Abstract
The Philippines is often highlighted as the global epicenter of marine biodiversity, yet surveys of reef-associated fishes in this region rarely extend beyond shallow habitats. Here, we improve the understanding of fish species diversity and distribution patterns in the Philippines by analyzing data from mesophotic coral ecosystems (MCEs; 30–150 m depth) obtained via mixed-gas rebreather diving and baited remote underwater video surveys. A total of 277 fish species from 50 families was documented, which includes thirteen newly discovered and undescribed species. There were 27 new records for the Philippines and 110 depth range extensions, indicating that many reef fishes have a broader geographic distribution and greater depth limits than previously reported. High taxonomic beta-diversity, mainly associated with family and genus turnover with depth, and significant effects of traits such as species body size, mobility and geographic range with maximum recorded depth, were observed. These results suggest that MCEs are characterized by unique assemblages with distinct ecological and biogeographic traits. A high proportion (60.5%) of the fish species are targeted by fishing, suggesting that Philippine MCEs are as vulnerable to overfishing as shallow reefs. Our findings support calls to expand conservation efforts beyond shallow reefs and draw attention to the need to explicitly include deep reefs in marine protected areas to help preserve the unique biodiversity of MCEs in the Philippines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Philippines is located within the Coral Triangle (CT), the area of highest coral reef biodiversity (Randall 1998; Roberts et al. 2002; Mora et al. 2003; Bellwood and Meyer 2009), and areas around its center are often identified as having the highest number of marine-fish species on Earth based on range-overlap analyses (Carpenter and Springer 2005; Allen 2008; Nañola et al. 2011; Sanciangco et al. 2013). Scientists have conducted fish collections of the shallow-water ichthyofauna in the Philippines for over a century (Herre 1953; Smith and Williams 1999), and extensive information about commercial fisheries and coral reef fish biodiversity for the region is widely available (Carpenter and Niem 1998–2001; Randall 1998; Allen and Erdmann 2012; Motomura et al. 2017). Many hypotheses have been proposed to explain the high biodiversity found in this region, and most include variations of: (1) center of origin of species (high speciation and low extinction rates within the CT); (2) center of overlap (species predominantly from the Indian and Pacific Oceans have overlapping distributions in the CT); and (3) center of accumulation (speciation originating in peripheral locations subsequently expand distributions into the CT) (Bellwood and Wainwright 2002; Carpenter and Springer 2005; Gaither and Rocha 2013). Lately, a consensus is building toward accepting these as co-existing rather than competing hypotheses (Bowen et al. 2013).
The high biodiversity combined with the large scope and importance of fishing activities have given rise to marine conservation and management initiatives in the Philippines at local and national levels (White et al. 2007; Horigue et al. 2015). The country has more than 1500 marine protected areas, most of which are small, community-based no-take reserves (Weeks et al. 2010; Cabral et al. 2014). Although progress has been made to set aside shallow habitats as reserves, overfishing remains a serious problem (Nañola et al. 2011; Lavides et al. 2016). Habitat loss from coral reef, seagrass beds and mangroves removal and degradation is also a concern (White et al. 2000). Recent studies in the Philippines have shown the importance of connectivity among different habitats for fishes that undergo ontogenetic habitat shifts and the fisheries that they support (Honda et al. 2013; Ramos et al. 2015). However, deeper ecosystems (depths > 30 m) in this region have received less attention and very little information about the biodiversity of coral reefs at these depths is available (Nacorda et al. 2017; Turner et al. 2017; Abesamis et al. 2018; Joseph Quimpo et al. 2018).
Recent studies of the deeper portions of coral reefs, known as mesophotic coral ecosystems (MCEs; widely defined as reef habitat between 30 and 150 m; Hinderstein et al. 2010; Loya et al. 2016), have significantly improved our understanding of the biodiversity and ecology of tropical reef ecosystems as well as their vulnerability to man-made threats (Holstein et al. 2015; Loya et al. 2016; Pinheiro et al. 2016; Baldwin et al. 2018; Rocha et al. 2018). A number of these studies report new records and new discoveries of fish species, which have enhanced our knowledge of the diversity and distribution of fishes inhabiting MCEs (Kane et al. 2014; Wagner et al. 2014; Pinheiro et al. 2015; Pyle and Kosaki 2016; Simon et al. 2016). This study documents the fish biodiversity of these deep and largely unexplored ecosystems in the Philippines and assesses how taxonomic beta-diversity and functional traits of the fish assemblage change with depth from upper (30–50 m deep) to lower (90–150 m) MCEs.
Methods
Study area
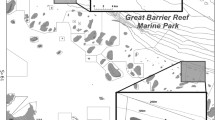
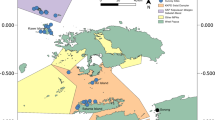
Deep reefs of the Verde Island Passage (VIP; the strait between Luzon Is. and Mindoro Is.) and Apo Island (off the southeast Negros Is.) were surveyed (Fig. 1). The VIP and Apo Island are situated in the sub-region of the Coral Triangle where fish species diversity reaches its peak (Carpenter and Springer 2005; Allen 2008; Nañola et al. 2011). Our research sites in the VIP are characterized by narrow, shallow fringing coral reefs which slope to a ledge at about 80–100 m depth, followed by a steep, vertical drop to lower MCEs. Water current and benthic cover vary among sites. While reef-building corals are abundant in the upper MCE of the VIP, azooxanthellate gorgonians, black corals, and solitary stony corals are the most abundant cnidarians in lower MCEs (Shepherd et al. 2018).
