Abstract
A series of silver nanoparticle-modified graphitic carbon nitride/N-doped TiO2 hybrids (Ag/g-C3N4/N-TiO2 or ACTs) were prepared by one-step in situ calcination process at 550 °C for 3 h in air atmosphere. The morphology, structure, and optical properties of ACTs with different Ag contents were characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, photoluminescence spectra, and UV–vis diffuse reflectance spectroscopy. Ag nanoparticles with grain size of about 5 nm are anchored on the surface of ACTs. In UV–vis absorption test, a red-shift of light absorption edge and more visible light absorption (compared to N-TiO2 and g-C3N4/N-TiO2) are observed for ACTs. Moreover, the dependence of photocatalytic activity on Ag contents was investigated, and ACTs-3 presents the highest photocatalytic activity as the optimal Ag content under visible light irradiation. Remarkably enhanced UV–visible light photoelectrochemical response was also confirmed for ACTs compared with TiO2, N-TiO2, and g-C3N4/N-TiO2. Ag-decorated g-C3N4/N-TiO2 composites result in enhanced light absorption capacity and improved transfer of charge carriers, which prominently contribute to the improvement of photoactivity. This work demonstrates that Ag/g-C3N4/N-TiO2 is a promising photocatalytic material for organic pollutant degradation under visible light irradiation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
TiO2 has been widely investigated in various research fields including purification of wastewater or air pollutions [1, 2], hydrogen production [3, 4], and dye-sensitized solar cells [5, 6]. However, TiO2 (with wide band gap of 3.0–3.2 eV) only activated efficiently under UV light irradiation [7], and hence lot of improvements are needed to extend the spectral response toward visible region [8, 9]. Among them, nitrogen doping has become one of the most studied focal points in the modification of TiO2 [10, 11] and the resulted N-TiO2 shows enhanced photocatalytic activities, because the doping of nitrogen impurities narrows the band gap and shifts the absorption edge of TiO2 into the visible range [12, 13]. However, it has to be further altered a lot in order to promote its practical application.
Recently, graphitic carbon nitride (g-C3N4) has emerged as a promising candidate for photocatalysts due to its narrow band gap (~2.7 eV). In addition, the conjugated carbon and nitrogen structure of g-C3N4, which helps in coupling with a wide range of materials, makes it act as a co-catalyst to enhance the visible photocatalytic activity of other photocatalysts [14, 15]. Wang et al. [16] prepared N-TiO2/g-C3N4 composites by a novel in situ microwave-assisted route, and such heterostructures exhibited better photodegradation performance of rhodamine B (RhB) and methyl blue (MB) compared with pristine N-TiO2. Han et al. [17] fabricated g-C3N4 hybridized N-TiO2 nanofibers composites by combined electrospinning with modified heat-etching approach, and the resultant showed highly efficient photocatalytic activity on H2 evolution and pollution degradation. Zhou et al. [18] synthesized a series of composites based on g-C3N4 and N-TiO2 by a pyrolysis process, and such hybrids showed excellent photocatalytic performance and photostability in photoreduction of CO2 to CO. In this concept, we also achieved higher photocatalytic activity for prepared g-C3N4/N-TiO2 homogeneous mixture via solid phase calcination process at 550 °C for 3 h in air [17, 19].
In general, Ag loading on any material can serve as trapping sites for electrons, thus improving the separation efficiency of electron–hole pairs [26], and hence it is a good alternative to improve the utilization efficiency of solar energy. Hence we tried here the modification of g-C3N4/N-TiO2 homogeneous mixture with Ag loading to yield Ag/g-C3N4/N-TiO2 nanocomposites via one-step in situ calcination process. The results indicated such composites showed a considerably enhanced activity of visible-light-induced photodegradation of MB.
Experimental section
Preparation of Ag/g-C3N4/N-TiO2 hybrids
Melamine (C3H6N6, >99 %), TiN (99.9 %, 40 nm), and silver nitrate (AgNO3, 99.8 %) were purchased from Sinopharm Chemical Reagent Entrepreneur Corp., China. All chemicals for synthesis and analysis were commercially available and used without further treatments.
