Abstract
Nickel selenide thin films were synthesized on the amorphous templates utilizing a dip method. In the synthesis process, ascorbic acid acts as complexing agent. Hydrazine hydrate too plays a role of complexing agent and/or catalyst. Structural, optical, microscopic, electrical and thermoelectrical parameters were estimated. The X-ray diffraction spectrum of nickel selenide shows polycrystalline nature having hexagonal structure. The absorptivity of nickel selenide films is highly absorptive with a direct type of transition. The optical gap of NiSe was found to be 2.18 eV. The Urbach energy was found to be 0.275 eV. The ratio of Ni: Se in the thin film was 1:1. Micrograph of nickel selenide thin film showing irregular shaped fine grains distributed to the whole surface. The electrical conductance of nickel selenide suggests that the activation energy was found to be 0.024 and 0.331 eV for lower and higher temperature areas, respectively. At 300 K, the carrier concentration is estimated to be 2.58 × 1019 and 3.55 × 1019 at 525 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, transition metal chalcogenides have applied as the absorbent for solar devices to substitute the silicon. They are projected as the most acceptable semiconductor materials for photoelectrochemical cell. It owns outstanding electronic, semiconducting and optical characteristics in the thin film [1, 2]. The thickness of such chalcogenides adopts the different characteristics. The features of thin film are dissimilar from bulk sample. It is crucial to choice the proper systems for the preparation of transition metal chalcogenide. [3,4,5]
Nickel chalcogenides becomes the novel thin film substances for the generation of solar power because of its substantial semiconductor and optical properties [6]. Nickel selenide (NiSe) has been extensively applied in diverse devices such as coating, IR detectors, solar cells, and photoconductors [7, 8]. Nickel selenide has some stable phases like NiSe, NiSe2, and Ni3Se2 [9,10,11]. A different complexing agent like triethanolamine [12], sodium salt of ethylene diammine tetra acetic acid [13], polyvinyl alcohol [14] was applied to prepare nickel sulphide thin film. Sharma et al. [15] synthesized nickel selenide thin film using a screen coating technique. The electrical resistivity was found to be 103 (Ω.cm) and activation energy around 0.18 eV.
In this research work, nickel selenide thin films were synthesized by the dip technique at room temperature. Ascorbic acid is utilized as a complexing agent. Structural, optical, microscopic, electrical, and thermoelectrical parameters were estimated.
2 Experimental details
2.1 Templates and chemicals
The deposition was carried on amorphous glass plates of dimensions 26 × 76× 2 mm. The templates were cleaned by using chromic acid, detergent, and alcohol. Analytical quality chemicals were utilized for the preparation of thin film. It consists of nickel sulphate octahydrate, ascorbic acid, hydrazine hydrate, sodium sulphite, selenium powder and ammonia. Sodium selenosulphate (~ 0.25 M) solution is ready by refluxing 2.5 g of selenium powder and 7.5 g sodium sulphite in 100 ml water for 6 h at 363 K. The hot solution was cooled and filtered [16]. Nickel selenide thin film was prepared utilizing dip technique.
2.2 Deposition of NiSe thin film
To prepare the bath, 10 mL (0.25 M) nickel sulphate octahydrate was poured in 100 mL beaker; other chemicals were used in the following sequence: 2.5 mL (1 M) ascorbic acid, 8 ml (15%) hydrazine hydrate, 10 mL (0.25 M) sodium selenosulphate. The pH of the reactive mixture is 10.95. 10 mL double distilled water is added in the bath. Reactive mixture kept in the ice bath. The non-conducting glass templates maintained vertically in a reaction mixture. The temperature of the reaction mixture surges up to 298 K. The deposition process gets completed after 6 h. The deposited templates were washed with water.
3 Results and discussion
3.1 Growth mechanism
The reaction mechanism of synthesis of NiSe thin film is shown below;
The nickel ions combine with ascorbic acid. Ascorbic acid binds the nickel ion to prevent the direct reaction between nickel and selenide ions. It plays the role as binding agent. pH of reaction mixture is greater than 7; sodium selenosulphate experiences decay generating free selenide ions. The quantity of nickel ions in the reaction mixture is conserved by ascorbic acid. The temperature of reaction bath upswings progressively; the disintegration of nickel ascorbate complex takes place. Free selenide and nickel ions were combined to form NiSe thin film. The deposition take place when the ionic product of is greater than solubility product. [Ksp = 2 × 10–26] [17]. Hydrazine hydrate is compulsory for the formation of NiSe thin film. It might be playing a complexing and/or catalytic role in the film process. It also improves compactness and adherences of the films [18, 19]. The as-grown NiSe films show grey colour [20]. The terminal thickness was found to be 0.14 μm for NiSe thin film.