Apo Island was declared a protected landscape and seascape in 1994, but community-based marine resource management started in 1982, with the establishment of a no-take marine reserve on the southeastern side of the island (Russ and Alcala 1999). It is one of the most successful MPAs in the Philippines and serves as a model for co-management nationwide (Alcala and Russ 2006). A recent study suggested that the fringing coral reef surrounding Apo Island extends to the MCE zone down to about 40 m, and between 40 and 80 m depth the benthic habitat is dominated by rock (dead coral), sand or rubble, with low cover of soft corals (octocorals and black corals), barrel and encrusting sponges, and macroalgae (Abesamis et al. 2018). Two typhoons (Washi in 2011 and Bopha in 2012) severely damaged shallow and upper MCE reefs on the eastern side of Apo Island, including the three-decade old no-take marine reserve (Russ et al. 2015).
Sampling
Eighty-two surveys were conducted in two regions of the Philippines (Fig. 1) between 2013 and 2017. Fifty-six surveys in twelve sites of the VIP (Table S1) were conducted during technical dives, each of which typically consisted of 10 min descent, 10–20 min at working depths, followed by 3–5 h of decompression. Mixed-gas, closed-circuit rebreathers were used to facilitate reaching operating depths down to 150 m. During each dive, divers spent 30–45 min in the MCE zone (30–150 m). One dive per day was conducted and each expedition consisted of 7–15 dives. Four to six divers surveyed each site, and each diver performed a specific fish survey method: visual, video or photo records, or collection of voucher specimens with hand nets or pole spears. At Apo Island, fishes were observed using baited remote underwater video (BRUV) systems that were deployed on the seafloor for up to 50 min. One site was situated within the storm-impacted marine reserve and the other site was an area open to fishing but was not damaged by the storms (Abesamis et al. 2018). A total of 26 BRUV deployments (11 in the reserve and 15 in the fished area) were performed during the day between depths of 10–80 m. Only the first 30 min of video were analyzed per deployment to standardize sampling effort.
Data analyses
Species nomenclature follows Eschmeyer and Fong (2018). Epinephelidae classification follows Craig and Hastings (2007), with Serranidae composed of Serraninae and Anthiadinae. Labridae classification follows Westneat and Alfaro (2005), which includes parrotfishes as the subfamily Scarinae.
Statistical analyses aimed to test for differences in biogeographic and ecological traits among fish species grouped according to their maximum depth of occurrence. Each fish species was categorized into one of four depth strata based on their maximum recorded depth (hereinafter MRD strata): 30–59 m, 60–89 m, 90–119 m, 120–150 m. These MRD strata were based to some extent on faunal breaks indicated by previous studies and therefore not entirely arbitrary (Slattery et al. 2011; Pinheiro et al. 2016; Semmler et al. 2016). The geographical distribution, depth range, maximum body size, and commercial importance of the species were compiled from Allen and Erdmann (2012), Froese and Pauly (2018), and museums records (www.fishnet2.net), supplemented with the authors’ personal observations. Geographical distribution was classified as: widely distributed (widespread within Pacific and Indian Oceans), central distribution (Coral Triangle, western Pacific and eastern Indian Oceans), Pacific Ocean predominance (central distribution and central/eastern Pacific), Indian Ocean predominance (central distribution, western Indian Ocean and/or Red Sea), localized (exclusive from one to three locations), and Philippines (only found in the Philippines). Species body size was classified as: small (< 10 cm), small-medium (10–25 cm), medium (26–50 cm) and large (> 50 cm). Commercial importance was categorized as: yes (fisheries and/or aquarium-trade resources) or no (no known commercial importance). Species were also classified into trophic guilds (macrocarnivores, mobile invertebrate feeders, sessile invertebrate feeders, corallivores, roving herbivores, territorial herbivores, omnivores, planktivores, cleaners) based on the available information on their diet (Froese and Pauly 2018). Mobility was classified as: sedentary (site-attached, short movements of few meters), roving (move among patch reefs and few kilometers), and highly mobile (not site-attached, most pelagic species that are able to move several kilometers). Species were further classified by their IUCN Red List status (IUCN 2017), which was used only for descriptive results, not in the statistical analyses.
Taxonomic (family and genera levels) beta-diversity analyses among MRD strata were performed using Sørensen dissimilarity index and its components: turnover (= Simpson dissimilarity) and nestedness (Baselga and Orme 2012). Beta-diversity analyses followed the method described by Baselga and Orme (2012), and were done using the package BETAPART in R. These metrics are appropriate for both presence-absence and quantitative data, and robust to small or unequal sample sizes (Koleff et al. 2003; Barwell et al. 2015).