ACTs were synthesized by one-step in situ calcination process. The corresponding preparation process is illustrated schematically in Scheme 1. Briefly, 0.2 g of TiN and melamine with a mass ratio of 1:1 were vigorously grinded to obtain a homogeneous mixture and then dispersed in 20 ml of silver nitrate solution. The suspension ultrasonically vibrated for 1 h. After that, the precursors were dried at 70 °C for 12 h to remove the solvent, followed by annealing at 550 °C for 3 h in air atmosphere. Thus, Ag/g-C3N4/N-TiO2 nanocomposites were obtained with light gray color. The loading amount of Ag was adjusted by controlling the concentration of AgNO3 solution (0, 0.003, 0.006, 0.012, and 0.024 M) in the process of preparing Ag/g-C3N4/N-TiO2 nanocomposites, and the relevant products were denoted as ACTs-0, ACTs-1, ACTs-2, ACTs-3, and ACTs-4, respectively. Blank N-TiO2 and g-C3N4 were synthesized via calcination of TiN and melamine at 550 °C for 3 h in air, respectively.
Characterizations
X-ray diffraction (XRD) was carried out on a PANalytical X’ Pert X-ray diffractometer (Holland) with Cu-Kα radiation at 40 kV and 40 mA. The morphologies of the samples were characterized by scanning electron microscopy (SEM, Model: JEOL JSM-7000F, Japan) and high-resolution transmission electron microscopy (HR-TEM, Model: FEI Tecnai F20, USA). The UV–vis diffuse reflectance spectra (UV–vis DRS) were recorded using a Shimadzu UV 3600 (Japan) spectrophotometer. The X-ray photoelectron spectroscopy (XPS) analysis was performed on a RBD upgraded PHI-5000 C ESCA system (PerkinElmer) with Al/MgKα as the radiation source. The photoluminescence (PL) spectra were obtained using an Edinburgh FLsp920 spectrophotometer at an excitation wavelength of 350 nm.
Photocatalytic tests
The photocatalytic activities of the prepared photocatalysts were tested for the degradation of MB under visible light irradiation. Typically, 15 ml of MB solution (5 mg/L) and 7.5 mg of photocatalysts were taken in a 50 ml reactor and the suspension was agitated in darkness for 2 h to ensure adsorption/desorption equilibrium, prior to irradiation. Subsequently, photodegradation process was carried out under visible light irradiation. A spherical Xe lamp (CHF-XM-500 W, Beijing) equipped with cut filters (λ > 420 nm) was used as the visible light source for photocatalysis. The light intensity of the lamp was 130 mW/cm2, which was measured by a light-intensity meter (FZ-A, Photoelectric Instrument Factory of Beijing Normal University). At given time intervals, 5 ml of the suspension was sampled and centrifuged to remove the photocatalyst. Then, the concentration of MB was analyzed using a UV–vis spectroscopy (Shimadzu UV 1601-PC, Japan) in the wavelength range of 400–800 nm. The photodegradation results were reported as C/C 0 and ln (C 0/C), where C 0 and C were the pollutants concentration after adsorption and photocatalysis, respectively.
Photoelectrochemical measurements
The photoelectrochemical performance was measured on a CHI 660E electrochemical workstation (Shanghai Chenhua Instruments, China) in a PEC cell with Pt foil as counter electrode and saturated calomel electrode (SCE) as reference electrode. 0.5 M Na2SO4 aqueous solution was used as the electrolyte. The working electrodes prepared on a FTO glass plate were illuminated under 500 Xe lamp, and the light intensity was 160 mW/cm2. Electrochemical impedance spectra (EIS) were recorded in the frequency range from 0.01 Hz to 100 kHz. All measurements were carried out under ambient conditions at room temperature.
