Abstract
Past studies have shown that the initiation of symbiosis between the Red-Sea soft coral Heteroxenia fuscescens and its symbiotic dinoflagellates occurs due to the chemical attraction of the motile algal cells to substances emanating from the coral polyps. However, the resulting swimming patterns of zooxanthellae have not been previously studied. This work examined algal swimming behaviour, host location and navigation capabilities under four conditions: (1) still water, (2) in still water with waterborne host attractants, (3) in flowing water, and (4) in flow with host attractants. Algae were capable of actively and effectively locating their host in still water as well as in flow. When in water containing host attractants, swimming became slower, motion patterns straighter and the direction of motion was mainly towards the host—even if this meant advancing upstream against flow velocities of up to 0.5 mm s−1. Coral-algae encounter probability decreased the further downstream of the host algae were located, probably due to diffusion of the chemical signal. The results show how the chemoreceptive zooxanthellae modify their swimming pattern, direction, velocity, circuity and turning rate to accommodate efficient navigation in changing environmental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zooxanthellae—symbiotic dinoflagellates of the genus Symbiodinium—inhabit a wide range of aquatic hosts (Trench 1993), most notably anthozoan cnidarians. The photosynthetic zooxanthellae provide the host with energy rich food, while the host provides inorganic carbon and other nutrients to the algae (Muscatine 1990); this symbiosis is essential for the existence and well being of oligotrophic tropical reef ecosystems (Levinton 1995). Acquisition of dinoflagellates in marine cnidarians, i.e., the initial infection event during which a new sexually produced offspring acquires its first complement of zooxanthellae, occurs via two main pathways: vertically (a “closed system”), in which new coral polyps are provided with algal cells by maternal inheritance, or horizontally (an “open system”), in which they acquire algal cells from the ambient environment (Trench 1987; Douglas 1994). Open systems are found in ~85% of cnidarian species hosting zooxanthellae (Harrison and Wallace 1990; Richmond 1997), the main advantage presumably being the ability to form partnerships that are best adapted to environmental conditions in the host’s eventual habitat (Buddemeier and Fautin 1993). Algal acquisition from the environment may take place at the embryonic stage (e.g., the scyphozoan Linuche unguiculata; Montgomery and Kremer 1995), the planula larval stage (e.g., the scleractinian coral Fungia scutaria; Krupp 1983) or the metamorphosed polyp stage (e.g., the soft coral Heteroxenia fuscescens; Benayahu et al. 1989a).
Several studies have established that azooxanthellate juvenile coral polyps (and adult anemones) are rapidly colonized by zooxanthellae in both the field and the laboratory, even when the algal cells are in low ambient concentration (Schwarz et al. 1999; Coffroth et al. 2001; Kinzie et al. 2001; Weis et al. 2001). H. fuscescens, the soft coral used in the present study, releases offspring that acquire algal symbionts in this way, soon after metamorphosis from planulae into primary polyps (Benayahu et al. 1989a, b; Yacobovitch 2001; Yacobovitch et al. 2003). Not much is known about Symbiodinium in its free-living state, and to date, there is only one report of Symbiodinium being isolated from the water column (Carlos et al. 1999), however, it seems highly likely that this motile phase is a dispersal or infectious form that functions in symbiosis onset (Trench 1980), and that the successful colonization process is due largely to the ability of the free-living algae to find their host and swim towards it. Zooxanthellae isolated from several hosts (e.g., stony corals, gorgonians and a giant clam) were observed swimming towards the mouth of primary polyps of the gorgonian Pseudopterogorgia bipinnata, followed by infection of these juvenile hosts (Kinzie 1974), and in another seminal study, zooxanthellae from a variety of hosts were likewise attracted to several types of aposymbiotic adult hosts, where they were consequently engulfed and presumably phagocytosed into endodermal cells (Fitt 1984).