Relationships between species traits (independent variables reflecting ecological and biogeographical characteristics) and MRD strata (dependent variable as an ordered categorical factor: 30–59 m < 60–89 m < 90–150 m) were investigated with cumulative linked mixed-effects models (CLMMs) (Christensen 2013). According to Luiz et al. (2016), CLMMs are commonly used for analyzing ranked categorical response variables, such as MRD strata, because they preserve the variance structure of the original ordinal ranks of the categories. Traits such as trophic guild, maximum body size, geographic distribution, and adult mobility were used as independent variables. These traits were chosen because they can potentially influence species dispersal and home-range sizes (Luiz et al. 2012). We expected that species with high dispersal abilities, often characterized by high trophic levels, large body size, wide geographic distribution, and high mobility would increase in importance with greater depth.
CLMMs were fitted using a logit link function. All independent variables were included in the models and further removed in a stepwise backward procedure: removing the one with the lowest Akaike information criteria (AIC) and rerunning the model until only significant variables remained (Luiz et al. 2012, 2016). We also performed a second independent CLMM to test the interaction between MRD strata and species targeted by fishing or the aquarium trade (target categories: yes or no). As many researchers have suggested deep reefs act as a refuge against human impacts, we tested whether there is a positive relationship between targeted species and MRD strata. Taxon (genus nested within family) was included as a random variable in all the analyses (Luiz et al. 2012). Analyses were conducted using the package “ordinal” (Christensen 2013) in R (R Core Team 2015).
Results
A total of 277 reef fish species belonging to 50 families was recorded in MCEs in the Philippines (Table S2). Eighty-one of these were recorded using BRUV in Apo Island, 57 of which were not recorded in the VIP. On the other hand, 220 species were recorded during diving activities in the VIP, 196 which were not recorded in Apo Island (Table S2). Thirteen taxa collected during rebreather dives represent newly discovered species, many of which have not yet been described (Table S2). Six records are undescribed species previously recorded elsewhere (Table S2). A total of 27 species constitute new records for the Philippines (Fig. 2), most of which were previously known from a few locations in the Indo-Pacific (Table S2). The maximum recorded depth of most species ranges between 60 and 119 m (72.4%). Our observations expand the depth range of 110 species (Table S2), which were previously only documented in shallower waters.
The family with the most species in our checklist is Labridae (39 species), followed by Serranidae (26), Pomacentridae (22), Gobiidae (15), Epinephelidae (13), Chaetodontidae (11), Pomacanthidae (11), Apogonidae (11), Lutjanidae (10) and Holocentridae (10) (Fig. 3; Table S2). Most species are planktivores (39.7%), followed by mobile invertebrate feeders (26.5%), macrocarnivores (18.4%), omnivores and sessile invertebrate feeders (5.1% each) (Table S2). Only two predominantly corallivore species, Arothron nigropunctatus and Chaetodon lineolatus, were recorded at MCE depths. Most of the species are widely distributed (39.7%) throughout both Indian and Pacific Oceans. Moreover, 20.6% are predominantly Pacific Ocean species while 8.6% are predominantly Indian Ocean species (Table S2). About 15.4% have a central distribution (western Pacific or Coral Triangle region), 12% are considered localized (recorded in few locations), and 3.7% only recorded in the Philippines.
Most are roving (51.5%) or sedentary (39.3%) species, and are small (< 10 cm; 27.1%) and small-medium (10–25 cm; 35.7%) sized. Many species have commercial importance (60.5%) for the aquarium trade (46.7%), or food fisheries (25.4%), with 11.6% of all species targeted by both the aquarium trade and food fisheries. Most species are listed on the IUCN Red List as Least Concern (LC) (59.9%) but three species of shark are listed as threatened by extinction: Alopias vulpinus (Vulnerable), A. pelagicus (Vulnerable) and Sphyrna lewini (Endangered). A total of 34.2% of the species has not been evaluated by the IUCN, and 2.6% are considered Data Deficient (Table S2).
Depth limits of the fish assemblage
The maximum recorded depth (MRD) of 61 species (22.4%) is between 30 and 59 m, MRD of 111 species (40.8%) is between 60 and 89 m, MRD of 86 species (31.6%) is between 90 and 119 m, and MRD of 14 species (5.1%) is between 120 and 150 m. The number of species of the richest reef fish families varied considerably among MRD strata (Fig. 3). The family Labridae is the most diverse with MRD between 30 and 89 m (Fig. 3). Most Serranidae and Pomacentridae species have MRD in the 90–119 m range. The family Serranidae was the most speciose in the 120–150 m MRD stratum, followed by Chaetodontidae and Epinephelidae (Fig. 3). Most Apogonidae and Pomacanthidae have MRD between 60 and 119 m, whereas Gobiidae, Lutjanidae and Holocentridae have MRD between 60 and 89 m (Fig. 3).
