. K+ concentration in leaves, fruit soluble, proximal, distal tissue (Table 4), and ratio P/D showed no differences between treatments.
There were no differences between treatments in cytoplasmic and apoplastic electrolyte leakage in proximal fruit tissue (Table 5). However, in the distal fruit tissue, the cytoplasmic electrolyte leakage was higher in control plants, whereas the apoplastic leakage was higher in EBL-treated plants.
Antioxidant Concentration and Activity in Leaf and Fruit Tissues
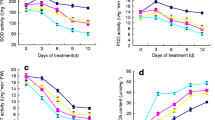
The concentration of AsA was 8% higher in EBL-treated plants (Fig. 2). There was no significant difference in malondialdehyde (MDA) content in both leaves and fruits, using EBL treatment, compared to control (Fig. 3). Hydrogen peroxide (H2O2) content decreased in response to EBL treatment in leaves and fruits, being higher in leaves in comparison to fruits (Fig. 3).
Oxidative damage induced by EBL treatment in tomato tissues expressed as MDA content (nmol g−1 fresh weight) and H2O2 µmol g−1 fresh weight. a MDA content in leaves; b MDA content in fruits; c H2O2 content in leaves; d H2O2 content in fruits. Fruit were harvested at 15 days after pollination. *Averages are statistically different according to the t test (5%). Data shown as mean ± standard deviation
On the other hand, the activity of SOD, CAT, and APX was higher in leaves and fruits of EBL-treated plants (Fig. 4). Focusing on the effects of their activity on fruits, SOD, CAT, and APX had 16, 12.5, and 18.4% increases after EBL treatment, respectively (Fig. 5).
Oxidative stress-related enzymes activity in tomato plants and fruits subjected to EBL treatments. a Superoxide dismutase activity in leaves; b Superoxide dismutase activity in fruits; c Catalase activity in leaves; d Catalase activity in fruits; e Ascorbate peroxidase activity in leaves; f Ascorbate peroxidase activity in fruits. Fruit were harvested at 15 days after pollination. *Averages are statistically different according to the t-test (5%). Data shown as mean ± standard deviation
Correlation Among Parameters Related to BER
The correlation analysis between the variables evaluated in the study revealed that some of the physiological variables correlated positively with BER (Table 6). The variables that had positive correlations with BER were cytoplasmic leakage, H2O2, soluble Ca2+-H2O2, cell wall-bound Ca2+, CAT, MDA, SOD, total variables related to triggering BER. The variables that had negative correlations with BER were AsA, apoplastic leakage, soluble Ca2++APX, soluble Ca2++AsA, APX, soluble Ca2+, soluble Ca2++SOD, soluble Ca2++CAT, total variables related to inhibiting BER.
Discussion
Blossom-end rot (BER) is a disorder that affects growing fruits. It increases oxidative compounds in fruit distal tissue, leading to membrane peroxidation and cell-wall damages, reaching all tissue in a later stage (Aktas et al. 2003). Some hormones, such as ABA, are being used to reduce BER damage, but focusing on adapting the plants to a restrictive environment (Riboldi et al. 2018c).
Recently, some hormones started to be tested, with new approaches, such as brassinosteroids. They are a class of hormones with many responses in plants and main effects are related to stress responses (Soares et al. 2016; Shahzad et al. 2018). In this study, EBL had a great effect on reducing BER incidence in developing tomato fruit by about 44.2%. Due to some well-known evidence, it was noted how EBL may modulate the antioxidant activity in plants (Liu et al. 2009; Xia et al. 2009; Soares et al. 2016). It is possible that these plants responded by increasing stress-related defenses, like the antioxidant response.
In addition to oxidative stress factors, other possible mechanisms related to morphological factors such as fruit size, weight, and number of xylem functionality, physiological factors such as stomatal conductance, water potential, and transpiration, as well as nutritional factors such as Ca2+, Mg2+, and K+ may also be possibly involved in determining fruit susceptibility to BER.
