Abstract
The sea urchin cardinalfish, Siphamia tubifer (Perciformes: Apogonidae), is unusual among coral reef fishes for its use of bioluminescence, produced by symbiotic bacteria, while foraging at night. As a foundation for understanding the relationship between the symbiosis and the ecology of the fish, this study examined the diel behavior, host urchin preference, site fidelity, and homing of S. tubifer in June and July of 2012 and 2013 at reefs near Sesoko Island, Okinawa, Japan (26°38′N, 127°52′E). After foraging, S. tubifer aggregated in groups among the spines of the longspine sea urchin, Diadema setosum, and the banded sea urchin, Echinothrix calamaris. A preference for D. setosum was evident (P < 0.001), especially by larger individuals (>25 mm standard length, P < 0.01), and choice experiments demonstrated the ability of S. tubifer to recognize and orient to a host urchin and to conspecifics. Tagging studies revealed that S. tubifer exhibits daily fidelity to a host urchin; 43–50 and 26–37 % of tagged individuals were associated with the same urchin after 3 and 7 days. Tagged fish also returned to their site of origin after displacement; by day two, 23–43 and 27–33 % of tagged individuals returned from displacement distances of 1 and 2 km. These results suggest that S. tubifer uses various environmental cues for homing and site fidelity; similar behaviors and cues might be used by larvae for recruitment to settlement sites and for the acquisition of luminous symbiotic bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many coral reef fishes have restricted home ranges and return to home sites daily after foraging and displacement (Sale 1978a). Having a home site can enhance an individual fish’s fitness through benefits associated with familiarity of local resources and the location of competitors, predators, and mates (Shapiro 1986; Noda et al. 1994; Brown and Dreier 2002). Furthermore, the diel homing behavior of fishes can directly affect nutrient transfer within a reef environment (e.g., Meyer et al. 1983; Bellwood 1995) as well as processes that influence population dynamics, such as mortality and recruitment (Sale 1978b). Among reef fishes, the cardinalfishes (Perciformes: Apogonidae) are one of the most abundant and species-rich groups in the Indo-Pacific (Allen 1993; Bellwood 1996). Cardinalfishes typically forage at night and form aggregations during the day around reef structures, such as branching corals (Greenfield and Johnson 1990; Gardiner and Jones 2005, 2010). Some cardinalfishes exhibit fidelity to their daytime home sites over the course of months (Kuwamura 1985; Okuda and Yanagisawa 1996; Marnane 2000), and few species are known to return to home sites when displaced substantial distances (Marnane 2000; Kolm et al. 2005). However, despite their abundance in reef communities, cardinalfishes remain one of the least studied families of reef fishes (Bellwood 1996). In particular, little is known of the behavioral ecology of members of the symbiotically luminous genus of cardinalfish, Siphamia.
Siphamia tubifer may be the most widespread Siphamia species; a recent taxonomic revision reclassified Siphamia versicolor (Smith and Radcliffe, in Radcliffe 1911; Tominaga 1964), reported from many locations throughout the Indo-West Pacific region, as a junior synonym of S. tubifer Weber 1909 (Gon and Allen 2012). Like other cardinalfishes, S. tubifer is a paternal mouth brooder; the adult male orally broods his fertilized clutch of eggs (Breder and Rosen 1966; Thresher 1984; Dunlap et al. 2012) and releases pre-flexion larvae into the plankton (Dunlap et al. 2009). Unusual for most cardinalfishes and other coral reef fishes, however, bioluminescence apparently plays a major role in the biology of S. tubifer. The abdominal light organ of S. tubifer, which is connected to the intestine by a duct, begins to develop in larvae after their release into the plankton and remains free of bacteria for at least 7 days of post-release development (Leis and Bullock 1986; Dunlap et al. 2009). The luminous bacteria, identified as members of clade II of Photobacterium mandapamensis (Kaeding et al. 2007; Urbanczyk et al. 2011), are then taken up from the environment and colonize the fish’s light organ (Dunlap and Nakamura 2011; Dunlap et al. 2012). The fish carries a large population of the symbiotic bacteria in the light organ and emits the bacterial light as an even glow over its ventrum while it forages at night (Dunlap and Nakamura 2011). After returning to an urchin from foraging, the fish release fecal material containing large numbers of the symbiotic bacteria (Dunlap and Nakamura 2011).
Despite progress in understanding the symbiosis of S. tubifer and P. mandapamensis, the behavioral ecology of the fish and the functional role of the symbiosis in its daily life remain largely unknown. During the day, S. tubifer associates in small to large groups with the longspine sea urchin, Diadema setosum, or the banded sea urchin, Echinothrix calamaris, remaining quiescent among the urchin’s spines (Lachner 1955; Eibl-Eibesfeldt 1961; Tamura 1982). A preference for a host urchin species would indicate which reef sites are suitable for incoming recruits, and predictable home sites could influence the distribution of competitors and predators at that reef. However, whether the fish exhibits the homing behavior and site fidelity seen in other cardinalfishes and whether the symbiosis is influenced by or contributes to these activities are not known. Therefore, to begin building a foundation for understanding the ecology of this group of apogonids with respect to the bioluminescent symbiosis, we examined the diel behavior, host urchin preference, site fidelity, and homing of S. tubifer at reefs in Okinawa, Japan.