The onset of symbiosis between H. fuscescens (Octocorallia: Alcyonacea) and its zooxanthellae begins with the chemical attraction of the motile algal cells to substances emanating from the juvenile, azooxanthellate coral polyps (Pasternak et al. 2004a). These substances do not affect the pattern of algal cell motility (Yacobovitch et al. 2004), but they do influence the directionality of the motion, with zooxanthellae swiming towards and aggregating in the vicinity of the mouth of the primary polyps (Yacobovitch 2001; Pasternak et al. 2004a). To date, however, there are no studies addressing the chemotactic navigational capabilities and the resulting directional swimming behaviour of free-living zooxanthellae as they search for their hosts. The aim of the present study was to elucidate the manner in which the symbiotic algal cells search for H. fuscescens host polyps, both in still water and in flow, and to determine the effect of distance from the host on search behaviour. We quantitatively characterized the swimming pattern, direction, velocity, net-to-gross displacement ratio (NGDR) and rate of change of direction (RCDi), as well as host-directed motion (HDM), of the algae. These factors were analysed in various combinations of flow and host attractants, and their effects on the host-location behaviour of the algae were examined.
Materials and methods
Mature colonies of H. fuscescens were haphazardly sampled from the Red-Sea coral reef near the InterUniversity Institute of Eilat, Israel, in June 2002. Planulae were collected as described in Ben-David-Zaslow and Benayahu (1996), and were grown into primary polyps following Yacobovitch et al. (2003). Zooxanthellae were extracted from mature H. fuscescens colonies, counted under a microscope and transformed into motile algal cells as described in Pasternak et al. (2004a). The motile cells derived from the short term culture of zooxanthellae were found to be a major (>90%) component of the cells originally isolated from parent colonies, thus providing confidence that the motile cells were representative of the algal population in the symbiosis. All experiments were conducted between 1000 and 1300 h (lights on 0800 h), since, under these conditions, Symbiodinium sp. cells exhibit motility with a diel rhythm lasting 8–9 h and peaking at 2.5–4 h after lights on (Yacobovitch et al. 2004).
The experiments were conducted in a transparent, round Plexiglas flume (external diameter 15 cm, channel width 2 cm and height 2 cm) in which 0.45 μm-filtered seawater was circulated by a paddlewheel to flow velocities of up to 0.5 mm s−1. We examined the water velocity profile by videotaping neutrally buoyant passive particles (Pliolite VT, Goodyear Co., USA) with and without a coral polyp, at a velocity of 0.5 mm s−1 in the centre of the flume. Twenty particles were filmed in the horizontal plane, i.e., from above, and another 20 in the vertical plane, i.e., from the side of the flume. Motion trajectories for the particles were drawn and the direction of motion was analysed (as explained below). The still water was analysed using the same methodology.
In the first experiment, algae were tested under the following four combinations of flow and host attractants: (1) still water without host attractants, (2) still water with host attractants—chemical compounds produced by the host in the flume, (3) flow (0.5 mm s−1) without host attractants, and (4) flow (0.5 mm s−1) with host attractants, in which algae were released downstream of the host. In the second experiment, algae were tested under flow conditions (0.5 mm s−1) at increasing distances (0–5 cm) downstream from the host polyp. In all experiments containing flow, flow speeds of >0.5 mm s−1 caused the algae to drift downstream. Still-water fluorescein-dye observations conducted prior to the actual experiments revealed that the dye reached the farthest edge of the viewing field after about 15 min from the insertion of the dye into the flume. Therefore, in all the experiments containing a host (in still water and flow), algae were gently pipetted into the centre of the viewing field 15 min after the insertion of the host into the flume. Algal motion was then recorded from above for 10 min using a stereomicroscope attached to a video camera. Contrary to most habitat selection experiments in flow, the algae were not passed over the host, but rather were only filmed in the viewing field (~3×3 cm) that was positioned ~2 cm downstream of it, sensing only those host attractants that drifted with the flow. After 10 min of videotaping, the flume was emptied, washed and filled with fresh filtered seawater, and the experiment began again using a fresh algal cohort and a new host polyp. Each experimental combination was repeated three times, recording 16 individual algae each time for a total of 48 algal trajectories per combination.