3.2 X-ray diffraction
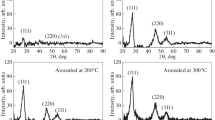
The X-ray diffraction pattern of the films was recorded by using Philips-PW-1710, X-ray diffractometer, in 2θ range from 10 to 80° using CuKα1 line (λ = 1.54056 Å). Nickel selenide exhibits wurtzite (hexagonal type), tetragonal and rhombohedral structure. This depends upon synthesis conditions such as the deposition temperature and precursor concentration. The spectra for pure NiSe [JCPDF Card No.75–0610] were used for identification purpose. The X-ray diffraction spectrum of NiSe thin film deposited on glass substrate is shown in Fig. 1.
The XRD pattern shows a large number of peaks indicate that the film are polycrystalline in nature having hexagonal structure. For NiSe, (100) (102) (110) (103) (112) planes of hexagonal are observed. Using Bragg’s equation, the interplanar distance of each peak became estimated. The highest intense reflection at d = 3.130 Å can be normally indexed as hexagonal (100). The interplanar distance became 1.984, 1.801, 1.540 and 1.499 Å for (102) (110) (103) and (112) planes, respectively. Lattice parameter of the NiSe thin film is estimated and found to be a = 3.614 and c = 5.129 Å. Umeyama et al. [21] reported lattice parameter a = 3.659 and c = 5.352 Å for NiSe thin film. Volume of unit cell of NiSe was found to be 66.989 (Å)3. The average crystallite size was determined by using Scherer’s formula. It was found to be 84.83 nm. The dislocation density of NiSe thin film was found to be 1.389 × 1014 m−2. The number of crystallite/unit area was estimated to be 2.293 × 1014 m−2.
3.3 Optical characterization
The absorbance of the NiSe thin film was measured with respect to wavelength. A Hitachi-330 (Japan) UV–VIS–NIR double-beam spectrophotometer was used for this purpose. The wavelength utilized to obtain the absorption spectra changes from 400 to 800 nm. The absorption spectra were used to calculate band gap, type of optical transition and absorption coefficient. The optical absorption spectra of representative films are shown in Fig. 2. Optical density of thin film decreases with the increase in wavelength. The absorption edge of exponential shape was observed in NiSe thin film. The absorptive nature of the NiSe thin film was measured by estimating absorption coefficient. At 400 nm, the absorptive coefficient was estimated to be 9.57 × 104 and 2.85 × 103 cm−1 at 800 nm. It decreases with the increase in wavelength. Higher magnitude of absorption coefficient indicates that NiSe thin film is highly absorptive. A plot of (α)2 against photon energy should be a straight line whose intercept to the x-axis gives the optical band gap. The graph of (α)2 vs. photon energy for nickel selenide is shown in Fig. 3. The band gap of NiSe thin film was found to be 2.18 eV [22]. The plot of (α)2 versus photon is linear in high energy region indicating direct band to band-type transition. The area close to optical edge is known as Urbach tail. Such tail was observed due to lower crystallinity, imperfections, some non-crystalline part of the materials and dislocations [23, 24]. Urbach energy can be found using the slope of the straight line of plotting ln (α) versus photon energy. The reciprocal of the slope gives Urbach energy. For NiSe thin films, the Urbach energy was found to be 0.275 eV. The graph of ln (α) versus photon energy is shown in Fig. 4.
3.4 Compositional and microscopic characterization
The composition of the NiSe thin film was investigated by using energy-dispersive X-ray analysis technique. The obtained pattern is represented in Fig. 5. The quantity of Ni present in the thin film was 51.36 and selenium 48.64%, respectively. The fraction of nickel to selenium is approximately 1. The microscopic analysis of NiSe thin film was carried out applying scanning electron microscopy. Figure 6 indicates the micrograph of NiSe thin film. The image indicates that the grains are fused with each other. No crack was observed in thin film. The irregular shaped fine grains were distributed to whole surface. The grains are compactly packed. The average grain dimension is used to estimate by way of applying Contrell’s technique. The mean grain dimension as calculated from scanning electron micrograph was applied to be observed in accord with these obtained from Scherer’s components in X-ray diffraction.
3.5 Electrical characterization
The dark DC conductivity of all samples was measured by using a two-probe method, in temperature range of 300–525 K for heating and cooling cycles. The change in electrical conductance is observed for the first cycle of heating and cooling. This is because for the first cycle NiSe thin film experiences irretrievable medications of non-equilibrium defects. The specific conductance at room temperature of NiSe was found to be of the order of 10–4(Ω cm)−1, respectively. The plot of conductivity vs. inverse temperature is shown in Fig. 7 for the film. The plot indicates the existence of two kinds of conduction mechanism. The lower-temperature region (300–430 K) was known as intrinsic having smaller slope. Above this temperature, it is known as extrinsic with characterized by a higher slope. The activation energy is calculated using the Arrhenius equation. The activation energies were obtained from the slope of straight lines of plot. For lower-temperature region, the activation energy was found to be 0.024 and 0.331 eV for higher-temperature area.