Materials and methods
Study sites
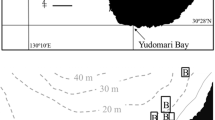
This study was carried out at shallow coral reefs at Sesoko Island, Okinawa, Japan (26°38′N, 127°52′E) and at nearby reefs on Motobu Peninsula (Fig. 1) during June and July of 2012 and 2013. Observations of diel behavior of Siphamia tubifer were made at reefs fronting Sesoko Station (Tropical Biosphere Research Center, University of the Ryukyus) on Sesoko Island, as were site fidelity experiments. Transects and homing experiments were carried out at a site in the vicinity of Motobu town, across the channel from Sesoko Island (Fig. 1). The protocols used here for the capture, care, and handling of S. tubifer were approved by the University of Michigan’s Institutional Animal Care and Use Committee, and they accord with animal handling guidelines of the University of the Ryukyus Guide for Care and Use of Laboratory Animals.
Map of the study area in Okinawa, Japan. Study sites are indicated with black circles and labeled as follows: a Sesoko Station (site fidelity study); b study site near Motobu, the point of origin for the homing study and site of all field transects; c 1 km release site for the homing study; and d 2 km release site for the homing study. Light gray shaded areas (left) indicate areas < 10 m in depth. Map modified from Hohenegger et al. (1999)
Diel behavior
Observations of groups of S. tubifer associated with Diadema setosum were made using SCUBA to determine the timing of departure from and return to a host urchin. On July 1, 2012, the group of fish at an urchin was monitored from approximately 15 min before sunset until no other fish left the urchin. An additional observation of the urchin was made at midnight to determine whether any fish had returned from foraging by this time. On July 4, 2012, the same urchin was monitored beginning at 1 h before sunrise until the time after which no additional fish returned to the urchin. One group of fish (n = 26) was collected immediately after their return to an urchin and examined for stomach fullness and contents.
Host preference
To determine the natural preference for S. tubifer to associate with D. setosum or Echinothrix calamaris (Fig. 2), surveys along randomly placed transects were carried out at a site approximately 40 m offshore where both species of urchin were abundant (Fig. 1). A total of six independent 50 m transects were surveyed using SCUBA for the number of urchins and associated S. tubifer along the backside of the reef and the adjacent sand flat. Transects were randomly placed, regardless of substrate (reef or sand), at least 20 m apart, and each urchin within two meters of either side of the transect tape was examined by divers. The urchin species and number of S. tubifer associated with each urchin were recorded along with the substrate type. The size of each S. tubifer observed was also estimated and recorded as either “small” (<25 mm standard length, SL) or “large” (>25 mm SL) for three of the transects, as a difference in size of fish associated with each host urchin species became evident during the first three transects.
For choice experiments, six groups of S. tubifer, which varied in standard lengths and number of fish, were collected with their urchins from reefs fronting Sesoko Station (Fig. 1) and maintained in aerated aquaria with flowing natural seawater. Individual fish were placed in the middle area of a large aquarium (2 m × 1 m × 1 m) that contained approximately 1,200 L of natural seawater. The tank was partitioned into three equal sections with square plastic mesh (20 × 20 mm2) through which the fish could swim. Different combinations of choices were presented to each fish in the two opposing sections of the aquarium, and the sides in which the stimuli were presented were randomly and periodically switched between fishes to ensure no side bias existed in the tank. For each trial, the side that an individual fish swam to and remained settled at for at least 30 s was recorded. Fish were allowed up to 2 min to choose a side, and any individual that did not choose a side within the 2 min was not included in the analysis. The aquarium was flushed with flowing seawater after each trial, and each fish was tested only once. The combination of choices presented and the number of fish tested for each combination were: a D. setosum urchin from a different patch of reef >20 m away from the collected fish (unfamiliar urchin) or no urchin (only seawater), n = 38; the D. setosum urchin collected with the fish (familiar urchin) or an unfamiliar D. setosum (unfamiliar urchin), n = 87; a group of ten S. tubifer collected from an urchin >20 m away (unfamiliar fish) or no fish (only seawater), n = 28; a group of ten S. tubifer collected from the same urchin (familiar fish) or an unfamiliar group of ten S. tubifer collected from a different urchin >20 m away, n = 57; a familiar D. setosum or a familiar group of ten S. tubifer, n = 19; and an unfamiliar D. setosum or an unfamiliar E. setosum (both collected >20 m away), n = 35. All groups of S. tubifer presented as a conspecific choice were kept in place in the aquarium with the same mesh structure used to partition the tank. On no occasion did any of these fish swim away from the group of fish or the mesh structure.