Algal paths were videotaped by an overhead camera, for as long as they remained within the camera-viewing field. Trajectories were manually drawn on acetate sheets at a sampling rate of one point per frame (25 points s−1), and were subsequently digitized at a 1 point pixel−1 resolution using ImageJ (http://www.rsb.info.nih.gov/ij). Digitized trajectories (see Fig. 1) were analysed using MATLAB (The MathWorks Inc., Natick, Massachusetts, USA) to identify their direction of motion at each point in time. These data were then pooled for all the trajectories in the same experimental combination and presented as polar-axis histograms (Fig. 2). Also shown in Fig. 2 are the combined percentages of motion direction when the horizontal space is divided into four sections: 45–135°, 135–225°, 225–315° and 315–45°; the host, if present, is located at 0° (i.e., the right-hand side); flow, if present, runs from 0° to 180° (i.e., from right to left). MATLAB was also used to calculate the following values for each trajectory: (1) swimming velocity (mm s−1), (2) NGDR, the ratio of the shortest linear distance between the start and endpoints of a path and the total travel distance. Increased NGDR indicates lower turning frequencies or straighter swimming paths. As an indicator of path circuity, NGDR has a maximum value of 100% when paths are completely straight and a minimum of zero when paths are totally circular; thus, a greater NGDR might also suggest movement that is more directed or less random, (3) RCDi, the total amount that the alga has turned divided by the path temporal duration (deg s−1, otherwise known as turning frequency, turning rate or angular velocity). RCDi is an indication of how straight the swimming paths are; a lower RCDi might suggest movement that is more directed or less random. For each of these three values, one-way anova tests (α=0.05) were used to determine possible difference between the four experimental combinations, and subsequent post hoc Scheffe tests (at 95% confidence interval) were used to determine the sources of these differences. In all four experimental combinations, additional 20 algae were randomly chosen and watched until they exited the viewing field or reached the host. HDM was calculated as the percentage of algae to reach the host or exit the viewing field to the direction of the host, and this was repeated five times for a total of 100 algae per combination.
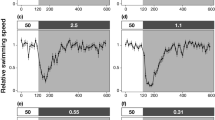
Typical examples of algal horizontal motion patterns, viewed from above. Typical motion patterns comprised at least 50% of motion patterns. The right column indicates flow condition and direction, the middle column indicates presence or absence of a host through which water passes en route to the zooxanthellae. The left column indicates typical observed algal motion, where motion trajectories begin at the X sign and end with a small arrow (in all experiments, cells were released at the same spot in the flume). Horizontal and vertical axes are equal and in mm. Duration and speed (respectively, mean ± SD) of motion at the different experimental conditions. a Still water without attractants 17.1±10.4 s, 0.4±0.1 mm s−1. b Still water with attractants 18.7±12.6 s, 0.2±0.1 mm s−1. c Flow without attractants 7.0±4.3 s, 0.7±0.2 mm s−1. d Flow with attractants 19.6±18.6 s, 0.3±0.1 mm s−1. e Passive particles in flow (Re=0.5) 4.2±0.5 s, 0.5±0.1 mm s−1
Directions of motion under the different experimental conditions: 45–135°, 135–225°, 225–315° and 315–45°. Y-axis is percentage out of total motion. Grey numbers are pooled percentages for each segment of the two-dimensional space (see text for further explanation). Full arrows indicate flow direction (in flow treatments), H indicates location of host (in host treatments). a Still water without attractants. b Still water with attractants. c Flow without attractants. d Flow with attractants
Results
Without a coral polyp in the flume, direction-analysis results of the passive particles from the vertical and horizontal planes did not vary significantly (Kruskal–Wallis test) and were therefore pooled. The particles exhibited a downstream motion (at 0.5 mm s−1), which was almost totally straight, implying laminar flow in both planes. When the cylindrical coral polyps were inserted (diameter 0.5–1 mm), the resulting Reynolds number at flow speeds of 0.5 mm s−1 was maximally Re=0.5, representing a laminar flow regime; this laminarity is reflected by the particle trajectories (Fig. 1e), where no recirculation around the polyp was observed. In still water, in the vertical plane, of the 20 passive particles, one remained in the water column, 14 sank to the bottom and five rose to the surface, indicating some degree of motion in the water; however, this degree was judged to be small, since in the horizontal plane (i.e., viewed from above), no noticeable motion of the particles could be detected.