Physiological/Morphological and Nutritional Factors Regulating BER Development
Although EBL is a hormone related to plant growth, its effect was only observed in the fruit and not on leaf area or plant dry weight. Ca2+ is transported through the transpiratory current, reaching the fruit and aerial parts of plants via the vascular system, especially through the xylem vessels. As suggested in other studies, functional xylem vessels are required to maintain proper fruit Ca2+ uptake to support the active growing cells at the fruit distal end (Bondada et al. 2005).
However, the EBL treatment had no effect on the number of functional xylem vessels in the fruit during growth and development. Some studies have shown that using BRs in tissue culture can lead to a greater differentiation of xylem vessels instead of phloem vessels (Nagata et al. 2001). It is also important to consider that xylem and phloem vessel differentiation depends on other plant hormones and their final ratio in the tissue, which may explain the lack of EBL effect on functional xylem vessels.
The effects of EBL controlling plant susceptibility to environmental stresses are important and this relation is becoming clearer with new studies (Singh and Shono 2005; Dobrikova et al. 2014; Soares et al. 2016; Shazad et al. 2018). Nevertheless, it was possible to see that EBL treatment reduced plant transpiration, changing leaf water potential. The movement of water and solutes in the plant takes place in response to water potential gradients. Therefore, the EBL reduction of leaf transpiration and the increase in water potential observed resulted in lower leaf Ca2+ accumulation in EBL-treated plants.
The content of Ca2+ in fruits was also affected by EBL treatment. In proximal and distal fruit parts, water-treated fruit showed greater tissue Ca2+ content. However, recent studies have shown that the concentration of total Ca2+ in the tissue cannot fully explain BER incidence, because other pools of Ca2+ at the cellular level are also involved (De Freitas et al. 2017).
Despite higher total fruit tissue Ca2+ content in water-treated plants, the content of soluble Ca2+ in the distal tissue was higher in EBL-treated fruit and also negatively correlated with BER. Furthermore, the Ca2+ bound to the cell wall was higher in water-treated plants. In that case, higher Ca2+ bound to the cell wall reduces the Ca2+ available for other cellular functions such as membrane structure and stability, potentially increasing fruit susceptibility to BER. The observed lower Ca2+ bound to the cell wall may be the result of lower pectin methylesterase (PME) activity and/or lower synthesis of desterified pectin in the tissue in response to EBL treatment (Peaucelle et al. 2012). De Freitas et al. (2012) have shown that reducing PME expression and activity can reduce Ca2+ binding to the cell wall, increasing other pools of Ca2+ in the cell and inhibiting BER development in tomato fruit.
The results of electrolytic leakage confirm some evidence of maintenance of membrane integrity and soluble Ca2+ content. The electrolytic leakage in the apoplastic portion is directly related to the solutes present in the apoplast and that the analytical solution is mixed in the first hours. In this case, there is a higher apoplast leakage in EBL-treated plants. Complementing what has already been discussed above, for Ca2+ to protect the membranes it must be in the soluble form, it becomes immobile once bound to the cell wall. Thus, there was a lower incidence of BER in EBL-treated plants, and this can be explained by the higher concentration of these solutes in the apoplastic portion, mainly composed of soluble Ca2+.
In the cytoplasmic leakage, which corresponds to the simplastic portion, there is greater leakage in the control plants and at the same time, a higher concentration of Ca2+ bound to the wall and lower soluble Ca2+. It is also the highest positive correlation, considering the main variables. This analysis corresponds directly to the stability of the membrane, since they represent the solutes that were mixed in the solution, passing through the membrane after several hours of analysis.
BER is believed to be triggered by a cell-localized Ca2+ deficiency that leads to plasma membrane damage, cell plasmolysis, and water-soaked tissue at the blossom-end region of the fruit that becomes dark-brown, as cells die (Ho and White 2005; Rached et al. 2018). Therefore, the loss of integrity of these membranes leads to higher cytoplasmic leakage.
Then, for the control plants, which exhibited a higher BER incidence, soluble and cell wall-bound Ca2+ levels regulated the onset of BER. On the other hand, plants treated with EBL presented lower rates of BER precisely because they had more stable membranes and soluble Ca2+ available in the apoplastic solution. Therefore, using physiological and nutritional parameters, it is possible to consider that the increase of cell wall-bound Ca2+, leaf Ca2+, cytoplasmic distal leakage, could have triggered BER in the fruit and EBL could lower the electrolytic leakage and improve the integrity of the membranes, which represented lower BER incidence in this treatment.