Tagging
Groups of S. tubifer, which varied in numbers and in standard lengths of individuals (Table 1), were collected from the reef with their associated host urchin, taken to the laboratory, and tagged. Individual fish were lightly anesthetized with 2-phenoxyethanol (Acros Organics) (0.2 mL per L of seawater) and measured to the nearest 0.5 mm SL prior to tagging. The standard length of all tagged fish (n = 313) ranged from 12.5 to 38.5 mm, with a mean length of 26.8 ± 5.1 (SD) mm. Brooding male fish were not included in these experiments, as they do not leave an urchin while brooding (Dunlap and Nakamura 2011; this study). Fluorescent visual implant elastomer (VIE) tags (Northwest Fisheries Supplies, Inc.) of different colors were injected subcutaneously at varying body locations to uniquely identify each group of S. tubifer collected with an individual urchin. After tagging, fish were given a 4-h recovery period in aquaria with aerated flowing seawater and were then released back into the field as a group with a D. setosum urchin. No fatalities occurred during this 4-h period in the experimental groups. To test for mortality associated with tagging, an additional group of S. tubifer (n = 41) was tagged and maintained in an aquarium for one week and fed daily with wild-caught zooplankton. Of this group of fish, one individual did not survive handling, and another fish was found dead in the aquarium one day after tagging. The remaining individuals (>95 %) were seemingly healthy by the end of one week after tagging, and all tags were clearly visible, indicating that mortality due to handling and tagging is less than 5 % and likely occurs during handling or by day one and that the tags remain in place and visible for this period of time.
Site fidelity
Analysis of site fidelity was carried out using groups of S. tubifer associated with individual D. setosum. Observations during this study indicated that divers could recognize individual urchins by their appearance and specific locations at reef sites, to which the urchins returned daily from short nocturnal foraging distances (generally <5 m) (Magnus 1967; this study). Three groups of S. tubifer (Table 1) were collected with their urchins from reefs fronting Sesoko Station in June 2013 and uniquely marked with VIE tags. After a 4-h recovery period, each group of fish was released with their urchin at its site of origin at least 2 h before sunset. The number of tagged fish from each group that were associated with their original urchin was determined on days one, two, three, and seven after release. In addition, the surrounding 10-m-radius area was surveyed for the presence of tagged fish on other urchins.
Homing behavior
To determine the homing ability of S. tubifer, three replicate groups of uniquely tagged S. tubifer (Table 1) were released with an unfamiliar D. setosum urchin (collected from a different reef) at sites 1 or 2 km from their reef of origin (Fig. 1) and monitored for their return over one week. The original urchin with which a group of fish was collected was returned back to its capture site at its reef of origin. Three control groups of fish were released with their urchin of origin at their capture site after tagging and recovery. Additional groups (three groups per displacement distance) were released 1 and 2 km from their reef of origin; the 2-km release site was located northeast of the site of origin, and the 1-km site was located southwest of the site of origin (Fig. 1). An additional 1-km release site northeast of the site of origin was also tested to determine whether the direction of the release site relative to the capture site influenced the homing ability of the fish. The percentage of fish that returned from this experimental group after one week (19 %) was within the range of those returning from the other 1-km site (19–24 %). Original urchins and the surrounding 10-m-radius area at the reef of origin were monitored for the presence of tagged individuals on days one, two, three, and seven after displacement.
Statistical analysis
Each transect at the study site was treated independently for the analysis of the distribution of S. tubifer on D. setosum and E. calamaris as host urchins. Because the data were not normally distributed, a Wilcoxon rank-sum test was performed, with correction for continuity, to test the preference of S. tubifer to associate with D. setosum or E. calamaris. To test whether small (≤25 mm SL) and large (>25 mm SL) fish associate more frequently with an urchin species, chi-square tests of independence were preformed on the proportion of fish of each size category in association with D. setosum or E. calamaris. In addition, Manly’s alpha scores (Manly et al. 1972; Chesson 1978) were calculated for all fish surveyed and converted into electivity indices (Chesson 1983; Shima 2001) to analyze the use of each urchin species as a host relative to their abundance on both reef and sand substrate at the study site. To analyze the choice experiments, chi-square tests of independence were performed on the proportion of fish that chose either stimulus for each pair of choices presented.
To analyze site fidelity data, a repeated-measures ANOVA followed by pairwise t tests between days was used to test for the effect of time on the proportion of individuals that returned to the same urchin daily. Homing data were analyzed using a generalized linear mixed model with a binomial distribution and a logit link function, with time in days, distance, and mean body length (mm, SL) of each group of fish (Table 1) as fixed effects and each replicate group as a random effect. The final model was chosen by stepwise selection based on lowest Akaike information criterion (AIC) scores. Individual body size was measured only during the initial tagging process; therefore, the correlation between homing success and fish body size was examined using metrics of size describing an entire group of tagged fish, such as the proportion of small individuals (<25 mm) and mean body length. All statistical analyses were performed in R, version 2.15.1 (R Development Core Team 2012).
Results
Diel behavior
Field observations of Siphamia tubifer associated with Diadema setosum revealed that the fish alternates between a non-feeding, protective association with an urchin during the day and foraging for zooplankton away from the urchin at night (Table 2); ambient light levels at dusk and dawn apparently cue this behavior. As dusk approached after sunset, the fish changed from a uniform nearly black, dark-brown color to a pattern of silver with three lengthwise dark stripes. At this time, the fish moved away from the urchin test toward the outer ends of the spines. The fish hovered at this position for several minutes, facing outward from the urchin. They then turned entirely silver in color and individually darted away from the urchin; approximately ten fish would leave the urchin within a few seconds of each other. All fish except brooding males had left the urchin, presumably to forage, within a few minutes (Table 2). Brooding males, identified by their swollen, distended jaws, remained dark-brown in color among the urchin spines throughout the night. As dawn approached, the foraging fish returned to the urchin, arriving singly or in pairs, and were silver in color. All fish arrived within several minutes of each other and had assembled among the urchin’s spines by approximately half an hour before sunrise. In one instance, a returning fish was chased by a larger, presumably predatory, fish (unknown species); the chased fish darted into a crevice of a Porites coral close to an urchin, remained still in this crevice for several minutes, and then darted among the spines of a nearby D. setosum urchin. Examination of the stomachs of the fish collected from an urchin immediately after their return at dawn revealed the stomachs to be full and to contain mostly benthic zooplankton. In contrast, the stomachs of brooding males were empty.