Results and discussion
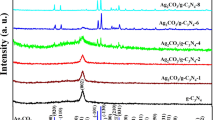
The crystal structures and phase of the obtained samples were characterized by XRD (Fig. 1). Figure 1a shows the XRD patterns of g-C3N4, N-TiO2, ACTs-0, and ACTs-3 samples. Pure g-C3N4 exhibits two distinct peaks at 13.1° and 27.4°, which are indexed to (100) and (002) diffraction planes, respectively [20]. The diffraction peaks of N-TiO2 observed at 2θ values of 25.4°, 27.5°, 37.8°, 48.0°, 53.9°, and 56.6o correspond to the (101), (110), (004), (200), (105), and (220) (JCPDS 03-065-5714; 03-065-0191) crystal planes of TiO2. Obviously, the as-prepared N-TiO2 is composed of anatase and rutile phase. The rutile phase is determined to be 15 % according to the integrated intensities of anatase (101) and rutile (110) peaks. Scherrer’s equation is applied to the anatase (101) planes, and the crystallite size is calculated to be around 30 nm for N-TiO2. Compared with XRD patterns of N-TiO2, ACTs-0 sample exhibits almost similar diffraction peaks but a stronger intensity at 27.4o, which may be attributed to the overlapping of (110) plane of TiO2 and (002) plane of g-C3N4. Furthermore, strong additional diffraction peaks at 38.1°, 44.3°, 64.4°, and 77.4° can be observed for the ACTs-3 sample, which are assigned to (111), (200), (220), and (311) plane of Ag (JCPDS 01-087-0597). Figure 1b shows XRD patterns of ACTs-x (x = 1, 2, 3, 4) composites with different Ag contents. The intensities of peak around 38.1o increase and the color of samples deepen gradually with the increase of Ag content in the composites. The XRD results imply the successful fabrication of Ag/g-C3N4/N-TiO2 nanocomposites. In addition, the insets of Fig. 1a are the corresponding color evolution of g-C3N4, N-TiO2, ACTs-0, and ACTs-3, which is a visual evidence for the successful synthesis of the target resultants. The existence of Ag and g-C3N4 in the composites could be further confirmed by XPS and TEM results, as described later.
The morphology and microstructure of the as-prepared samples were examined by FE-SEM (Fig. 2a–c) and TEM (Fig. 3d–f). As shown in Fig. 2a–c, sphere nanoparticles with grain sizes around 50 nm are observed. It is difficult to tell the difference of morphologies between pure N-TiO2, ACTs-0, and ACTs-3. The TEM images provide a more clear observation. Figure 2d displays the HR-TEM images of ACTs-0 sample. The lattice fringes of 0.352 and 0.233 nm are ascribed to the (101) and (112) planes of anatase TiO2 [21], respectively, while the value of 0.310 nm is attributed to the (110) planes of rutile [22]. This observation confirms the co-existence of anatase and rutile TiO2, which further corroborates the results of XRD. In addition, it is also clearly observed that g-C3N4 shows stacked layer structure. Figure 2e, f presents the HR-TEM images of ACTs-3. As shown in Fig. 2e, it is clearly seen that Ag nanoparticles, loading on the surface of g-C3N4/N-TiO2, present spherical structure with an average diameter of 5 nm. Moreover, layered g-C3N4, characteristic lattice fringes of TiO2, and spherical Ag nanoparticles are observed for ACTs-3 in Fig. 2f, demonstrating the successful fabrication of Ag/g-C3N4/N-TiO2.