In still water and the absence of attractants, algae exhibited a swimming pattern consisting of segments of fairly straight-path helical motion, with angle-changes between segments sometimes coupled with tight cyclic motion (Fig. 1a). Swimming was uniformly directed (Fig. 2a) and pooling of the motion directions revealed that the algae did not swim significantly in one direction over the others (Fig. 2a). In still water with host attractants, algae swam in a helical pattern (Fig. 1b), typical for chemotactic dinoflagellates (Fenchel 2001). Motion was significantly directed towards the host (Fig. 2b; df=3, F=36.93, P<0.01) and HDM was 52±17% (Fig. 3b). In flow without host attractants, algae again swam helically, but this time punctuated with segments of tight cyclic motion (Fig. 1c). Swimming was directed downstream to some extent, but pooling of the motion directions revealed that the algae did not swim significantly in one direction over the others (Fig. 2c). In flow with host attractants, algae swam mostly helically (Fig. 1d); Swimming was significantly directed upstream, towards the host (Fig. 2d, df=3, F=13.46, P<0.01). HDM was 64±22%, significantly higher than both no-host controls, but not significantly different than that with a host in still water (Fig. 3b; one-way anova test, df=3, F=19.22, P<0.01, with subsequent Scheffe post hoc tests).
The swimming velocity of the algae in flow without attractants was two to three times higher than in all other combinations (Fig. 3a; df=3, F=56.68, P<0.01). It is important to note that the swimming speeds we refer to in this study are the net speeds seen through the camera, and do not take into account the velocities needed in order to oppose the water flow; as a result, the actual swimming speed was faster. RCDi displayed the same pattern: in flow without attractants, RCDi was over 1,500 deg s−1, while in the other treatments, RCDi decreased to less than a third of that value (Fig. 3a; df=3, F=64.55, P<0.01). Swimming velocity and RCDi were highly correlated (r 2=0.97). NGDR levels were found to be significantly different between the various experimental conditions (Fig. 3b; df=3, F=2.79, P=0.05). The presence of host attractants (whether in still water or flow) caused algae to swim in straighter paths than in their absence. NGDR level and HDM were also found to be highly correlated (r 2=0.89).
Algal behaviour was also tested at increasing distances (0–5 cm) downsteam of the host (flow speed was 0.5 mm s−1, Re=0.5). The results indicate that the host-location ability of the zooxanthellae became less pronounced the more downstream of the host they were located: swimming became more uniformly directed (Fig. 4a) and HDM decreased sharply (Fig. 4c). Algal swimming velocity and RCDi downstream of the host, on the other hand, did not change as sharply but rather gradually (Fig. 4b; linear trendline significance: r 2=0.95 for swimming velocity and r 2=0.91 for RCDi).
Zooxanthellae direction of motion (a), swimming velocity and rate of change of direction (RCDi) (b) and net to gross displacement ratio (NGDR) and host-directed motion (HDM) (c) in different experimental conditions. The water flows from 0° to 180°, collecting the host’s chemical attractant(s) and carrying them downstream to varying distances (0–5 cm from the host). Values are mean ± SD. N.S., no significant difference between directions; Asterisk indicates significant difference (α=0.05); Perp., perpendicular to flow direction. Linear trendline significance is r 2=0.95 for swimming velocity and r 2=0.91 for RCDi
Discussion
The present study illustrates the ways in which the symbiont zooxanthellae of Red-Sea H. fuscescens corals navigate in the chemical concentration gradient of attractants emanating from the host. The swimming behaviour was modified according to the presence or absence of host coral polyps, with flow conditions playing a much less important role. According to the motion parameters that were studied, the algal motion can be classified into two distinct classes: “search motion” and “guided motion”. In “search motion”, which occured when the algae were in water without attractants (regardless of flow conditions), the symbionts swam equally in all directions. Their RCDi was high, resulting in a very curved path, and causing the motion NGDR to be low. Together with the high swimming velocity, this resulted in an efficient search—the exploring of more space in less time. The only effect flow appeared to have on algal motion was that, in the absence of host attractants, it caused them to swim faster and in more curved paths than in still water. In “guided motion”, which occured when the algae were in water containing host attractants (regardless of flow conditions), host-finding was very efficient: more than half the algal cells reached the host in our experimental setup (10 min., max. 10 cm). Swimming was directed mainly towards the host, even if this meant advancing upstream in flow velocities lower than the algal swimming speed (up to 0.5 mm s−1). It is interesting to note the subtle difference between motion directions in still water (Fig. 2b) and flow (Fig. 2d): the flow concentrated the attractants plume in the 0–180° axis, converting it from a sphere to an ellipse, and as a result, the algae swam in a more directed path towards the host. Swimming velocity in the “guided motion” was slow, perhaps in order to facilitate better receiving or processing of the chemical cue. The RCDi was also low, i.e., the path was straighter, making it easier to follow the concentration gradient and reach the chemical source as quickly as possible. A similar navigation strategy is seen in larger aquatic searchers, such as the larvae of the parasitic barnacle Heterosaccus dollfusi, which inhabits the brachyuran crab Charybdis longicollis (Pasternak et al. 2004b). In our experiments, the zooxanthellae of H. fuscescens swam upstream when they encountered host attractants and were able to oppose water flows of up to 25 body lengths s−1, mirroring the behaviour of H. dolfusi larvae.