Oxidative Stress-Related Factors Regulating BER Development
Brassinosteroids can stimulate growth, as well as enhance the plant’s ability to overcome stresses, such as drought, high temperatures, or oxidative stress (Liu et al. 2009; Soares et al. 2016). In this way, EBL could improve the relationship between plants and the environment (Singh and Shono 2005).
In normal conditions, ROS are produced by cell metabolism, which is aggravated under stress conditions. Generally, in these conditions, there is an increase in the rate of lipid peroxidation, resulting from an increase in accumulation of ROS. In this way, some compounds such as singlet oxygen, hydroxyl radical, anion superoxide, or hydrogen peroxides attack the unsaturated lipids, especially fatty acids, and cause their peroxidation (Van Breusegem and Dat 2006), leading to the liberation of malondialdehyde (MDA) (Yamauchi et al. 2008).
Brassinosteroids are well known as acting to increase the antioxidant defense. In this study, EBL treatment increased ascorbic acid (AsA) content in the fruit, which could help explaining the reduction in BER incidence in EBL-treated fruit. Ascorbic acid is the most abundant, powerful, and water-soluble antioxidant that acts to prevent or minimize the damage caused by ROS in plants (Athar et al. 2008), protecting and preventing membrane damage and leakage. It is also possible to observe that AsA had the highest negative correlation, showing the importance of this compound in inhibiting BER development.
Furthermore, our results show a great increase in the activity of the enzymes SOD, CAT, and APX, in both leaves and fruit. Our study reveals an important stimulation of SOD activity in EBL-treated leaves and fruits and a high increase of CAT activity in EBL-treated tomato leaves and fruits. APX is part of the ascorbate–glutathione cycle and is responsible for the elimination of hydrogen peroxide. The stimulation of the APX activity has been reported to be triggered as a plant response to stress conditions (Liu et al. 2009; Borges et al. 2018), such as ROS generation during BER development in fruits. Therefore, these enzymes act as scavengers, transforming ROS into less dangerous compounds, such as water.
Indeed, studies have shown that high expression and activity of these enzymes in response to brassinosteroids resulted in plants more tolerant to stresses such as drought, oxidative, salt, and some metals conditions (Liu et al. 2009). In this study, total enzyme activities were considered, but it is important to bear in mind that most of these enzymes are present as isoenzymes in a number of plants, including tomato (Gratão et al. 2005, 2015; Pompeu et al. 2017; Carvalho et al. 2018). Therefore, it is not possible to establish whether the changes observed in this study are due to an overall change by all isoenzymes of a particular enzyme, for instance SOD, or to only one or another isoenzyme. Ongoing research in our laboratories is considering these possibilities particularly in the case of SOD whose distinct isoenzymes reallocate in distinct cell compartments, and therefore respond to specific changes in superoxide in different organelles.
Our study was the first to show the effects of EBL on different pools of Ca2+ at the cellular level as well as on antioxidant mechanisms inhibiting BER development in fruit tissue. Therefore, the combined effect of specific pools of Ca2+ and enzymes involved in stress resistance resulted in fruits less susceptible to BER in response to EBL treatment.
Possible Mechanism Inhibiting BER in Response to EBL
For many years, researchers have considered BER as a disorder caused only by Ca2+ depletion in the tissues in the distal portion of the fruit. However, more recently, it has been concluded that localized Ca2+ deficiency may lead to membrane leakage, which results in BER symptom development (Saure 2014).
Under stress conditions, increasing tissue ROS production to levels greater than the cells can metabolize may result in lipid peroxidation and cell death. In this case, we can treat BER as a direct consequence of ROS accumulation in the tissue (Rached et al. 2018), because the main symptoms observed during the development of the disorder are the loss of membrane stability and tissue necrosis.
In this study, EBL increased tissue antioxidant capacity, minimizing the effects of ROS on tissue oxidation and BER incidence. Therefore, spraying the plants with EBL increased plant resistance to low Ca2+ availability and stress conditions. In addition, EBL reduced cell wall-bound and increased water-soluble Ca2+ contents, as well as increased AsA leaves, both showing a strong negative correlation with BER incidence.