Host preference
The natural and apparently exclusive daytime hosts of S. tubifer are D. setosum and Echinothrix calamaris in the Motobu Peninsula area. During the day, we found S. tubifer primarily in association with the longspine urchin, D. setosum, but also frequently with the banded urchin E. calamaris, which has shorter spines (Fig. 2). Despite extensive observations, we did not find the fish during the day in association with any other urchin species, with corals, with the crown-of-thorns seastar Acanthaster (Stier et al. 2009), or in other areas of the reef. The transect site (Fig. 1) contained more E. calamaris than D. setosum, and both urchin species occurred on the backside of the reef as well as on adjacent sand flats; however, 85 % of all urchins surveyed were located on the sand flat (Fig. 3). Of the D. setosum surveyed, 65 % were found on the reef, whereas only 3 % of E. calamaris were on reef substrate (Fig. 3). The distribution of S. tubifer at this site was therefore influenced by the distribution of host urchins.
The proportion of host urchin species surveyed along transects that were associated with sand or reef as substrate (top). The proportion of large (>25 mm SL) and small (<25 mm SL) Siphamia tubifer surveyed that were associated with each host urchin species (bottom). Total numbers of individuals surveyed are indicated at the top of each bar
Of all urchins surveyed, 41 % had S. tubifer associated with them, but fish were found more frequently in association with D. setosum; 56 % of all fish surveyed were associated with D. setosum despite its low relative abundance at the study site (Fig. 3, Wilcoxon ranked-sum test, T = 7,911.5, P < 0.001). When comparing host urchins occupied by small (<25 mm SL) and large (>25 mm SL) S. tubifer, more small fish were associated with E. calamaris than large fish (χ 2 = 78.7, df = 1, P < 0.0001); 82 % of fish associated with E. calamaris were small (Fig. 3). Conversely, there was little difference in the numbers of small and large fish associated with D. setosum (χ 2 = 0.30, df = 1, P < 0.58); 53 and 47 % of the fish surveyed with D. setosum were small and large, respectively (Fig. 3). An electivity score (ε) of 0.68 for all fish surveyed over both substrates indicate that S. tubifer selectively associate with D. setosum, although this preference is stronger for large fish (ε = 0.37) than for small fish (ε = 0.15) (Table 3). In contrast, all electivity scores calculated for fish associated with E. calamaris were negative, which indicates a lack of preference for E. calamaris as a host urchin. On the reef, all S. tubifer surveyed appeared to avoid E. calamaris as a host; no fish were seen in association with E. calamaris on the reef, and consequently the electivity scores were −1.00 for all fish, regardless of size (Table 3).
The results of choice experiments in the aquarium confirmed the observed preference of S. tubifer for D. setosum. Compared to an empty area with no urchin, S. tubifer associated more frequently with D. setosum (χ 2 = 13.88, P < 0.0001) as well as with conspecifics (χ 2 = 4.95, P < 0.05) (Fig. 4). The fish also exhibited a preference for D. setosum over E. calamaris; 71 % of S. tubifer tested associated with D. setosum (χ 2 = 2.85, P = 0.09). Although not statistically significant, the preference for D. setosum (Fig. 4) is consistent with the higher numbers of S. tubifer associated with D. setosum compared to E. calamaris in the wild. With respect to choosing between familiar and unfamiliar urchins and conspecifics, S. tubifer showed no obvious preference; 49 and 54 % of fish tested associated with familiar urchins (χ 2 = 0.03, P = 0.86) and conspecifics (χ 2 = 0.15, P = 0.70), respectively (Fig. 4).
Choices made by Siphamia tubifer when provided two choices on opposing sides of an aquarium. Numbers of fish tested that made a choice (and the number that did not make a choice) for each experiment, from top to bottom were: 34(4), 80(7), 25(3), 52(5), 16(3), 31(4). All urchins were Diadema setosum with the exception of the choice between host urchin species (bottom). Significant differences in choices made by fish (P < 0.05) are indicated by *
Site fidelity
Consistent with field observations, S. tubifer exhibits daily fidelity to an individual urchin at a site. Tagged fish were re-sighted on their original urchin 7 days after tagging, with an average of 55, 51, 46, and 33 % of tagged individuals re-sighted on the same urchin on days one, two, three, and seven, respectively (Fig. 5). Time after release had a significant effect on proportion of fish found with the same urchin (P < 0.01); a lower proportion of fish were re-sighted at the same urchin after one week than on days one and two (P < 0.05). In some instances, up to 5 % of tagged fish were sighted with other D. setosum within five meters from their original urchin.