Free-living zooxanthellae, at least in laboratory experiments, move in space to explore their environment for hosts. Their success depends on optimizing their search strategy, which is reflected by a typical geometry of the trajectories followed by the individuals. The diffusion approximation, where movement of individuals is assumed to be random (similar to Brownian particles), has been applied for some organisms (Turchin 1998). In other cases, it has been found that the movement is better characterized as a Levy walk, where the trajectory is composed of successive displacement steps of length l i , which are not equal but taken from a power-law distribution P(l) ~l -α and are separated by random angles (Schlesinger et al. 1993). In two dimensions, a Levy walk is an optimal search strategy that has been identified in a variety of organisms (e.g., albatrosses, Viswanathan et al. 1996). A rigorous analysis of the motion of zooxanthellae revealed that their motion is neither random nor a Levy walk (Blasius and Pasternak, unpublished data), and efforts are currently underway to fully elucidate their search strategy.
The results of the second experiment, where algal behaviour was tested at increasing distances downstream of the host, revealed that the swimming velocity and RCDi decreased gradually downstream of the host, while HDM dropped more sharply. Thus, we may conclude that when algae are located farther downstream of the host, the probability of them finding the host decreases substantially, probably due to dispersion of the chemical signal. Almost nothing is known about the ecology of free-living zooxanthellae in the natural environment (LaJeunesse 2001; Knowlton and Rohwer 2003), but we may assume that the “window of opportunity” during which searching for a host can be performed is limited both in space and time: algae can only efficiently locate a host when they are at a distance of cm to dm downstream of the host (this study), and only search 2–4 h after sunrise (Yacobovich et al. 2004). Despite these constraints, many algal cells reach their destination. Numerous symbiotic reef invertebrates utilize horizontal transmission (i.e., each generation obtains its algal symbionts from the environment rather than maternally), indicating that there must be substantial populations of potential zooxanthellae partners in reef waters (Goulet and Coffroth 1997). These potential symbiotic partners exist outside of hosts and may occur in fish faeces (Muller-Parker 1984), be released from corals (Stimson and Kinzie 1991) or occur free living (Carlos et al. 1999). It is probable, therefore, that settled H. fuscescens polyps are immediately populated by algal cells from the nearby benthos. However, since the algal chemoreceptive range in flow decreases downstream, and the maximum flow velocity in which they are able to navigate is 0.5 mm s−1, this scenario has two prerequisites. First, that the host-finding process be conducted where water turbulence is minimal, e.g., within the boundary layer [Goldshmid et al. (2004) have shown that flow in the Eilat natural environment can be very slow and sometimes halts altogether]. Second, that potential algal settlers must be very close, in the order of centimetre to decimetre, to a newly settled polyp. Alternatively, water motion may expose corals to free-living zooxanthellae, potentially resulting in the establishment of symbiosis even when zooxanthellae are at very low concentrations (Kinzie 1999).