Higher activity of oxidative stress-related enzymes such as APX, CAT, and SOD, possibly helped reducing H2O2 concentration and lipid peroxidation, which resulted in the observed lower distal fruit tissue membrane leakage and BER incidence. Accordingly, the correlation analysis showed that cytoplasmic leakage and H2O2 levels were the main factors triggering BER in the fruit. In that case, ROS possibly acted by disrupting membranes, increasing membrane leakage, and triggering BER in the fruit.
Conclusions
In this study, EBL inhibited BER development in ‘BRS Montese’ tomato fruit. EBL maintained higher soluble Ca2+ and lower cell wall-bound Ca2+ contents in fruit tissue, reducing fruit susceptibility to BER. EBL also increased ascorbic acid content and decreased hydrogen peroxide contents, as well as increased the activity of the three main antioxidant enzymes known as ascorbate peroxidase, catalase, and superoxide dismutase in fruit tissue.
The results show that not only Ca2+ content plays a role in determining fruit susceptibility to BER, but also the oxidative activity in distal fruit tissues.
Although brassinosteroids are still not commercially used in agriculture, there is a great potential use of these hormones to improve plant stress resistance and reduce tomato fruit susceptibility to BER.
Future studies should be carried out to optimize doses, timing of applications, and possible interactions between brassinosteroids and other hormones. Furthermore, studies are also required to better understand the mechanisms regulating the activity of the main antioxidant enzymes, as well as increasing soluble Ca2+ and decreasing cell wall-bound Ca2+.
References
Aktas H, Karni L, Aloni B, Bar-Tal A (2003) Physiological and biochemical mechanisms leading to blossom-end rot in greenhouse-grown peppers, irrigated with saline solution. Acta Hortic 609:81–88
Aktas H, Karni L, Chang DC, Turhan E, Bar-Tal A, Aloni B (2005) The suppression of salinity-associated oxygen radicals production in pepper (Capsicum annuum) fruit by manganese, zinc and calcium in relation to its sensitivity to blossom-end rot. Physiol Plant 123:67–74
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Alves LR, Monteiro CC, Carvalho RF, Ribeiro PC, Tezotto T, Azevedo RA, Gratão PL (2017) Cadmium stress related to root-to-shoot communication depends on ethylene and auxin in tomato plants. Environ Exp Bot 134:102–115
Athar HR, Khan A, Ashraf M (2008) Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ Exp Bot 63:224–231
Azevedo RA, Alas RM, Smith RJ, Lea PJ (1998) Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol Plant 104:280–292
Bondada BR, Matthews MA, Shackel KA (2005) Functional xylem in the post-veraison grape berry. J Exp Bot 56:2949–2957
Borges KLR, Salvato F, Alcântara BK, Nalin RS, Piotto FA, Azevedo RA (2018) Temporal dynamic responses of roots in contrasting tomato genotypes to cadmium tolerance. Ecotoxicology 27(3):245–258
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Campbell A, Huysamer M, Stotz HU, Greve LC, Labavitch JM (1990) Comparison of ripening processes in intact tomato fruit and excised pericarp discs. Plant Physiol 94:1582–1589
Carvalho CRL, Mantovani DMB, Carvalho PRN, Moraes RM (1990) Análises químicas de alimentos. Instituto de Tecnologia de Alimentos, Campinas, p 121
Carvalho MEA, Piotto FA, Nogueira ML, Gomes-Junior FG, Chamma MCP, Pizzaia D, Azevedo RA (2018) Cadmium exposure triggers genotype-dependent changes in seed vigor and germination of tomato offspring. Protoplasma 255(4):989–999
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867
Constantine GN, Ries SK (1977) Superoxide dismutases: I. occurrence in higher plants. Plant Physiol 59:309–314
Cuypers A, Hendrix S, Amaral dos, Reis R, De Smet S, Deckers J, Gielen H, Jozefczak M, Loix C, Vercampt H, Vangronsveld J, Keunen E (2016) Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front Plant Sci 7:470