Beta-diversity among maximum recorded depth (MRD) strata was generally high in both family (0.49–0.94) and genus (0.73–1.0) levels, mainly driven by taxonomic turnover (Table 1). All comparisons including the 120–150 m MRD stratum showed the highest rates of beta-diversity, reaching 90% in the genus level (Table 1). Comparisons including the 120–150 m MRD stratum also reveal differences in the beta-diversity components, with high values of nestedness (Table 1), suggesting that families and genera with the deepest depth limits make up a small subset of those limited to the shallower MCE zones. The CLMM results are presented in Table 2, and they show the importance of body size, mobility, and geographical distribution among traits that could influence the ability of species to disperse and inhabit deeper MCEs in the Philippines (Table 2a). Small and small-medium fishes are more numerous among species with MRD between 90 and 150 m (Fig. 4a; Table 2a). Sedentary fishes are more numerous among species with MRD between 30 and 59 m, while roving fishes (but not significant in the CLMM analysis) among species with MRD between 90 and 150 m (Fig. 4b; Table 2a). For geographical distribution, species that are only known to occur in the Philippines and species with localized distribution (the latter not significant in the CLMM analysis) showed increasing proportion among species with MRD between 60 and 119 m, and 120–150 m, respectively (Fig. 4c; Table 2a). Commercially targeted species were more numerous among species with MRD between 30 and 89 m (Fig. 4d), and although our CLMM analysis did not show any significant result (Table 2b), their proportion and numbers decrease toward the deepest MRD zones.
Relative changes of the significant fish variables (ecological and biogeographic traits) in the CLMM analysis among maximum recorded depth strata. a Body size, b Mobility (SED sedentary, ROV roving, HMO highly mobile); c Geographical distribution (Indian Ocean and Pacific Ocean predominance are clumped with widely distributed species); d Targeted species (yes = targeted, no = not targeted)
Discussion
The number of species recorded (277) in our study is higher than that reported on MCEs from the Northwestern Hawaiian Islands [162 species; (Fukunaga et al. 2017)], Central Pacific [100 species; (Pyle 2000)], Johnston Atoll [99; (Wagner et al. 2014)], main Hawaiian Islands [52–60; (Pyle et al. 2016)], and Atlantic locations such as the Vitoria-Trindade Seamount Chain [128; (Pinheiro et al. 2015)], Puerto Rico [103; (Bejarano et al. 2014)], Abrolhos Shelf [74; (Simon et al. 2016)], and St Paul’s Rocks [19; (Rosa et al. 2016)], but within the range expected for the central Philippines, for the level of sampling effort (Nañola et al. 2011). The higher number reported here, composed exclusively by primary data, may also be a consequence of the advantage of using two complementary sampling techniques: diving and BRUV surveys (Langlois et al. 2010). However, the number of species recorded in the Philippines was only slightly higher than an extensive compilation reported for the entire Hawaiian Archipelago [259; (Pyle et al. 2016)]. Thus, based on the difference of the total number of fish species that are known to occur within these two biogeographical provinces (Bellwood and Meyer 2009; Kulbicki et al. 2013), and on the high number of fish species recorded exclusively in each of the two study regions, the number of reef fishes recorded in Philippine MCEs is expected to increase as more sites are surveyed.
The higher diversity observed in Philippines MCEs is consistent with previous findings that documented a high marine biodiversity in the region (Carpenter and Springer 2005; Nañola et al. 2011). Similar to shallow coral reef fish diversity (Randall 1998; Mora et al. 2003; Bellwood and Meyer 2009), MCE fish diversity comprises both widespread species and local and regional endemics, the latter predominantly found in either the Indian or Pacific Oceans. Many MCE species are only known from the Coral Triangle, western Pacific, or from a few isolated localities. This observation was corroborated by our analyses, which suggest restricted geographic distribution as an important driver of fish diversity in lower MCEs. Although high endemism rates have been suggested for some MCEs (Kane et al. 2014), the small reported ranges do not necessarily equate to restricted distribution. Since available knowledge of biodiversity in MCEs is scant, expeditions to explore deep reefs are still revealing new records and new species (Wagner et al. 2014; Pinheiro et al. 2015; Simon et al. 2016; Baldwin et al. 2018; Coleman et al. 2018; Shepherd et al. 2018), and, as with this study, extending the known distribution of deep reef species, including many species that were previously considered endemics elsewhere.
In addition to the species overlap from different biogeographical provinces, we also observed some vertical overlap between shallower and deeper taxa within Philippines MCEs, with several depth range extensions. However, the high beta-diversity found among depth limits, predominantly driven by turnover, indicates a high distinctness at both family and genus level reaching the lower depths of MCEs in the Philippines. For instance, while families such as Apogonidae, Pomacentridae and Labridae have species exclusively from MCEs and some genera that are shared with shallow reefs, other families such as Symphysanodontidae and Callanthiidae are exclusive to lower MCE zones (90–150 m). Serranidae and Gobiidae showed high turnover at the genus level, where taxa such as Odontanthias, Sacura, and Tryssogobius are only found in the lower MCE zones (90–150 m). Moreover, we also found significant changes with depth in traits such as body size and mobility, indicating that most deeper fishes are small-sized rovers. This combination of taxonomic overlap and turnover indicates a complex evolutionary history for deep reef fishes in the Philippines, possibly driven by historic and recent colonization events, likely including both vertical and horizontal dispersal. Similar to the Philippines, the high taxonomic turnover with depth of MCE fish communities in the Caribbean Sea is hypothesized to be driven by multiple colonization events in its evolutionary history (Tornabene et al. 2016; Baldwin et al. 2018).