Most cnidarian-microalgae symbioses exhibit some degree of specificity in host-symbiont pairing. Symbionts within the diverse genus Symbiodinium are classified into groups or clades (A, B, C, etc.) on the basis of sequence variation in the small-subunit ribosomal gene (Rowan and Powers 1991), and most cnidarians preferentially establish and maintain a stable symbiosis with either a specific clade (Goulet and Coffroth 2003) or a subset of the clades that vary with environmental gradients such as light intensity (Rowan et al. 1997). The question of specificity of the algal activity, i.e. is the altered swimming behaviour a general response or does it occur only when a suitable host is in the vicinity, is still very much open. However, evidence from three species of octocorals suggests that dinoflagellates from several clades initially enter the host, and later a “sorting” process occurs that changes the algal population genotypes to the specificity observed in the adult host (Coffroth et al. 2001; Lewis and Coffroth 2004).
References
Benayahu Y, Achituv Y, Berner T (1989a) Metamorphosis of an octocoral polyp and its infection by algal symbiont. Symbiosis 7:159–169
Benayahu Y, Berner T, Achituv Y (1989b) Development of planulae within mesogaleal coat in the soft coral Heteroxenia fuscescens. Mar Biol 100:203–210
Ben-David-Zaslow R, Benayahu Y (1996) Longevity, competence and energetic content of the soft coral Heteroxenia fuscescens. J Exp Mar Biol Ecol 206:55–68
Buddemeier RW, Fautin DG (1993) Coral bleaching as an adaptive mechanism: a testable hypothesis. BioScience 43:320–326
Carlos AA, Baillie BK, Kawachi M, Maruyama T (1999) Phylogenetic position of Symbiodinium (Dinophycaeae) isolates from tridacnids (Bivalvia), cardiids (Bivalvia), a sponge (Porifera), a soft coral (Anthozoa), and a free-living strain. J Phycol 35:1054–1062
Coffroth MA, Santos SR, Goulet TL (2001) Early ontogenetic expression of specificity in a cnidarian-algal symbiosis. Mar Ecol Prog Ser 222:85–96
Douglas AE (1994) Symbiotic interactions. Oxford University Press, Oxford
Fenchel T (2001) How dinoflagellates swim. Protist 152:329–338
Fitt WK (1984) The role of chemosensory behavior of Symbiodinium microadriaticum, intermediate hosts, and host behavior in the infection of coelenterates and molluscs with zooxanthellae. Mar Biol 81:9–17
Goldshmid R, Holzman R, Weihs D, Genin A (2004) Aeration of corals by sleep-swimming fish. Limnol Oceanog 49:1832–1839
Goulet TL, Coffroth MA (1997) A within colony comparison of zooxanthella genotypes in the Caribbean gorgonian Plexaura kuna. Proc 8th Int Coral Reef Symp, Panama 2:1331–1334
Goulet TL, Coffroth MA (2003) Genetic composition of zooxanthellae between and within colonies of the octocoral Plexaura kuna, based on small subunit rDNA and multilocus DNA fingerprinting. Mar Biol 142:233–239
Harrison PL, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed) Ecosystems of the world, vol 25. Coral reefs. Elsevier, Amsterdam, pp 133–207
Kinzie RA (1974) Experimental infection of aposymbiotic gorgonian polyps with zooxanthellae. J Exp Mar Ecol 15:335–345
Kinzie RA (1999) Sex, symbiosis and coral reef communities. Am Zool 39:80–91
Kinzie RA, Takayama M, Santos SR, Coffroth MA (2001) The Adaptive Bleaching Hypothesis: Experimental Tests of Critical Assumptions. Biol Bull 200:51–58
Knowlton N, Rohwer F (2003) Microbial mutualisms on coral reefs. Am Nat 162:51–62
Krupp DA (1983) Sexual reproduction and early development of the solitary coral Fungia scutaria (Anthozoa: Scleractinia). Coral Reefs 2:159–164
LaJeunesse TC (2001) Investigating the biodiversity, ecology and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a “species” level marker. J Phycol 37:866–880
Levinton JS (1995) Marine Biology: Function, Biodiversity, Ecology. Oxford University Press, NY