De Freitas ST, Padda M, Wu Q, Park S, Mitcham E (2011a) Dynamic alterations in cellular and molecular components during blossom-end rot development in tomatoes expressing sCAX1, a constitutively active Ca2+/H+ antiporter from Arabidopsis. Plant Physiol 156:844–855
De Freitas ST, Shackel KA, Mitcham EJ (2011b) Abscisic acid triggers whole-plant and fruit-specific mechanisms to increase fruit calcium uptake and prevent blossom-end rot development in tomato fruit. J Exp Bot 62:2645–2656
De Freitas ST, Handa AK, Wu Q, Park S, Mitcham EJ (2012) Role of pectin methylesterases in cellular calcium distribution and blossom-end rot development in tomato fruit. Plant J 71:824–835
De Freitas ST, Martinelli F, Feng B, Reitz NF, Mitcham EJ (2017) Transcriptome approach to understand the potential mechanisms inhibiting or triggering blossom-end rot development in tomato fruit in response to plant growth regulators. J Plant Growth Regul 37(1):183–198
Dobrikova AG, Vladkova RS, Rashkov GD, Todinova SJ, Krumova SB, Apostolova EL (2014) Effects of exogenous 24-epibrassinolide on the photosynthetic membranes under non-stress conditions. Plant Physiol Biochem 80:75–82
Gallie DR (2013) L-ascorbic acid: a multifunctional molecule supporting plant growth and development. Scientifica. https://doi.org/10.1155/2013/795964
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal- stressed plants a little easier. Funct Plant Biol 32:481–494
Gratão PL, Monteiro CC, Tezotto T, Carvalho RF, Alves LR, Peters LP, Azevedo RA (2015) Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. Biometals 28:803–816
Heath RL, Packer L (1968) Photoperoxidation in isoled chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:11
Hepler PK, Winship LJ (2010) Calcium at the cell wall-cytoplast interface. J Integr Plant Biol 52:147–160
Ho LC, White PJ (2005) A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Ann Bot 95:571–581
Ho LC, Belda R, Brown M, Andrews J, Adams P (1993) Uptake and transport of calcium and the possible causes of blossom end rot in tomato. J Exp Bot 44:509–518
Ikeda H, Shibuya T, Nishiyama M, Nakata Y, Kanayama Y (2017) Physiological mechanisms accounting for the lower incidence of blossom-end rot in tomato introgression line IL8-3 fruit. Hortic J 86(3):327–333
Jiang W, Bai J, Yang X, Yu H, Liu Y (2012) Exogenous application of abscisic acid, putrescine, or 2,4-epibrassinolide at appropriate concentration effectively alleviate damage to tomato seedlings from suboptimal temperature stress. Horttechnology 22(1):137–144
Kraus TE, Fletcher RA, Evans RC, Pauls KP (1995) Paclobutrazol enhances tolerance to increased levels of UV-B radiation in soybean (Glycine max) seedlings. Can J Bot 73:797–806
Liu Y, Zhao Z, Si J, Di C, Han J, An L (2009) Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. Plant Growth Regul 59:207–214
Maia CF, Silva BRS, Lobato AKS (2018) J Plant Growth Regul. https://doi.org/10.1007/s00344-018-9802-2
Malavolta E, Vitti GC, Oliveira AS (1997) Avaliação do estado nutricional das plantas- princípios e aplicações. 2 ed, POTAFOS, Piracicaba, p 309
Mestre TC, Garcia-Sanchez F, Rubio F, Martinez V, Rivero RM (2012) Glutathione homeostasis as an important and novel factor controlling blossom-end rot development in calcium-deficient tomato fruits. J Plant Physiol 169:1719–1727
Nagata N, Asami T, Yoshida S (2001) Brassinazole, an inhibitor of brassinosteroid biosynthesis, inhibits development of secondary xylem in cress plants (Lepidium sativum). Plant Cell Physiol 42:1006–1011
Nakano Y, Asada K (1981) Hydrogen-peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Ogweno JO, Song XS, Shi K, HU WH, Mao WH, Zhou YH, YU JQ, Nogués S (2008) Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57