The surface composition and chemical states of the as-prepared sample (ACTs-3) were detected by XPS (Fig. 3a–f), and the binding energies analysis is corrected for specimen charging by referencing C 1 s to 284.6 eV. Figure 3a provides the general XPS for ACTs-3 sample over a large energy range at low resolution. It shows the presence of C 1 s, Ag 3d, N 1 s, Ti 2p, O 1 s, and Ti 2 s. Figure 3b shows the Ag 3d core-level XPS spectrum. Two individual peaks at around 368.1 and 374.0 eV are attributed to Ag 3d 5/2 and 3d 3/2, respectively, indicating Ag particles exist in the form of zero-valence [28]. Figure 3c shows the Ti 2p core-level spectrum: the binding energies for Ti 2p 3/2 and Ti 2p 1/2 appear at 458.4 and 464.2 eV, respectively. According to earlier report [23], the Ti 2p 3/2 of pure TiO2 should be located at the binding energy of 458.8 eV. Apparently, nitrogen doping results in a decrease of Ti 2p 3/2-binding energy, suggesting a change in the electron density around the Ti atoms. This phenomenon can be ascribed to the lower electronegativity of N compared to O, partial electron transfer can occur from the N to the Ti [24]. Figure 3d shows that the O 1 s peaks center at the binding energies of 529.6 and 531.4 eV. The peak at 529.6 eV is associated with the Ti–O bond in N-TiO2 [25], while the other O 1 s peak at 531.4 eV is probably related to the hydroxyl groups (H–O bonds) on the surface of ACTs-3 or the presence of Ti–O–N bonds as in previous reports [14, 26]. Two peaks are observed from the C 1 s core-level spectrum shown in Fig. 3e. The peak at 284.6 eV is ascribed to the carbon contamination, and the peak at 288.2 eV is assigned to the N–C=N group in graphitic carbon nitride [27]. As shown in Fig. 3f, the N 1 s core-level spectrum can be fitted with three peaks. The peak centered at 398.8 eV is attributed to the sp 2-hybridized nitrogen (C=N–C) group of g-C3N4 [28]. And the other two peaks located at 399.7 and 401.2 eV are derived from N-TiO2, which indicates N atoms may locate at the interstitial sites of the TiO2 lattice and form Ti–O–N or Ti–N–O [23]. Based on the above XPS analysis, we can draw the conclusion that the Ag/g-C3N4/N-TiO2 composites were successfully obtained.
UV–vis absorption spectra (a) and UV–vis reflectance spectra (b) of the as-prepared samples are shown in Fig. 4. All the ACTs-x (x = 1, 2, 3, 4) samples show strong absorption in visible region, and the visible light absorption capacity gradually enhances with the increase of Ag loading content. The band gaps of ACTs-0, ACTs-1, ACTs-2, ACTs-3, ACTs-4, N-TiO2, and P25 are estimated to be about 2.75, 2.70, 2.65, 2.40, 2.30, 2.90, and 3.10 eV, according to the absorption edges at about 450, 459, 468, 516, 540, 428, and 400 nm, respectively. As shown in Fig. 4b, UV–vis diffuse reflectance spectra reveal that the ACTs-x (x = 1, 2, 3, 4) samples exhibit a wider and stronger absorption of visible light than that of bare ACTs-0, N-TiO2, and P25 samples, which is consistent with the absorption spectra results. These results indicate that the red-shift of absorption edge and narrower band gap are achieved for TiO2 with modification of Ag loading, g-C3N4 coupling, and N doping, which is beneficial to the visible-light-driven photocatalytic performance.
Figure 5 shows the PL spectra of ACTs-0, ACTs-1, ACTs-2, ACTs-3, and ACTs-4 samples. All the samples show two emission peaks at 460 and 510 nm. The emission at 510 nm with band gap energy of 2.44 eV is attributed to the transition from isolated N 2p level to the conduction band of TiO2 [29]. The emission at 460 nm can be assigned to the g-C3N4 photoluminescence [24]. It is also observed that the intensity of the luminescence peaks becomes weaker as bits of Ag deposited on g-C3N4/N-TiO2 sample (ACT-1, ACT-2, and ACT-3). This can be ascribed to efficient interfacial electron transfer from the conduction band of ACTs-0 to the Ag species, which act as electron sinks and suppress the recombination of photoinduced carriers [30]. The lower intensity of PL peak implies that the fewer recombinations of electron and hole pairs occur. However, the PL intensity of ACT-4 is obviously higher than ACTs-0 (See inset of Fig. 5), which indicates that excess amount of Ag on g-C3N4/N-TiO2 surface will act as the recombination center of electrons and holes, leading to the low quantum efficiency.