As scientific technical diving evolves, the lower portion of the MCEs are becoming more accessible to scientists, and a high number of new species are being discovered (Anderson et al. 2016; Rocha et al. 2017). For instance, a total of 13 new species was discovered in the Philippines during the rebreather dives in this study (~ 6% of the species recorded in the VIP), with steady progress publishing the formal taxonomic descriptions (Anderson et al. 2016; Rocha et al. 2017; Arango et al. 2019) or in development. The discovery rate of the first two expeditions was approximately two species per hour of diving, which, although lower than expeditions conducted in 1980’s and 1990’s (Pyle 2000), is still much higher than current shallow reef species discovery (Allen and Werner 2002; authors pers. obs.). Time spent collecting new species is also revealing many new records and depth range extensions (Pinheiro et al. 2015). Although richness generally decreases with depth in MCEs (Kahng et al. 2010; Pinheiro et al. 2016), we found a higher number of species deeper than previously reported, suggesting that a greater number of species have depth ranges that include the lower limits of coral reefs. Nevertheless, at an ecological level, most of these species are rare and vagrants on MCEs, and the community is generally very distinct from shallow reefs as observed elsewhere (Pinheiro et al. 2016; Rocha et al. 2018).
Many studies suggest that deep reefs can serve as a refuge for depth generalist species against natural disturbances and human impacts such as fishing (Bongaerts et al. 2010; Goetze et al. 2011; Lindfield et al. 2014, 2016). Although a great number of commercial species was recorded, our analysis did not indicate any expected characteristics of depth generalists, such as high mobility, high trophic levels or large sizes, to increase in importance with recorded depth. While commercially targeted fishes were mostly limited to shallower depths (30–90 m), we observed a unique assemblage composed of smaller and many unknown species at deeper depths. These findings suggest limitations to the deep reefs refuge hypothesis. Furthermore, the lack of many widespread and depth generalist endangered species (Froese and Pauly 2018) and the high proportion of fishes targeted by fisheries found in this study suggest that MCEs in the Philippines are vulnerable to overfishing. For perspective, a recent study showed that nearly 60 commercial fish species disappeared from catches in five key biodiversity areas in the Philippines between 1950s and 2014 (Lavides et al. 2016). Many of these species inhabit both shallow and deep reefs, and only eight were recorded in the deep reefs we studied (Table S2).
The Philippines has one of the largest networks of MPAs in the world (Weeks et al. 2010; Horigue et al. 2015). However, most of these MPAs are very small (90% < 1 km2) and collectively they protect less than 3.5% of the total shallow coral reef area of the Philippines (Weeks et al. 2010). While many of these MPAs are likely to include deeper reefs, the very low proportion of reef area that they protect suggests that the current network of MPAs in the Philippines offers little protection to MCEs. There is also a serious concern that many of these MPAs are not being effectively managed (Alcala et al. 2008; Maypa et al. 2012). At the recent United Nation’s Ocean Conference, the Philippine government committed to invest US $80 million in marine conservation, enhancing MPA networks in key biodiversity areas (oceanconference.un.org/commitments). Here we call attention to the importance of explicitly including MCEs in this MPA expansion program to more comprehensively protect the unique biological communities that occur in the global epicenter of marine biodiversity.
References
Abesamis RA, Langlois T, Birt M, Thillainath E, Bucol AA, Arceo HO, Russ GR (2018) Benthic habitat and fish assemblage structure from shallow to mesophotic depths in a storm-impacted marine protected area. Coral Reefs 37:81–97
Alcala A, Bucol A, Nillos-Kleiven P (2008) Directory of marine reserves in the Visayas, Philippines
Alcala AC, Russ GR (2006) No-take marine reserves and reef fisheries management in the Philippines: a new people power revolution. Ambio 35:245–254
Allen G (2008) Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquat Conserv Mar Freshw Ecosyst 18:541–556
Allen GR, Erdmann MV (2012) Reef fishes of the East Indies, vol I-III. Tropical Reef Research, Perth
Allen GR, Werner TB (2002) Coral reef fish assessment in the ‘coral triangle’ of southeastern Asia. Environ Biol Fishes 65:209–214