Homing behavior
In addition to host urchin preference and site fidelity, S. tubifer is able to return to its home reef site from substantial distances, regardless of the direction of displacement. When fish were displaced 1 km and 2 km (Fig. 1), an average of 35 and 29 %, respectively, were re-sighted on an urchin within a ten meter radius of their original urchin at their capture site by day two, with up to 24 % of individuals returning to their original urchin. On day seven, an average of 34 and 24 % of fish from the 1- and 2-km groups, respectively, were re-sighted within a ten meter radius of their original urchin (Fig. 6a). Averages of control group fish, tagged and released at their capture site with their original urchin, re-sighted on days 1, 2, 3, and 7 after release, were 48, 35, 42, and 17 %, respectively (Fig. 6a). Thus, displacement distance had a significant effect on the proportion of fish that returned to their site of origin (P < 0.01). There was also a strong effect of mean group standard length (Table 2) on homing (P < 0.0001); a smaller proportion of fish homed from groups with a lower mean standard length than from groups with a higher mean standard length, irrespective of release distance (Fig. 6b). The proportion of small fish in a group, however, did not have a significant effect on the proportion of fish that homed (P = 0.38), and its relationship with homing was weaker (R 2 = 0.17, F = 6.85, P = 0.01) than that of mean body length (R 2 = 0.45, F = 29.9, P < 0.001) (Fig. 6b).
Results from homing experiments in which Siphamia tubifer were tagged and released distances of 0, 1, or 2 km. a The mean proportion of tagged fish per group that were observed at their site of origin over 7 days. Error bars indicate standard error. b The mean proportion of fish from a group that were recovered at their site of origin by mean body size (standard length) of the group (F = 29.9, P < 0.001). Bars indicate the range of the mean proportion of fish recovered across all time points sampled for each group
Discussion
Together with the ability to emit ventral luminescence, the behaviors and preferences described here for Siphamia tubifer appear to function to minimize predation. The daytime association with an urchin allows the fish, which typically is dark in coloration at that time, to be cryptic. Consistent with our field observations, Tamura (1982) observed S. tubifer at dusk and documented the fish’s body color change, from dark brownish black to silver striped to all silver, as the fish left an urchin. The fish remained silver all night, which presumably helps S. tubifer avoid detection while foraging. Ventral luminescence, which begins to be emitted at dusk (Dunlap and Nakamura 2011), might complement the silver coloration, helping the fish remain cryptic while foraging. Nonetheless, predation rates on S. tubifer are probably high; direct predation by lionfish has been observed (Michael 2013), and during this study, predatory fish, including larger apogonid species, were often sighted near urchins occupied by S. tubifer and observed preying on fish leaving and returning to an urchin.
The preference of S. tubifer for Diadema setosum as its daytime host over Echinothrix calamaris, a shorter-spined urchin (Fig. 2), is consistent with the observation that the fish prefer urchins with longer spines (Tamura 1982). Longer spines presumably provide better protection from predators, especially for larger S. tubifer. When both E. calamaris and D. setosum are present at a reef, small fish may be able to find adequate protection from predators among the shorter spines of E. calamaris. It is also possible that learning occurs with age; larger fish might have learned that the longer spines of D. setosum provide better protection than those of E. calamaris. Intraspecific interference competition (e.g., Holbrook and Schmitt 2002) could also influence the distribution of small and large S. tubifer associated with both urchin species; larger fish may outcompete smaller fish for space among the more protective D. setosum spines, and consequently displace smaller individuals to take residence among the shorter spines of E. calamaris. Additional studies, e.g., testing different size classes of the fish, “small” and “large”, with D. setosum versus E. calamaris in choice experiments, would provide further insight on host characteristics important for the fish throughout development.
This study establishes that S. tubifer exhibits daily site fidelity and returns to a home site after being displaced one and two km. Like host preference, site fidelity and homing by S. tubifer are likely to be shaped by the need to avoid predators. Knowledge of the local reef structure and the location of urchins and resident predators presumably enhances survival of fish departing from and returning to a home reef site and urchin. In this study, the percentage of tagged fish re-sighted at home reef sites for the control group of the homing study was similar to the proportion of fish re-sighted in the site fidelity experiment, which suggests that the lower numbers of fish returning over time to a home site and urchin reflect losses due to predation. The natural mortality rate of S. tubifer might also be relatively high, as the life spans of other apogonids are short (<1–2 years) (Chrystal et al. 1985; Marnane 2000; Kingsford et al. 2014). Another factor that could have influenced the proportion of fish recovered during the homing study was the study site itself; the collection (control) site was selected due to the high abundance of S. tubifer, which correlated with a high density of host urchins. Consequently, more tagged fish may have returned to the general area but were not re-sighted in the surveyed home site radius.
Consistent with our results, previous studies have shown that various apogonids can return to a home reef site when displaced substantial distances (Marnane 2000; Kolm et al. 2005). Marnane (2000) showed that between 33 and 63 % of three apogonid species returned to their site of origin within three days when translocated two km. Additional studies have shown that other apogonids, including members of Siphamia, exhibit site fidelity and remain at the same reef site for weeks to months (Strasburg 1966; Allen 1972; Kuwamura 1985; Okuda and Yanagisawa 1996; Marnane 2000), the consequences of which may directly affect nutrient distribution within a reef as well as the assembly of predator and prey species at that reef (Marnane 2000; Marnane and Bellwood 2002). The daily site fidelity and homing by S. tubifer might also lead to a local enrichment of their luminous symbiont in the water at a home site because excess symbiont cells are released daily with the fish’s feces (Dunlap and Nakamura 2011).