The photocatalytic activity of ACTs was investigated using the model pollutant MB in aqueous solution. As shown in Fig. 6, the photodegradation of MB in the absence of photocatalyst is negligible. It is found that ACTs-3 sample is optimum to achieve the highest photodegradation efficiency of MB. The photodegradation rate of MB generally follows pseudo-first-order kinetic process, which can be expressed by the equation −ln(C/C 0 ) = k α t, where k α is the pseudo-first-order rate constant. The calculated reaction rate constants of MB degradation under visible light irradiation are 0.0094, 0.0137, 0.0163, 0.0201, 0.0152, 0.0070, and 0.0036 min−1 for ACTs-0, ACTs-1, ACTs-2, ACTs-3, ACTs-4, N-TiO2, and P25 samples, respectively (See inset of Fig. 6b). It is concluded that the photocatalytic activity of g-C3N4/N-TiO2 can be enhanced by the loading of appropriate Ag content. The sample of ACTs-3 presents the highest reaction rate constant, which is 2.1 times higher than that of pure ACTs-0 sample. Excess amount of Ag is unfavorable for the photocatalytic process. The reaction rate constant for ACTs-4 is even smaller than that of ACTs-2 sample. There are two main reasons for the effect of Ag loading content on the photocatalytic activity of ACTs sample. On the one hand, excess amount of Ag loading on the surface of catalyst may influence the absorption of visible light, thus decrease the quantum efficiency, leading to lower concentration of photoinduced charge carrier [31]. On the other hand, excess amount of Ag species can act as recombination center of photogenerated electrons and holes, decreasing the separation efficiency of charge carriers [32].
The improved separation of photogenerated electron–hole pairs and prolonged lifetime of carriers can be demonstrated by the photoelectrochemical investigation (Fig. 7). It is observed that the photocurrent densities of P25, N-TiO2, ACTs-0, and ACTs-3 samples are 0.74, 6.89, 8.83, and 23.8 μA·cm−2, respectively. The photocurrent density of ACTs-3 sample is about 3 times more than that of the ACTs-0 electrode, confirming that longer life span is achieved with the introduction of Ag into g-C3N4/N-TiO2 composites. This result is consistent with the PL analysis. The advantage of Ag loading on the surface of ACTs can be further confirmed by the electrochemical impedance spectra (EIS) results, as displayed in Fig. 8. It is observed that the semicircle diameter in Nyquist plots for ACTs-3 electrodes obviously decreased as compared to that of ACTs-0, N-TiO2, and P25 samples, indicating a faster charge-carrier transfer rate in the Ag/g-C3N4/N-TiO2 nanocomposite [33].
On the basis of the above results, a possible mechanism of visible light photocatalytic degradation of pollutants over the Ag/g-C3N4/N-TiO2 is illustrated in Scheme 2. The conduction band (CB) and valence band (VB) potentials of TiO2 are at −0.29 and +2.91 eV, while they are estimated to be −1.12 and +1.57 eV for g-C3N4 [34], respectively. The N doping can efficiently enhance the visible light absorption of TiO2. Electrons are easily excited from the N impurity energy level to the conduction band of TiO2 under visible light irradiation. In addition, g-C3N4 can also activate under visible light irradiation. Due to the matching of the band structure between g-C3N4 and N-TiO2, the photogenerated electrons from the CB of g-C3N4 readily inject into the CB of N-TiO2, and the holes from the VB of N-TiO2 can transfer to the VB of g-C3N4. Therefore, the recombination activity of photogenerated electrons and holes is suppressed, leaving more holes in the VB of g-C3N4 and more electrons in the CB of N-TiO2. Subsequently, the electrons can accumulate on the Ag nanoparticles loaded on the surface of g-C3N4/N-TiO2 which reduce the adsorbed O2 rapidly to superoxide anion radicals (•O2 −). Simultaneously, the photogenerated holes in the VB of g-C3N4 react with OH− to form •OH. The highly active species •O2 − and •OH are responsible for the degradation of MB.