Peaucelle A, Braybrook SA, Höfte H (2012) Cell wall mechanics and growth control in plants: the role of pectins revisited. Front Plant Sci 3:121
Pompeu GB, Vilhena MB, Gratão PL, Carvalho RF, Rossi ML, Martinelli AP, Azevedo RA (2017) Abscisic acid-deficient sit tomato mutant responses to cadmium-induced stress. Protoplasma 254(2):771–783
Rached M, Pierre B, Yves G, Matsukura C, Ariizumi T, Ezura H, Fukuda N (2018) Differences in blossom-end rot resistance in tomato cultivars is associated with total ascorbate rather than calcium concentration in the distal end part of fruits per se. Hortic J. https://doi.org/10.2503/hortj.OKD-150
Riboldi LB, Araújo SHC, De Freitas ST, Castro PRC (2018a) Blossom-end rot incidence in elongated tomato fruit. Botany 96(10):663–673
Riboldi LB, Araújo SHC, De Freitas ST, Castro PRC (2018b) Fruit shape regulates susceptibility of tomato to blossom-end rot. Acta Sci Agron
Riboldi LB, Araújo SHC, Múrcia JAG, De Freitas ST, Castro PRC (2018c) Abscisic acid (ABA) and 24-epibrassinolide regulate blossom-end rot (BER) development in tomato fruit under Ca2+ deficiency’. Aust J Crop Sci 12(9):1440–1446
Saltveit ME (2002) The rate of ion leakage from chilling-sensitive tissue does not immediately increase upon exposure to chilling temperatures. Postharvest Biol Technol 26:295–304
Saure MC (2001) Blossom-end rot of tomato (Lycopersicon esculentum Mill.): a calcium or a stress-related disorder? Sci Hortic 90:193–208
Saure MC (2014) Why calcium deficiency is not the cause of blossom-end rot in tomato and pepper fruit–a reappraisal. Sci Hortic 174:151–154
Shahzad B, Tanveera M, Cheb Z, Rehmanc A, Cheemac SA, Sharmad A, Songb H, Rehmane S, Singh I, Shono M (2005) Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid on thermotolerance of tomato. Plant Growth Regul 47:111–119
Soares C, De Sousa A, Pinto A, Azenha M, Teixeira J, Azevedo RA, Fidalgo F (2016) Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ Exp Bot 122:115–125
Turhan E, Karni L, Aktas H, Deventurero G, Chang DC, Bar-Tal A, Aloni B (2006) Apoplastic antioxidants in pepper (Capsicum annuum L.) fruit and their relationship to blossom-end rot. J Hortic Sci Biotechnol 81:661–667
Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141:384–390
Wu W, Zhang Q, Ervin EH, Yang Z, Zhang X (2017) Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-epibrassinolide. Front Plant Sci 8:1017
Xia X-J, Wang Y-J, Zhou Y-H, Tao Y, Mao W-H, Shi K, Asami T, Chen Z, Yu J-Q (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150(2):801–814
Yadav S, Hayat S, Wani AS, Irfan M, Ahmad A (2012) Comparison of the influence of 28-homobrassinolide and 24-epibrassinolide on nitrate reductase activity, proline content, and antioxidant enzymes of tomato. Int J Veg Sci 18(2):161–170
Yamauchi Y, Furutera A, Seki K, Toyoda Y, Tanaka K, Sugimoto Y (2008) Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol Biochem 46:786–793
Zheng Q, Liu J, Liu R, Wu H, Jiang C, Wang C, Guan Y (2016) Temporal and spatial distributions of sodium and polyamines regulated by brassinoesteroids in enhancing tomato salt resistance. Plant Soil 400:147–164
Acknowledgements
The Coordination of Improvement of Higher Education Personnel (CAPES) and the Department of Biological Sciences of the University of São Paulo (ESALQ/USP) supported this study. We also thank the Laboratory of Plant Ecophysiology, Laboratory of Plant Genetics and Biochemistry (ESALQ/USP), and Laboratory of Plants Mineral Nutrition (CENA/USP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Riboldi, L.B., Gaziola, S.A., Azevedo, R.A. et al. 24-Epibrassinolide Mechanisms Regulating Blossom-End Rot Development in Tomato Fruit. J Plant Growth Regul 38, 812–823 (2019). https://doi.org/10.1007/s00344-018-9892-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9892-x