Previous studies have also shown that site fidelity and homing behavior of fishes can vary with ontogeny (Yoshiyama et al. 1992; Shima et al. 2012; White and Brown 2013); older fish are more likely to risk the return to a home site across unfamiliar waters, although this is not always the case for all fishes (White and Brown 2013). Our homing results appear to be consistent with this view, but additional studies are needed to empirically test whether larger S. tubifer are actually more successful at homing than smaller fish. The lower proportion of fish that homed from groups with smaller mean body size, however, may reflect a greater loss of smaller fish to predation. High predation risk could, therefore, play a critical role in shaping the highly cryptic life history of S. tubifer and provide incentive for the homing behavior observed in this study; prior knowledge of the predator and urchin communities in an area could outweigh the risks of making the return trip home.
The mechanisms used by fishes to navigate daily to home sites and those used by recruitment-stage larvae to find suitable settlement sites may involve visual, olfactory, and auditory cues. From short distances, S. tubifer likely uses visual cues to recognize and navigate within a familiar area to its daytime urchin host and probably has some spatial memory of a home site (e.g., White and Brown 2013), including the location of the host urchins in the area. However, to navigate back to a home site after displacement or to find a settlement site as a larva, S. tubifer presumably uses additional cues. Other cardinalfishes use olfaction to discriminate between familiar and unfamiliar reef waters, and settlement-stage apogonids might use chemical cues to recruit to their natal reefs (Atema et al. 2002; Døving et al. 2006; Gerlach et al. 2007). Previous studies have also shown that apogonids are attracted to reef sounds, which could also serve as cues for larval fish to navigate to a settlement site (Leis et al. 2003; Simpson et al. 2004, 2005). Sound can propagate relatively long distances through water, regardless of the direction of current flow (Rogers and Cox 1988), and urchins produce distinct sounds at frequencies detectable by fish against the background noises of coral reef communities (Radford et al. 2008, 2010). Therefore, S. tubifer could use a combination of olfactory and auditory cues for homing, which could also convey habitat quality to incoming S. tubifer recruits searching for a suitable settlement site.
The homing and site fidelity behavior of S. tubifer described here, together with other studies that suggest settling fishes might use environmental cues to navigate to natal reefs (Atema et al. 2002; Leis et al. 2003; Simpson et al. 2004, 2005; Gerlach et al. 2007), leads us to speculate that S. tubifer larvae could use similar environmental cues to recognize and recruit to reefs inhabited by adult conspecifics. If so, the larvae might encounter higher numbers of symbiotic bacteria near the reef compared to in the plankton, due to the daily release of the bacteria by adults at their daytime home sites (Dunlap and Nakamura 2011). Depending on the developmental timing of recruitment, light organ development, and the timing of symbiont acquisition by S. tubifer larvae (Leis and Bullock 1986; Dunlap et al. 2012), this interaction might function to ensure the successful initiation of the symbiosis, by establishing a quasi-vertical, adult to larvae, form of symbiont transfer. However, the environmental cues used by S. tubifer larvae for settlement and the relationship between settlement and initiation of the symbiosis remain to be determined.
References
Allen GR (1972) Observations on a commensal relationship between Siphamia fuscolineata (Apogonidae) and the crown-of-thorns starfish, Acanthaster planci. Copeia 1972(3):595–597
Allen GR (1993) Cardinalfishes (Apogonidae) of Madang Province, Papua New Guinea, with descriptions of three new species. Revue Française d’aquariologie 20(1):9–20
Atema J, Kingsford MJ, Gerlach G (2002) Larval reef fish could use odour for detection, retention, and orientation to reefs. Mar Ecol Prog Ser 241:151–160. doi:10.3354/meps241151
Bellwood DR (1995) Carbonate transport and within-reef patterns of bioerosion and sediment release by parrotfishes (family Scaridae) on the Great Barrier Reef. Mar Ecol Prog Ser 117:127–136. doi:10.3354/meps117127
Bellwood DR (1996) The Eocene fishes of Monte Bolca: the earliest coral reef fish assemblage. Coral Reefs 15:11–19. doi:10.1007/BF01626074
Breder CM Jr, Rosen DE (1966) Modes of Reproduction in Fishes. Natural History Press, Garden City
Brown GE, Dreier VM (2002) Predator inspection behaviour and attack cone avoidance in a characin fish: the effects of predator diet and prey experience. Anim Behav 63:1175–1181. doi:10.1006/anbe.2002.3024