Conclusion
In this paper, a series of composites composed of Ag, g-C3N4, and N-TiO2 were successfully prepared by a facile in situ calcination process, which are confirmed by characterizations including XRD, TEM, and XPS. The loading amount of Ag can be adjusted by controlling the concentration of AgNO3 solution in the synthesis process. Enhanced visible light absorption and improved visible-light-induced photocatalytic performance were demonstrated for ACTs for photodegradation of methylene blue. The highest photocatalytic performance is observed for ACTs-3, which is about 2.1, 2.9, and 5.6 times larger than that of bare ACTs-0, N-TiO2, and P25, respectively. The positive effects of Ag loading, g-C3N4 coupling, and N doping on TiO2 are also confirmed by the photoelectrochemical measurements. The enhanced photoactivity of Ag/g-C3N4/N-TiO2 is related to the effective separation of electro–hole pairs and an enhanced absorption of visible light, due to the synergetic interactions of TiO2, Ag, and N dopant in the as-prepared composites. This work promotes the application of Ag/g-C3N4/N-TiO2 in solar energy conversion and environmental remediation.
References
Sun MX, Li WB, Sun SF et al (2014) One-step in situ synthesis of graphene-TiO2 nanorod hybrid composites with enhanced photocatalytic activity. Mater Res Bull 61:280–286
Sun MX, Fang YL, Wang Y et al (2015) Synthesis of Cu2O/graphene/rutile TiO2 nanorod ternary composites with enhanced photocatalytic activity. J Alloy Compd 650:520–527
Xiang QJ, Yu JG, Jaroniec M (2012) Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J Am Chem Soc 134:6575–6578
Zhang XY, Sun YJ, Cui XL et al (2012) A green and facile synthesis of TiO2/graphene nanocomposites and their photocatalytic activity for hydrogen evolution. Int J Hydrogen Energ 37:811–815
Zhu K, Neale NR, Miedaner A et al (2007) Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett 7:69–74
Sun MX, Cui XL (2013) Needle-shaped 3D dye-sensitized solar cells using anodized Ti wire and Pt nanoparticle/carbon fiber electrodes. J Power Sources 223:74–78
Zhu J, Yang D, Geng J et al (2008) Synthesis and characterization of bamboo-like CdS/TiO2 nanotubes composites with enhanced visible-light photocatalytic activity. J Nanopart Res 10:729–736
Shanmugasundaram S, Horst K (2003) Daylight photocatalysis by carbon-modified titanium dioxide. Angew Chem Int Edit 42:4908–4911
Yu JX, Liu SW, Xiu ZL et al (2009) Synthesis of sulfur-doped TiO2 by solvothermal method and its visible-light photocatalytic activity. J Alloy Compd 471:23–25
Asahi R, Morikawa T, Ohwaki T et al (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271
Valentin CD, Finazzi E, Pacchioni G et al (2007) N-doped TiO2: theory and experiment. Chem Phys 339:44–56
Sun MX, Song P, Li J et al (2013) Preparation, characterization and applications of novel carbon and nitrogen codoped TiO2 nanoparticles from annealing TiN under CO atmosphere. Mater Res Bull 48:4271–4276
Sun SF, Sun MX, Fang YL et al (2016) One-step in situ calcination synthesis of g-C3N4/N-TiO2 hybrids with enhanced photoactivity. RSC Adv 6:13063–13071
Wang XC, Blechert S, Antonietti M (2012) Polymeric graphitic carbon nitride for heterogeneous photocatalysis. Acs Catal 2:1596–1606
Wang Y, Wang XC, Antonietti M (2012) Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry. Angew Chem Int Edit 51:68–89
Wang XJ, Yang WY, Li FT et al (2013) In situ microwave-assisted synthesis of porous N-TiO2/g-C3N4 heterojunctions with enhanced visible-light photocatalytic properties. Ind Eng Chem Res 52:17140–17150