Anderson WD, Greene BD, Rocha LA (2016) Grammatonotus brianne, a new callanthiid fish from Philippine waters, with short accounts of two other Grammatonotus from the Coral Triangle. Zootaxa 4173:289–295
Arango BG, Pinheiro HT, Rocha C, Greene BD, Pyle RL, Copus JM, Shepherd B, Rocha LA (2019) Three new species of Chromis (Teleostei, Pomacentridae) from mesophotic coral ecosystems of the Philippines. Zookeys 835:1–15
Baldwin CC, Tornabene L, Robertson DR (2018) Below the Mesophotic. Sci Rep 8:4920
Barwell LJ, Isaac NJB, Kunin WE (2015) Measuring β—diversity with species abundance data. J Anim Ecol 84:1112–1122
Baselga A, Orme CDL (2012) Betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812
Bejarano I, Appeldoorn RS, Nemeth M (2014) Fishes associated with mesophotic coral ecosystems in La Parguera, Puerto Rico. Coral Reefs 33:313–328
Bellwood DR, Meyer CP (2009) Searching for heat in a marine biodiversity hotspot. J Biogeogr 36:569–576
Bellwood DR, Wainwright PC (2002) The history and biogeography of fishes on Coral Reels. Coral Reef Fishes. Academic Press, San Diego, pp 5–32
Bongaerts P, Ridgway T, Sampayo EM, Hoegh-Guldberg O (2010) Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29:309–327
Bowen BW, Rocha LA, Toonen RJ, Karl SA (2013) The origins of tropical marine biodiversity. Trends Ecol Evol 28:359–366
Cabral RB, Aliño PM, Balingit ACM, Alis CM, Arceo HO, Nanola CL Jr, Geronimo RC, Partners MSN (2014) The Philippine marine protected area (MPA) database. Philipp Sci Lett 7:300–308
Carpenter KE, Niem VH. FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. FAO, Rome
Carpenter KE, Springer VG (2005) The center of the center of marine shore fish biodiversity: the Philippine Islands. Environ Biol Fishes 72:467–480
Christensen RHB (2013) Package “Ordinal”: regression models for ordinal data. Denmark, Copenhagen
Coleman RR, Copus JM, Coffey DM, Whitton RK, Bowen BW (2018) Shifting reef fish assemblages along a depth gradient in Pohnpei, Micronesia. PeerJ 6:e4650
Craig MT, Hastings PA (2007) A molecular phylogeny of the groupers of the subfamily Epinephelinae (Serranidae) with a revised classification of the Epinephelini. Ichthyol Res 54:1–17
Eschmeyer, Fong J (2018) Species by family/subfamily in the catalog of fishes
Froese R, Pauly D (2018) Fishbase. World Wide Web electronic publication. www.fishbase.org, version (06/2018)
Fukunaga A, Kosaki RK, Wagner D (2017) Changes in mesophotic reef fish assemblages along depth and geographical gradients in the Northwestern Hawaiian Islands. Coral Reefs 36:785–790
Gaither MR, Rocha LA (2013) Origins of species richness in the Indo-Malay-Philippine biodiversity hotspot: evidence for the centre of overlap hypothesis. J Biogeogr 40:1638–1648
Goetze JS, Langlois TJ, Egli DP, Harvey ES (2011) Evidence of artisanal fishing impacts and depth refuge in assemblages of Fijian reef fish. Coral Reefs 30:507–517
Herre AW (1953) Checklist of Philippine fishes. U.S. Printing Office. 119., Washington, DC
Hinderstein LM, Marr JCA, Martinez FA, Dowgiallo MJ, Puglise KA, Pyle RL, Zawada DG, Appeldoorn R (2010) Theme section on “Mesophotic coral ecosystems: characterization, ecology, and management”. Coral Reefs 29:247–251
Holstein DM, Smith TB, Gyory J, Paris CB (2015) Fertile fathoms: deep reproductive refugia for threatened shallow corals. Sci Rep 5:12407
Honda K, Nakamura Y, Nakaoka M, Uy WH, Fortes MD (2013) Habitat use by fishes in coral reefs, seagrass beds and mangrove habitats in the Philippines. PLoS ONE 8:e65735
Horigue V, Pressey RL, Mills M, Brotánková J, Cabral R, Andréfouët S (2015) Benefits and challenges of scaling up expansion of marine protected area networks in the Verde Island Passage, Central Philippines. PLoS One 10:e0135789
IUCN (2017) The IUCN red list of threatened species. http://www.iucnredlist.org
Joseph Quimpo TR, Cabaitan PC, Dionnie Olavides RD, Dumalagan EE, Munar J, Siringan FP (2018) Preliminary observations of macrobenthic invertebrates and megafauna communities in the upper mesophotic coral ecosystems in Apo Reef Natural Park, Philippines. Raffles Bull Zool 7600:1–11
Kahng SE, Garcia-Sais JR, Spalding HL, Brokovich E, Wagner D, Weil E, Hinderstein L, Toonen RJ (2010) Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29:255–275
Kane C, Kosaki RK, Wagner D (2014) High levels of mesophotic reef fish endemism in the Northwestern Hawaiian Islands. Bull Mar Sci 90:693–703
Koleff P, Gaston K, Lennon J (2003) Measuring beta diversity for presence–absence data. J Anim Ecol 72:367–382
Kulbicki M, Parravicini V, Bellwood DR, Arias-Gonzàlez E, Chabanet P, Floeter SR, Friedlander A, McPherson J, Myers RE, Vigliola L, Mouillot D (2013) Global biogeography of reef fishes: a hierarchical quantitative delineation of regions. PLoS ONE 8:e81847