Chesson J (1978) Measuring preference in selective predation. Ecology 211–215. doi:10.2307/1936364
Chesson J (1983) The estimation and analysis of preference and its relationship to foraging models. Ecology 1297–1304. doi:10.2307/1937838
Chrystal PJ, Potter IC, Loneragan NR, Holt CP (1985) Age structure, growth rates, movement patterns and feeding in an estuarine population of the cardinalfish Apogon rueppellii. Mar Biol 85:185–197. doi:10.1007/BF00397437
Døving KB, Stabell OB, Östlund-Nilsson S, Fisher R (2006) Site fidelity and homing in tropical coral reef cardinalfish: are they using olfactory cues? Chem Senses 31:265–272. doi:10.1093/chemse/bjj028
Dunlap PV, Nakamura M (2011) Functional morphology of the luminescence system of Siphamia versicolor (Perciformes: Apogonidae), a bacterially luminous coral reef fish. J Morphol 272:897–909. doi:10.1002/jmor.10956
Dunlap PV, Kojima Y, Nakamura S, Nakamura M (2009) Inception of formation and early morphogenesis of the bacterial light organ of the sea urchin cardinalfish, Siphamia versicolor (Perciformes, Apogonidae). Mar Biol 156:2011–2020. doi:10.1007/s00227-009-1232-z
Dunlap PV, Gould AL, Wittenrich ML, Nakamura M (2012) Symbiosis initiation in the bacterially luminous sea urchin cardinalfish Siphamia versicolor. J Fish Biol 81:1340–1356. doi:10.1111/j.1095-8649.2012.03415.x
Eibl-Eibesfeldt I (1961) Eine Symbiose zwischen Fischen (Siphamia versicolor) und Seeigeln. Zeitschrift fur Tierpsychologie 18:56–59
Gardiner NM, Jones GP (2005) Habitat specialization and overlap in a guild of coral reef cardinalfishes (Apogonidae). Mar Ecol Prog Ser 305:163–175. doi:10.3354/meps305163
Gardiner NM, Jones GP (2010) Synergistic effects of habitat preference and gregarious behaviour on habitat use in coral reef cardinalfish. Coral Reefs 29:845–856. doi:10.1007/s00338-010-0642-1
Gerlach G, Atema J, Kingsford MJ, Black KP, Miller-Sims V (2007) Smelling home can prevent dispersal of reef fish larvae. PNAS 3(104):858–863. doi:10.1073/pnas.0606777104
Gon O, Allen GR (2012) Revision of the Indo-Pacific cardinalfish genus Siphamia (Perciformes: Apogonidae). Zootaxa 3294:1–84
Greenfield DW, Johnson RK (1990) Heterogeneity in habitat choice in cardinalfish community structure. Copeia 4:1107–1114
Hohenegger J, Yordanova E, Nakano Y, Tatzreiter F (1999) Habitats of larger foraminifera on the upper reef slope of Sesoko Island, Okinawa, Japan. Mar Micropaleontol 36(2):109–168
Holbrook SJ, Schmitt RJ (2002) Competition for shelter space causes density-dependent predation mortality in damselfishes. Ecology 83(10):2855–2868. doi:10.2307/3072021
Kaeding AJ, Ast JC, Pearce MM, Urbanczyk H, Kimura S, Endo H, Nakamura M, Dunlap PV (2007) Phylogenetic diversity and cosymbiosis in the bioluminescent symbioses of Photobacterium mandapamensis. Appl Environ Microbiol 73(10):3173–3182. doi:10.1128/AEM.02212-06
Kingsford MJ, Finn MD, O’Callaghan MD, Atema J, Gerlach G (2014) Planktonic larval duration, age and growth of Ostorhinchus doederleini (Pisces: Apogonidae) on the southern Great Barrier Reef, Australia. Mar Biol 161(2):245–259. doi:10.1007/s00227-013-2331-4
Kolm N, Hoffman EA, Olsson J, Berglund A, Jones AG (2005) Group stability and homing behavior but no kin group structures in a coral reef fish. Behav Ecol 16:521–527. doi:10.1093/beheco/ari022
Kuwamura T (1985) Social and reproductive behaviour of three mouthbrooding cardinalfishes, Apogon doederlini, A. niger and A. notatus. Environ Biol Fishes 13:17–24
Lachner EA (1955) Inquilinism and a new record for Paramia bipunctata, a new cardinal fish from the Red Sea. Copeia 1955:53–55
Leis JM, Bullock S (1986) The luminous cardinalfish Siphamia (Pisces, Apogonidae), development of larvae and the luminous organ. In: Uyeno T, Arai R, Taniuchi T, Matsuura K (eds) Indo-pacific fish biology: proceedings of the second international conference on Indo-pacific fish. Japanese Ichthyol Soc, Toyko, pp 703–714
Leis JM, Carson-Ewart BM, Hay AC, Cato DH (2003) Coral reef sounds enable nocturnal navigation by some reef-fish larvae in some places and at some times. J Fish Biol 63:724–737. doi:10.1046/j.1095-8649.2003.00182.x
Magnus DB (1967) Ecological and ethological studies and experiments on the echinoderms of the Red Sea. Stud Trop Oceanogr 5:635–664
Manly BFJ, Miller P, Cook LM (1972) Analysis of a selective predation experiment. Am Nat 719–736
Marnane MJ (2000) Site fidelity and homing behaviour in coral reef cardinalfishes. J Fish Biol 57:1590–1600. doi:10.1111/j.1095-8649.2000.tb02234.x
Marnane MJ, Bellwood DR (2002) Diet and nocturnal foraging in cardinalfishes (Apogonidae) at One Tree Reef, Great Barrier Reef, Australia. Mar Ecol Prog Ser 231:261–268. doi:10.3354/meps231261
Meyer JL, Shultz ET, Helfman GS (1983) Fish schools: an asset to corals. Science 220:1047–1049