3.6 Thermoelectrical power measurement
The temperature range 300 to 525 K is selected to measure electromotive force produced by the thin film. Thermoelectric power of NiSe thin film with temperature is signified in Fig. 8. The graph indicates the uniform nature of thin film because of upsurge in thermoelectric power with temperature. The nature of conductivity was also decided by thermoelectric power measurement. In this study, positive terminal is associated with the cold end, so the thin film confirms p-type conductance. At two extreme points of temperature, thermoelectric power was observed to be 6.0 and 49.5 µV/K. Carrier density is determined using the mathematical form [25]
Carrier density is estimated for working temperature. At 300 K, the carrier density is obtained to be 2.58 × 1019 cm−3 and 3.55 × 1019 cm−3 at 525 K. The carrier density of the thin film became a surge with temperature. Ionized nickel vacancies (Ni+2) are responsible for p-type conductivity in NiSe thin films. This increase in ionized Ni vacancies with surge temperature enhances the conductivity of the films. The electrical conductivity rise as shown in Fig. 7 with respect to temperature can be correlated to carrier density. Conductivity increases as the carrier density increases with temperature.
4 Conclusions
The preparation of NiSe thin film on glass templates is carried out by applying the dip method. The hump in the X-ray diffraction spectra of NiSe thin film indicates some amorphous phase that exists in it. The optical characteristics showed that the NiSe thin film has band tail energy of 0.275 eV. From scanning electron micrograph images, it is suggested that the NiSe thin film that has irregular shaped grains was attached to each other. The film shows semiconducting nature having conductance of the order of 10–4 (Ω cm)−1. Tungsten diselenide and molybdenum disulphide have conductivity in the same range as of NiSe. These films have layer structure and better optical properties. The thin film confirms p-type conductivity.
References
T. Anand, R. Rajan, M. Said, L. Tee, Adv. Energy Convers. Mater. 1, 55 (2020)
T. Anand, S. Shariza, Electrochim. Acta. 81(81), 64 (2012)
G. Chandra, D. Singh, D. Shrivastava, S. Sahu, J. Phys. D 17, 2125 (1984)
S. Kale, C. Lokhande, Mater. Chem. Phys. 62, 103 (2000)
P. Kalita, B. Sarma, H. Das, Bull. Mater. Sci. 23, 313 (2000)
T. Anand, M. Zaidan, S. Shariza, Proc. Eng. 53, 555 (2013)
A. Hamad, Z. Elmandouch, H. Elmeleegi, Acta Physica Polonia A 127, 901 (2015)
P. Nwofe, R. Chikwenze, P. Agbo, H. Igwe, Asian. J. Sci. Res. 10, 43 (2017)
W. Zhang, Z. Hui, Y. Cheng, L. Zhang, Y. Xie, Y. Qian, J. Cry. Growth 209, 213 (2000)
M. Xue, Z. Fu, Electrochem. Comm. 8, 1855 (2006)
Y. Liu, Q. Xu, R. Wang, Y. Zhang, L. Zhu, Z. Wang, W. Zheng, J. Mater. Chem. A 8, 797 (2020)
K. Anuar, W. Tan, A. Abdullah, H. Jelas, N. Saravanan, S. Ho, M. Yazid, Oriental J. Chem. 25, 813 (2009)
K. Anuar, M. Rosli, S. Ho, Int. J. Chem. Res. 3, 21 (2011)
P. Agbo, P. Nwofe, R. Chikwenze, O. Ozibo, IOSR J. Appl. Phys. 7, 99 (2015)
K. Sharma, D. Sharma, V. Kumar, Optik 182, 519 (2019)
P. Hankare, A. Manikshete, D. Sathe, P. Chate, Mater. Sci. Mater. Electron. 21, 698 (2010)
G. Hodes, Chemical solution deposition of semiconductor films (Marcel Dekker Inc., New York, 2003)
C. Lokhande, P. Patil, H. Tributsch, A. Ennaoui, Sol. Ener. Mater. Sol. Cells 55, 379 (1998)
P. Hankare, P. Chate, S. Delekar, M. Asabe, I. Mulla, J. Phys. Chem. Solids 67, 2310 (2006)
P. Hankare, B. Jadhav, K. Garadkar, P. Chate, I. Mulla, S. Delekar, J. Alloys Compd 490, 228 (2010)
N. Umeyama, M. Tokumoto, S. Yagi, M. Tomura, K. Tokiwa, T. Fujii, R. Toda, N. Miyakawa, S. Ikeda, Jpn. J. Appl. Phys. 51, 053001 (2012)
A. Kassim, M. Rosli, H. Min, Inter. J. Chem. Res. 3, 21 (2011)
S. Ikhmayies, R. Ahmad-Bitar, J. Mater. Res. Technol. 2, 221 (2013)
K. Aly, A. Elnaeim, M. Uosif, O. Abdel-Rahim. Physica B 406, 4227 (2011)
D. Sonavane, S. Jare, R. Survawanshi, R. Kathare, R. Bulakhe. Int. Res. J Sci Eng A2, 61 (2018)
Funding
The authors would like to thank to the University of Mumbai, Mumbai, for financial support for this project (Research Project No. 62 of 2012-13).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chate, P.A., Sathe, D.J. & Hake, S.L. Nickel selenide thin films: opto-electric and thermoelectric properties. Appl. Phys. A 128, 904 (2022). https://doi.org/10.1007/s00339-022-06064-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06064-0