Lewis C, Coffroth MA (2004) The acquisition of exogenous algal symbionts by an octocoral after bleaching. Science 304:1490–1492
Montgomery MK, Kremer PM (1995) Transmission of symbiotic dinoflagellates through the sexual cycle of the host scyphozoan Linuche unguiculata. Mar Biol 124:147–155
Muller-Parker G (1984) Dispersal of zooxanthellae on coral reefs by predators on cnidarians. Biol Bull 167:159–167
Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinsky Z (ed) Ecosystems of the world, vol 25. Coral reefs. Elsevier, Amsterdam, pp 75–87
Pasternak Z, Bachar A, Abelson A, Achituv Y (2004a) Initiation of symbiosis between the soft coral Heteroxenia fuscescens and its zooxanthellae. Mar Ecol Prog Ser 279:113–116
Pasternak Z, Blasius B, Abelson A (2004b) Host location by larvae of a parasitic barnacle: larval chemotaxis and plume tracking in flow. J Plankton Res 26(3):1–7
Richmond RH (1997) Reproduction and recruitment in corals: critical links in the persistence of reefs. In: Birkeland C (ed) Life and death of coral reefs. Chapman Hall, NY, pp 175–197
Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269
Rowan R, Powers D (1991) Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae). Mar Ecol Prog Ser 71:65–73
Schlesinger MF, Zaslavsky GM, Klafter J (1993) Strange kinetics. Nature 363:31–37
Schwarz JA, Dave AK, Weis VM (1999) Late larval development and onset of symbiosis in the scleractinian coral Fungia scutaria. Biol Bull 196:70–79
Stimson J, Kinzie R (1991) The diel pattern of release of zooxanthellae by colonies of Pocillopora damicornis maintained under control and nitrogen-enriched conditions. J Exp Mar Biol Ecol 153:63–74
Trench RK (1980) Integrative mechanisms in mutualistic endosymbiosis. In: Cook CB, Rudolph E, Pappas PW (eds) Cellular interactions in symbiosis and parasitism. Ohio State University Press, Columbus, pp 275–297
Trench RK (1987) Dinoflagellates in non parasitic symbioses. In: Taylor FJR (ed) The Biology of dinoflagellates. Blackwell, pp 530–570
Trench RK (1993) Microalgal-invertebrate symbiosis: a review. Endocyt Cell Res 9:135–175
Turchin P (1998) Quantitative analysis of movement. Sinauer, Massachusetts
Viswanathan GM, Afanasyev V, Buldyrev SV, Murphy EJ, Prince PA, Stanley HE (1996) Levy flight search patterns of wandering albatrosses. Nature 381:413–415
Weis VM, Reynolds WS, deBoer MD, Krupp DA (2001) Host–symbiont specificity during onset of symbiosis between the diniflagellates Symbiodinium spp. and planula larvae of the scleractinian coral Fungia scutaria. Coral Reefs 20:301–308
Yacobovitch T (2001) Acquisition of zooxanthellae by a sexuallly-produced offspring of the soft coral Heteroxenia fuscescens. Tel Aviv University, Master’s Thesis
Yacobovitch T, Benayahua Y, Weis VM (2004) Motility of zooxanthellae isolated from the Red Sea soft coral Heteroxenia fuscescens (Cnidaria). J Exp Mar Biol Ecol 298:35–48
Yacobovitch T, Weis VM, Benayahu Y (2003) Development and survivorship of zooxanthellate and azooxanthellate primary polyps of the soft coral Heteroxenia fuscescens: laboratory and field comparisons. Mar Biol 142:1055–1063
Acknowledgements
The authors would like to thank Shay Glazer and Shiri Klarfeld from Bar-Ilan University and Prof. Yehuda Benayahu, Anat Maoz and Ettie Sapir from Tel-Aviv University. We thank Michal Gur for the trajectory digitalization and Duet Kaparot for her help with the graphics. This work is part of a dissertation of Z.P. towards his PhD degree.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor H.R. Lasker
Rights and permissions
About this article
Cite this article
Pasternak, Z., Blasius, B., Abelson, A. et al. Host-finding behaviour and navigation capabilities of symbiotic zooxanthellae. Coral Reefs 25, 201–207 (2006). https://doi.org/10.1007/s00338-005-0085-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-005-0085-2