Langlois TJ, Harvey ES, Fitzpatrick B, Meeuwig JJ, Shedrawi G, Watson DL (2010) Cost-efficient sampling of fish assemblages: comparison of baited video stations and diver video transects. Aquat Biol 9:155–168
Lavides MN, Molina EPV, De Rosa GE (2016) Patterns of coral-reef finfish species disappearances inferred from fishers’ knowledge in global epicentre of marine shorefish diversity. PLoS ONE 11:e0155752
Lindfield SJ, Harvey ES, Halford AR, McIlwain JL (2016) Mesophotic depths as refuge areas for fishery-targeted species on coral reefs. Coral Reefs 35:125–137
Lindfield SJ, McIlwain JL, Harvey ES (2014) Depth refuge and the impacts of SCUBA spearfishing on coral reef fishes. PLoS ONE 9:e92628
Loya Y, Eyal G, Treibitz T, Lesser MP, Appeldoorn R (2016) Theme section on mesophotic coral ecosystems: advances in knowledge and future perspectives. Coral Reefs 35:1–9
Luiz OJ, Madin JS, Robertson DR, Rocha LA, Wirtz P, Floeter SR (2012) Ecological traits influencing range expansion across large oceanic dispersal barriers: insights from tropical Atlantic reef fishes. Proc R Soc B Biol Sci 279:1033–1040
Luiz OJ, Woods RM, Madin EMP, Madin JS (2016) Predicting IUCN extinction risk categories for the world’s data deficient groupers (Teleostei: Epinephelidae). Conserv Lett 9:342–350
Maypa AP, White AT, Caňares E, Martinez R, Eisma-Osorio RL, Aliňo P, Apistar D (2012) Marine protected area management effectiveness: progress and lessons in the Philippines. Coast Manag 40:510–524
Mora C, Chittaro PM, Sale PF, Kritzer JP, Ludsin SA (2003) Patterns and processes in reef fish diversity. Nature 421:933–936
Motomura H, Alama UB, Muto N, Babaran RP, Ishikawa S (2017) Commercial and bycatch market fishes of Panay Island, Republic of the Philippines. The Kagoshima University Museum, Kagoshima, University of the Philippines Visayas, Iloilo, and Research Institute for Humanity and Nature, Kyoto
Nacorda HME, Dizon RM, Menez LAB, Nanola CL, Roa-Chio PBL, De Jesus DO, Hernandez HB, Quimpo FTR, Licuanan WRY, Alino PM, Villanoy CL (2017) Beneath 50 m of NW Pacific water: coral reefs on the Benham Bank Seamount off the Philippine Sea. J Environ Sci Manag 20:110–121
Nañola CL, Aliño PM, Carpenter KE (2011) Exploitation-related reef fish species richness depletion in the epicenter of marine biodiversity. Environ Biol Fishes 90:405–420
Pinheiro HT, Goodbody-Gringley G, Jessup ME, Shepherd B, Chequer AD, Rocha LA (2016) Upper and lower mesophotic coral reef fish communities evaluated by underwater visual censuses in two Caribbean locations. Coral Reefs 35:139–151
Pinheiro HT, Mazzei E, Moura RL, Amado-Filho GM, Carvalho-Filho A, Braga AC, Costa PAS, Ferreira BP, Ferreira CEL, Floeter SR, Francini-Filho RB, Gasparini JL, Macieira RM, Martins AS, Olavo G, Pimentel CR, Rocha LA, Sazima I, Simon T, Teixeira JB, Xavier LB, Joyeux J-C (2015) Fish biodiversity of the Vitória-Trindade Seamount Chain, southwestern Atlantic: an updated database. PLoS ONE 10:e0118180
Pyle R (2000) Assessing undiscovered fish biodiversity on deep coral reefs using advanced self-contained diving technology. Mar Technol Soc J 34:82–91
Pyle RL, Boland R, Bolick H, Bowen BW, Bradley CJ, Kane C, Kosaki RK, Langston R, Longenecker K, Montgomery A, Parrish FA, Popp BN, Rooney J, Smith CM, Wagner D, Spalding HL (2016) A comprehensive investigation of mesophotic coral ecosystems in the Hawaiian Archipelago. PeerJ 4:e2475
Pyle RL, Kosaki RK (2016) Prognathodes basabei, a new species of butterflyfish (Perciformes, Chaetodontidae) from the Hawaiian Archipelago. Zookeys 614:137–152
Ramos DAE, Aragones LV, Rollon RN (2015) Linking integrity of coastal habitats and fisheries yield in the Manta lip Reef System. Ocean Coast Manag 111:62–71
Randall JE (1998) Zoogeography of shore fishes of the indo-pacific region. Zool Stud 37:227–268
Roberts CM, Mcclean CJ, Veron JEN, Hawkins JP, Allen GR, Mcallister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C, Werner TB (2002) Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 80(295):1280–1284
Rocha LA, Pinheiro HT, Shepherd B, Papastamatiou YP, Luiz OJ, Pyle RL, Bongaerts P (2018) Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 80(361):281–284
Rocha LA, Pinheiro HT, Wandell M, Rocha CR, Shepherd B (2017) Roa rumsfeldi, a new butterflyfish (Teleostei, Chaetodontidae) from mesophotic coral ecosystems of the Philippines. Zookeys 709:127–134
Rosa MR, Alves AC, Medeiros DV, Coni EOC, Ferreira CM, Ferreira BP, de Souza Rosa R, Amado-Filho GM, Pereira-Filho GH, de Moura RL, Thompson FL, Sumida PYG, Francini-Filho RB (2016) Mesophotic reef fish assemblages of the remote St. Peter and St. Pauls Archipelago, Mid-Atlantic Ridge, Brazil. Coral Reefs 35:113–123