Han C, Wang YD, Lei YP et al (2015) In situ synthesis of graphitic-C3N4 nanosheet hybridized N-doped TiO2 nanofibers for efficient photocatalytic H2 production and degradation. J Mater Res 8:1199–1209
Zhou S, Liu Y, Li JM et al (2014) Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl Catal B 158:20–29
Sun MX, Fang YL, Sun SF et al (2016) Surface co-modification of TiO2 with N doping and Ag loading for enhanced visible-light photoactivity. RSC Adv 6:12272–12279
Ge L, Han CC, Liu J (2011) Novel visible light-induced g-C3N4/Bi2WO6 composite photocatalysts for efficient degradation of methyl orange. Appl Catal B 108:100–107
Liang YY, Wang HL, Casalongue HS et al (2010) TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res 3:701–705
Ramchiary A, Samdarshi SK (2015) Hydrogenation based disorder-engineered visible active N-doped mixed phase titania. Sol Energ Mat Sol C 134:381–388
Wang HQ, Yan JP, Chang WF et al (2009) Practical synthesis of aromatic amines by photocatalytic reduction of aromatic nitro compounds on nanoparticles N-doped TiO2. Catal Commun 10:989–994
Parida KM, Pany S, Naik B (2013) Green synthesis of fibrous hierarchical meso-macroporous N doped TiO2 nanophotocatalyst with enhanced photocatalytic H2 production. Int J Hydrogn Energ 38:3545–3553
Górska P, Zaleska A, Kowalska E et al (2008) TiO2 photoactivity in Vis and UV light: the influence of calcination temperature and surface properties. Appl Catal B 84:440–447
Liu L, Ma D, Zheng H et al (2008) Synthesis and characterization of microporous carbon nitride. Micropor Mesopor Mat 110:216–222
Wang SP, Li CJ, Wang T et al (2014) Controllable synthesis of nanotube-type graphitic C3N4 and their visible-light photocatalytic and fluorescent properties. J Mater Chem A 2:2885–2890
Yan SC, Li ZS, Zou ZG (2010) Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 26:3894–3901
Nakamura R, Tanaka T, Nakato Y (2004) Mechanism for visible light responses in anodic photocurrents at N-Doped TiO2 film electrodes. J Phys Chem B 108:10617–10620
Georgekutty R, Seery MK, Pillai SC (2008) A highly efficient Ag-ZnO photocatalyst: synthesis, properties, and mechanism. J Phys Chem C 112:13563–13570
Lu WW, Liu GS, Gao SY et al (2008) Tyrosine-assisted preparation of Ag/ZnO nanocomposites with enhanced photocatalytic performance and synergistic antibacterial activities. Nanotechnology 19:82–85
Ren L, Zeng YP, Jiang D (2009) Preparation, characterization and photocatalytic activities of Ag-deposited porous TiO2 sheets. Catal Commun 10:645–649
Pan XY, Xu YJ (2015) Graphene-templated bottom-up fabrication of ultralarge binary CdS-TiO2 nanosheets for photocatalytic selective reduction. J Phys Chem C 119:7184–7194
Wang X, Maeda K, Thomas A et al (2008) A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat Mater 8:76–80
Acknowledgements
This work was financially supported by Innovation Program of Shanghai Municipal Education Commission (15ZZ092), Training Program for Young Teachers in Shanghai Colleges and Universities (ZZgcd14010), Startup Foundation of Shanghai University of Engineering Science (NO. XiaoQi 2014-22), and National Natural Science Foundation of China (No. 51375294 and 51508026).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, S., Sun, M., Kong, Y. et al. One-step thermal synthesis of Ag-modified g-C3N4/N-doped TiO2 hybrids with enhanced visible-light photocatalytic activity. J Mater Sci 52, 1183–1193 (2017). https://doi.org/10.1007/s10853-016-0414-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0414-6