Michael SW (2013) Lionfish: risky but rewarding. Lionfish are beautiful, but stay away from their sting. FishChannel.com. http://www.fishchannel.com/saltwater-aquariums/species-info/lionfish/lionfish-rewards.aspx. Accessed 22 April 2014
Noda M, Gushima K, Kakuda S (1994) Local prey search based on spatial memory and expectation in the planktivorous reef fish, Chromis chrysurus (Pomacentridae). Anim Behav 47:1413–1422. doi:10.1006/anbe.1994.1188
Okuda N, Yanagisawa Y (1996) Filial cannibalism by mouthbrooding males of the cardinalfish, Apogon doederlini, in relation to their physical condition. Environ Biol Fish 45:397–404
R Development Core Team (2012) R: a language and environment for statistical computing. R 2.15.1 edn. R Foundation for Statistical Computing, Vienna, Austria
Radcliffe L (1911) Notes on some fishes of the genus Amia, family of Cheilodipteridae, with descriptions of four new species from the Philippine Islands. Proc US Natl Mus 41(1853):245–261
Radford CA, Jeffs AG, Tindle CT, Montgomery JC (2008) Resonating sea urchin skeletons create coastal choruses. Mar Ecol Prog Ser 362:37–43. doi:10.3354/meps07444
Radford CA, Stanley JA, Tindle CT, Montgomery JC, Jeffs AG (2010) Localised coastal habitats have distinct underwater sound signatures. Mar Ecol Prog Ser 401:21–29. doi:10.3354/meps08451
Rogers PH, Cox M (1988) Underwater sound as a biological stimulus. In: Atema J, Fay RR, Popper AN, Travolga WN (eds) Sensory biology of aquatic animals. Springer, New York, pp 131–149
Sale PF (1978a) Reef fishes and other vertebrates: a comparison of social structures. In: Reese ES, Lichter FJ (eds) Contrasts in behaviour: adaptations in the aquatic and terrestrial environments. Wiley, New York, pp 313–346
Sale PF (1978b) Coexistence of coral reef fishes: a lottery for living space. Environ Biol Fish 3:85–102. doi:10.1007/BF00006310
Shapiro DY (1986) Intra-group home ranges in a female-biased group of sex changing fish. Anim Behav 34:865–870. doi:10.1016/S0003-3472(86)80072-0
Shima JS (2001) Recruitment of a coral reef fish: roles of settlement, habitat, and postsettlement losses. Ecology 82:2190–2199. doi:10.2307/2680225
Shima JS, McNaughtan D, Geange SW, Wilkinson S (2012) Ontogenetic variation in site fidelity and homing behaviour of a temperate reef fish. J Exp Mar Biol Ecol 416:162–167. doi:10.1016/j.jembe.2012.02.020
Simpson SD, Meekan MG, McCauley RD, Jeffs A (2004) Attraction of settlement-stage coral reef fishes to reef noise. Mar Ecol Prog Ser 276:263–268. doi:10.3354/meps276263
Simpson SD, Meekan MG, Montgomery JC, McCauley RD, Jeffs AG (2005) Homeward sound. Science 308(5719):221. doi:10.1126/science.1107406
Stier AC, Steele MA, Brooks AJ (2009) Coral reef fishes use crown-of-thorns seastar as habitat. Coral Reefs 28(1):227. doi:10.1007/s00338-008-0445-9
Strasburg DW (1966) Observations on the ecology of four apogonid fishes. Pacific Sci 20:338–341
Tamura R (1982) Experimental observations on the association between the cardinalfish (Siphamia versicolor) and the sea urchin (Diadema setosum). Galaxea 1:1–10
Thresher RE (1984) Reproduction in reef fishes. TFH Publications, Neptune City, p 399
Tominaga Y (1964) Notes on the fishes of the genus Siphamia (Apogonidae), with a record of S. versicolor from the Ryukyu Islands. Jpn J Ichthyol 12:10–17
Urbanczyk H, Ogura Y, Hendry TA, Gould AL, Kiwaki N, Atkinson JT, Hayashi T, Dunlap PV (2011) Genome sequence of Photobacterium mandapamensis strain svers. 1.1, the bioluminescent symbiont of the cardinal fish Siphamia versicolor. J Bacteriol 193(12):3144–3145. doi:10.1128/JB.00370-11
Weber M (1909) Diagnosen neuer Fische der Siboga-Expedition. Notes Leyden Museum 31:143–169
White GE, Brown C (2013) Site fidelity and homing behaviour in intertidal fishes. Mar Biol 160:1365–1372. doi:10.1007/s00227-013-2188-6
Yoshiyama RM, Gaylord KB, Philippart MT, Moore TR, Jordan JR, Coon CC, Schalk LL, Valpey CJ, Tosques I (1992) Homing behavior and site fidelity in intertidal sculpins (Pisces: Cottidae). J Exp Mar Biol Ecol 160:115–130. doi:10.1016/0022-0981(92)90114-P
Acknowledgments
We thank K. Dougan (University of Michigan) and S. Kadena (Sesoko Station) for technical assistance. This study is a contribution from Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus. Support was provided by the University of Michigan’s Rackham Graduate School and International Institute, and the Department of Ecology and Evolutionary Biology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Clements.
Rights and permissions
About this article
Cite this article
Gould, A.L., Harii, S. & Dunlap, P.V. Host preference, site fidelity, and homing behavior of the symbiotically luminous cardinalfish, Siphamia tubifer (Perciformes: Apogonidae). Mar Biol 161, 2897–2907 (2014). https://doi.org/10.1007/s00227-014-2554-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2554-z