Russ GR, Alcala AC (1999) Management histories of Sumilon and Apo Marine Reserves, Philippines, and their influence on national marine resource policy. Coral Reefs 18:307–319
Russ GR, Miller KI, Rizzari JR, Alcala AC (2015) Long-term no-take marine reserve and benthic habitat effects on coral reef fishes. Mar Ecol Prog Ser 529:233–248
Sanciangco JC, Carpenter KE, Etnoyer PJ, Moretzsohn F (2013) Habitat availability and heterogeneity and the Indo-Pacific warm pool as predictors of marine species richness in the tropical Indo-Pacific. PLoS One 8:e56245
Semmler RF, Hoot WC, Reaka ML (2016) Are mesophotic coral ecosystems distinct communities and can they serve as refugia for shallow reefs? Coral Reefs 36:433–444
Shepherd B, Pinheiro HT, Rocha LA (2018) Ephemeral aggregation of the benthic ctenophore Lyrocteis imperatoris on a mesophotic coral ecosystem in the Philippines. Bull Mar Sci 94:101–102
Simon T, Pinheiro HT, Moura RL, Carvalho-Filho A, Rocha LA, Martins AS, Mazzei EF, Francini-Filho RB, Amado-Filho GM, Joyeux J-C (2016) Mesophotic fishes of the Abrolhos Shelf, the largest reef ecosystem in the South Atlantic. J Fish Biol 89:990–1001
Slattery M, Lesser MP, Brazeau D, Stokes MD, Leichter JJ (2011) Connectivity and stability of mesophotic coral reefs. J Exp Mar Bio Ecol 408:32–41
Smith DG, Williams JT (1999) The great Albatross Philippine expedition and its fishes. Mar Fish Rev 61:31–41
Tornabene L, Van Tassell JL, Robertson DR, Baldwin CC (2016) Repeated invasions into the twilight zone: evolutionary origins of a novel assemblage of fishes from deep Caribbean reefs. Mol Ecol 25:3662–3682
Turner JA, Babcock RC, Hovey R, Kendrick GA (2017) Deep thinking: a systematic review of mesophotic coral ecosystems. ICES J Mar Sci 74:2309–2320
Wagner D, Kosaki RK, Spalding HL, Whitton RK, Pyle RL, Sherwood AR, Tsuda RT, Calcinai B (2014) Mesophotic surveys of the flora and fauna at Johnston Atoll, Central Pacific Ocean. Mar Biodivers Rec 7:e68
Weeks R, Russ GR, Alcala AC, White AT (2010) Effectiveness of marine protected areas in the Philippines for biodiversity conservation. Conserv Biol 24:531–540
Westneat MW, Alfaro ME (2005) Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol Phylogenet Evol 36:370–390
White AT, Gomez E, Alcala AC, Russ G (2007) Evolution and lessons from fisheries and coastal management in the Philippines. In: McClanahan TR, Castilla JC (eds) Fisheries management: progress towards sustainability. Blackwell Publishing Ltd, Chapter 5
White AT, Vogt HP, Arin T (2000) Philippine Coral Reefs under threat: the economic losses caused by reef destruction. Mar Pollut Bull 40:598–605
Acknowledgements
This work was funded by the generous support of donors who endorsed the California Academy of Sciences’ Hope for Reefs Initiative, and through grants from the National Science Foundation to T. Gosliner, R. Mooi, G. Williams and L.A. Rocha (DEB 12576304), and Brian W. Bowen (OCE-15-58852), and a grant from the Seaver Institute to Brian W. Bowen and Richard L. Pyle. We also thank the Australian Research Council Center of Excellence for Coral Reef Studies at James Cook University, the Oceans Institute and School of Biological Sciences at University of Western Australia, G.R. Russ, A.C. Alcala and T. Langlois for support. We are grateful to many colleagues who helped in the field and with discussions: M. Bell, E. Jessup, M. Lane, N. Nazarian, T. Phelps, B. Bowen, C. Ka‘apu-Lyons, S. Longo. Hollis, Poseidon and Anilao Beach Club provided gear and logistical support. HTP thanks CNPq for his PhD fellowship (Ciência sem Fronteiras; GDE 202475/2011-5). Philippines research and collecting permits (GP-0072-13, GP-0077-14, and GP-0085-15) were provided by the Department of Agriculture-Bureau of Fisheries and Aquatic Resources. Additional permits were provided by Apo Island Protected Area Management Board. This is a collaborative research initiative with key Philippine partners including: former Secretary of Agriculture P. J. Alcala; former Philippine Consul General M. Paynor and the Consular staff in San Francisco; former BFAR Director A. G. Perez; BFAR colleagues, especially A. Vitug and L. Labe; and NFRDI colleagues especially, Acting Director D. Bayate and N. Romena.
Author information
Authors and Affiliations
Corresponding author
Additional information
Topic Editor Morgan S. Pratchett
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pinheiro, H.T., Shepherd, B., Castillo, C. et al. Deep reef fishes in the world’s epicenter of marine biodiversity. Coral Reefs 38, 985–995 (2019). https://doi.org/10.1007/s00338-019-01825-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-019-01825-5